 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Varicella Surveillance Practices --- United States, 2004Varicella became a reportable disease in the United States in 1972, with states reporting weekly aggregate data to the National Notifiable Disease Surveillance System (NNDSS) (1). In 1981, varicella reporting was removed from the national notifiable diseases list (2) because reporting of this common disease was becoming a burden in the absence of a vaccine. This action was followed by additional changes in varicella surveillance practices (Box). In 1995, varicella vaccine was licensed and added to the routine childhood vaccination schedule. In 2002, the Council of State and Territorial Epidemiologists (CSTE) recommended that varicella case-based surveillance be implemented in all states by 2005; in 2003, varicella again was added to the national notifiable diseases list (3) to allow for monitoring of the effect of varicella vaccine on varicella incidence. In 2004, to assess the progress in varicella surveillance in the United States, CDC surveyed immunization program managers in selected public health jurisdictions. This report describes the results of that survey, which indicated that substantial progress has been made toward the implementation of case-based surveillance as recommended by CSTE in 2002. As of 2004, however, 28 jurisdictions still had not implemented case-based surveillance. To monitor the effect of the vaccination program on the changing epidemiology of varicella disease, every state should now be conducting case-based surveillance for varicella. This is particularly important in light of the 2006 recommendation by the Advisory Committee on Immunization Practices for a routine second dose of varicella vaccine for children aged 4--6 years because enhanced surveillance is needed to further monitor varicella epidemiology. In September 2004, a self-administered survey was distributed to immunization program managers in all 50 states, the District of Columbia (DC), and five cities (Chicago, Illinois; New York, New York; Philadelphia, Pennsylvania; Houston, Texas; and San Antonio, Texas). The survey included questions about varicella reporting and surveillance practices, existing or planned legislation mandating varicella reporting, and barriers to and strategies for implementing varicella case-based surveillance. For the survey, case-based surveillance was defined as the collection and reporting of data on individual cases of varicella. Statewide case-based surveillance was defined as solicitation of varicella case reports from any reporting source within the state, including but not limited to health-care providers, hospitals, and schools. Sentinel-site case-based surveillance was defined as the solicitation of individual varicella case reports from designated reporting sites, such as selected physician offices or schools. Mandated varicella reporting was defined as the existence of a reporting law or regulation requiring reporting of varicella cases to public health officials. Fifty-one (91%) of the 56 jurisdictions responded to the survey, including 46 states (all but Alaska, Mississippi, Nebraska, and New York), DC, and four cities (Chicago, New York, Philadelphia, and San Antonio). Twenty-three (45%) of the respondents (from 19 states, three cities, and DC) reported having established case-based surveillance. Of the 19 states, 15 reported that they had implemented statewide reporting, two reported that they received reports only from sentinel sites, and two did not respond regarding methods for case-based surveillance. Of the three city respondents reporting establishment of case-based surveillance, one was located in a state that reported having established case-based surveillance; the other two cities were in states that were planning to implement case-based surveillance. DC received reports from throughout the jurisdiction. Seventeen (33%) of the respondents indicated that they were planning to implement case-based surveillance, although the time frame for implementation was unknown (Figure 1). The respondents who reported that they had established case-based surveillance stated that they were receiving varicella case reports from hospitals (70% of respondents), emergency departments (68%), outpatient clinics (68%), physician offices (68%), laboratories (68%), elementary and high schools (61%), colleges (58%), and day care facilities (52%). Thirty (59%) of 51 respondents reported that they had mandated varicella case reporting, including 26 states, DC, and three cities; two of the cities are in states that reported having varicella reporting laws. Twenty-one (70%) of these 30 jurisdictions had implemented case-based surveillance, but only four (20%) of the 21 jurisdictions without mandated varicella reporting had implemented case-based surveillance. When queried about the various varicella incidents that are reported (e.g., outbreaks, deaths, individual cases, adult hospitalizations, child hospitalizations, laboratory-confirmed cases, and aggregate cases), a greater proportion of respondents from jurisdictions with a reporting mandate stated that they were being informed about hospitalizations, laboratory-confirmed cases, and aggregate cases than respondents from jurisdictions without a mandate. However, outbreaks and deaths were reported most frequently and at nearly the same rate, regardless of a reporting mandate; 90% of outbreaks and 83% of deaths were reported from states with varicella reporting mandates, and 82% of outbreaks and 77% of deaths were reported from states without varicella reporting mandates (Figure 2). Respondents were questioned about barriers to and strategies for the implementation of case-based surveillance. The most frequently reported barrier was lack of staffing resources (39%). Respondents also identified other barriers, including the absence of a reporting mandate (16%), difficulty establishing support for varicella surveillance among local health department staff and community partners (10%), the perception that varicella is not serious enough to be reported (4%), and the lack of usefulness of the individual case data (1%). The most frequently cited successful strategy in facilitating case-based surveillance implementation was partnering with the reporting community (i.e., groups that report varicella cases, such as physician groups, day care centers, or school nurses) through meetings, e-mails, and newsletters (41%). Reported by: F Averhoff, MD, L Zimmerman, MPH, R Harpaz, MD, D Guris, MD, Epidemiology and Surveillance Div, National Center for Immunization and Respiratory Diseases (proposed); A Rue, MPH, EIS Officer, CDC. Editorial Note:The results of the survey described in this report provide a snapshot of case-based varicella surveillance implementation in the United States in 2004 and help interpret trends in surveillance data as case-based surveillance implementation increases. The findings suggest that substantial progress has been made toward the implementation of case-based surveillance as recommended by the CSTE in 2002; however, 28 jurisdiction respondents still had not implemented case-based surveillance as of 2004. In 2006, 31 jurisdictions are conducting case-based surveillance for varicella. Implementation of a national varicella vaccination program in 1995 resulted in reestablishment of national varicella surveillance. In 1995, only 17 states were reporting varicella to NNDSS, and levels of reporting in states that had reported continually since 1970 had been declining (2). In 1995, CDC initiated support for active surveillance in three geographic areas in the United States; two of these sites continue to provide data that have demonstrated the effect of the vaccination program and the changing epidemiology of varicella disease. These geographic areas have vaccination coverage that is higher than the national average and is therefore not representative of the U.S. population (4). Supplementary surveillance data from national varicella mortality reports and outbreak investigations provide additional information. However, mortality reports measure only severe disease. In addition, although outbreak investigations provide critical data about vaccine effectiveness, they provide more limited data on varicella epidemiology. Because numbers of infected persons usually are small, not all outbreaks are investigated, and the settings in which they are investigated are inconsistent, leading to variable findings. Although morbidity and outbreak data have been important for guiding the varicella vaccination program during the initial years of implementation, more complete national data are necessary to maintain an effective and efficient vaccination program. Case-based surveillance is necessary to 1) assess the effect of vaccination on the epidemiology of varicella, including changes in age distribution and severity of cases; 2) evaluate the proportion of cases in persons who are vaccinated (i.e., breakthrough disease); and 3) assess and monitor vaccine effectiveness. Because the considerable number of cases in the early vaccine era made case-based surveillance impractical in states with limited resources, CSTE initially did not recommend implementation of case-based surveillance in 1995. As a first step to implementing national surveillance, varicella deaths became reportable in 1999 (5), and states were also encouraged by CSTE to establish some form of morbidity reporting (6). In 2002, with rapidly decreasing varicella rates (4), CSTE recommended that national case-based surveillance be implemented by 2005 (7). States with mandatory varicella notification more frequently reported implementing case-based surveillance and receiving reports of varicella incidents. Reports of deaths and outbreaks were less affected by the presence or absence of reporting mandates in the jurisdiction and were the most frequently cited type of report received by public health officials. In addition to the lack of mandatory reporting laws, other barriers to the implementation of case-based surveillance, such as insufficient staffing resources, were identified. A gradual approach to full implementation of case-based surveillance might mitigate the burden of implementation on jurisdictions. CSTE has previously recommended that states consider initiating case-based surveillance using sentinel-site surveillance if statewide case-based surveillance was not feasible initially. Case-based surveillance might also be initiated by collecting only data for key variables (e.g., age of the patient, varicella vaccination history, and severity of disease). Collecting and analyzing this information locally will enable jurisdictions to monitor their own programs. When feasible, jurisdictions can incorporate additional variables, such as other symptoms, complications, and diagnostic laboratory data. While states have been planning and implementing varicella case-based surveillance, CDC has been developing an electronic system capable of accepting individual case reports through NNDSS; this system is expected to be operational in 2006. These data will allow for the monitoring of national trends and will help guide national varicella vaccination policy. As a guide for case-based varicella surveillance, a new varicella worksheet is available from CDC at http://www.cdc.gov/nip/diseases/varicella/default.htm. State and local public health officials with questions regarding implementation of case-based surveillance can contact CDC by telephone, 404-639-8230. Acknowledgments The findings in this report are based, in part, on contributions by state and local health departments. References
Figure 1 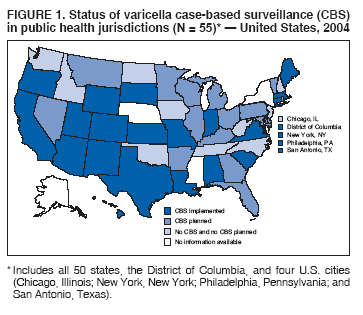 Return to top. Figure 2 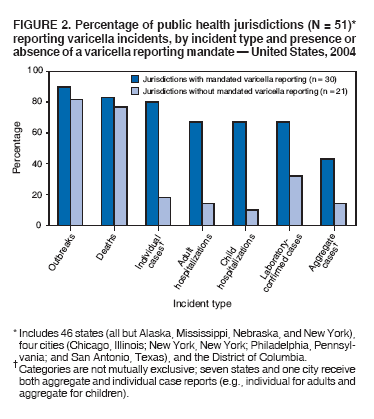 Return to top. Box 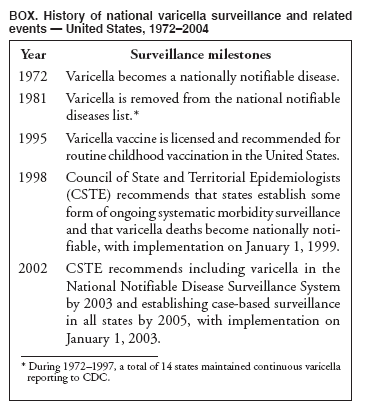 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/19/2006 |
|||||||||
|