 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Achievements in Public Health: Elimination of Rubella and Congenital Rubella Syndrome --- United States, 1969--2004On March 21, this notice was posted as an MMWR Early Release on the MMWR website (http://www.cdc.gov/mmwr). In October 2004, CDC convened an independent panel* of internationally recognized authorities on public health, infectious disease, and immunization to assess progress toward elimination of rubella and congenital rubella syndrome (CRS) in the United States, a national health objective for 2010 (1). Since rubella vaccine licensure in 1969, substantial declines in rubella and CRS have occurred, and the absence of endemic transmission in the United States is supported by recent data: 1) fewer than 25 reported rubella cases each year since 2001 (Figure), 2) at least 95% vaccination coverage among school-aged children, 3) estimated 91% population immunity, 4) adequate surveillance to detect rubella outbreaks, and 5) a pattern of virus genotypes consistent with virus originating in other parts of the world. Given the available data, panel members concluded unanimously that rubella is no longer endemic in the United States. This report summarizes the history and accomplishments of the rubella vaccination program in the United States and the Western Hemisphere and the challenges posed by rubella for the future. Usually a mild rash illness, rubella (also called German measles) can have devastating effects when a pregnant woman is infected, especially during her first trimester. During the 1962--1965 worldwide rubella epidemic, an estimated 12.5 million cases of rubella occurred in the United States, resulting in 2,000 cases of encephalitis, 11,250 fetal deaths, 2,100 neonatal deaths, and 20,000 infants born with CRS, a constellation of birth defects that often includes blindness, deafness, and congenital heart defects. The economic impact of this epidemic in the United States was estimated at $1.5 billion (2). The global epidemic spurred development of rubella vaccines and emphasized the need to develop and implement strategies for using these vaccines to prevent this devastating health burden (3). Rubella Vaccination in the United StatesIn 1969, live, attenuated rubella vaccines were first licensed in the United States (4), and a vaccination program was established with the goal of preventing congenital infections, including CRS. Before the introduction of vaccine, rubella incidence was highest among children aged <9 years (5). The new rubella vaccination program targeted a dose of vaccine to children aged 1 year to puberty (6). Although the greatest impact from rubella results from infections during pregnancy, vaccination of women of childbearing age was not advised because data were not available to assess the possible risk to the fetus if live, attenuated rubella virus vaccine was administered to a pregnant woman. Because of the possible risk to the fetus in women who were vaccinated while unknowingly pregnant, a registry was established to collect pregnancy outcomes (7). To increase coverage among school-aged children rapidly, mass campaigns were conducted, particularly in schools. In some places, these campaigns were also open to younger children. During 1969--1977, an estimated 80 million doses of live, attenuated rubella virus vaccines were distributed in the United States. By 1977, reported vaccination levels were approximately 60% for children aged 1--4 years, 71% for those aged 5--9 years, and 64% for those aged 10--14 years (8). The number of reported rubella cases declined 78%, from 57,686 cases in 1969 to 12,491 cases in 1976. As anticipated, the greatest decreases in rubella occurred among persons aged <15 years; however, incidence declined in all age groups, including adults. This decrease in rubella also resulted in a decline in the number of reported CRS cases, from 68 cases reported in 1970 to 23 reported in 1976 (9). The total number of rubella cases continued to decline overall during the late 1970s; however a resurgence of rubella occurred among older adolescents and young adults, with outbreaks occurring among students in high schools, colleges, universities, and among persons on military bases and workers in hospitals. Rubella incidence was highest among young adults (8,10). In addition, the number of reported CRS cases increased, from 23 in 1976 to 57 in 1979; however, the annual number of CRS cases never reached the level reported during the 1960s in the prevaccine era. Serologic studies at that time suggested that 10%--20% of adults remained susceptible to rubella (11). The resurgence of rubella and its increased incidence among young adults focused attention on the need for additional strategies. In 1978, the changing epidemiology of rubella prompted the Advisory Committee on Immunization Practices (ACIP) to additionally recommend that rubella vaccine be targeted to susceptible postpubertal females, in addition to adolescents, persons in military service, college students, and persons in certain work settings (e.g., hospitals) (12). During 1978--1981, data from rubella vaccinations administered in the public sector (40%--50% of all rubella vaccinations) revealed that the number of doses of rubella vaccine administered to persons aged >15 years had doubled (13). Efforts to increase overall childhood vaccination coverage to greater than 90% for all vaccine-preventable diseases, including rubella, had begun in 1977, with the first National Childhood Immunization Initiative (13). In 1978, a program was undertaken to eliminate indigenous measles in the United States; the use of combined vaccines, either measles-rubella (MR) vaccine or measles-mumps-rubella (MMR) vaccine was encouraged. During 1978--1979, a review of the immunization records of approximately 28 million school-aged children indicated that 83% of students in kindergarten through 12th grade had received rubella vaccine (13). Unvaccinated children were offered vaccine. These efforts to increase immunity among selected adults and children resulted in substantial decreases in the numbers of both rubella and CRS cases. During 1977--1981, reported rubella cases declined from 20,395 to 2,077. During 1979--1981, reported CRS cases decreased from 57 to 10 (9). For the 1981--82 school year, rubella vaccination coverage was 96% for children entering school (i.e., into kindergarten or first grade) in the 50 states and the District of Columbia (14). Efforts to maintain high coverage through enforcement of school immunization laws produced a continuing decrease in reported rubella cases. In 1979, a new formulation of live, attenuated, rubella vaccine (RA 27/3) replaced the previous rubella vaccines in the United States. RA 27/3 vaccine had been determined to induce higher antibody titers and produce an immune response more closely paralleling natural infection than previous vaccines (15). By 1979, rubella vaccination had eliminated the characteristic 6--9 year epidemic cycle of rubella in the United States (9). In 1980, national health objectives for 1990 were established for rubella and CRS, calling for reductions in the annual number of rubella cases to fewer than 1,000 and CRS cases to fewer than 10 (16). During the 1980s, the number of reported rubella cases continued to decline steadily, and overall incidence continued to decrease in all age groups. By 1983, the 1990 objectives already had been achieved, with 970 rubella cases and four CRS cases reported (9,13). During the early 1980s, outbreaks continued to be reported in health-care settings, universities, workplaces, and prisons. In 1981, ACIP recommendations increased emphasis on targeting these settings to ensure vaccination coverage among students and staff members (17). By 1984, with outbreaks continuing (18), ACIP recommendations were expanded to include workers in government offices and at industrial sites (19). In 1988, state health departments reported an all-time low of 225 cases of rubella; however, in 1989, a total of 396 cases were reported, and in 1990, the number increased to 1,125 (20). Most cases were associated with outbreaks that occurred in settings where unvaccinated adults congregated, including colleges, workplaces, prisons, and in religious communities that did not accept vaccination. Outbreaks among these populations accounted for 56% of CRS cases in the 1990s. In 1989, a goal was established to eliminate indigenous rubella transmission and CRS in the United States by 2000 (21). In 1990, recommendations included a new 2-dose schedule (22). Three years later, with establishment of the 1993 Childhood Immunization Initiative, efforts to attain high vaccination coverage were intensified (23). With these efforts, the number of annual rubella cases continued to decline in the mid-1990s. Outbreaks continued to be associated with settings where adults had close contact; however, the demographic characteristics of rubella patients changed. Before 1995, most persons with rubella were non-Hispanic; beginning in 1995, most were Hispanic (24). Beginning in 1998, data on country of origin were collected for rubella patients. These data revealed that, during 1998 and 1999, approximately 79% and 65% of patients whose country of origin was known were foreign-born. Of these, 91% in 1998 and 98% in 1999 were born in the Western Hemisphere, and 43% in 1998 and 81% in 1999 were born in Mexico. These persons were either unvaccinated or their vaccination status was unknown. Although no new recommendations were implemented, emphasis was increased on identifying and vaccinating foreign-born adults. During 1998--2000, a total of 23 CRS cases were reported to CDC. The infants in 22 (96%) of these cases were born to Hispanic women, and 22 of the mothers with known country of birth were born outside the United States. The countries of origin of these mothers were Mexico (14 mothers), Dominican Republic (four), Honduras (two), Colombia (one), and Philippines (one). A nationwide rubella seroprevalence study during 1988--1994 demonstrated overall rubella seropositivity of 89% (25), which, according to a mathematical model, is above the level needed to interrupt transmission of rubella virus and sustain elimination. Since 2001, the annual numbers of rubella cases have been the lowest ever recorded in the United States: 23 in 2001, 18 in 2002, seven in 2003, and nine in 2004. Approximately half of these cases have occurred among persons born outside the United States, of whom most were born outside the Western Hemisphere. During 2001--2004, four CRS cases were reported to CDC; the mothers of three of the children were born outside the United States. Low numbers of cases and geographic and temporal distribution of cases support the conclusion that rubella is no longer endemic in the United States. Specifically, CDC defines absence of endemic transmission as the lack of existence of any continuous U.S.-acquired chain of transmission that persists for >12 months in any defined geographic area. In 2004, the panel convened by CDC concluded that sustained transmission of rubella has been interrupted. Rubella Vaccination in the Western HemisphereThe changing epidemiology of rubella in the United States during the preceding 10 years reflected efforts to control the disease elsewhere in the Western Hemisphere. The burden of rubella was increasingly recognized in countries as their vaccination programs succeeded in controlling measles, whose symptoms can resemble rubella. In 1997, a Technical Advisory Group for the Pan American Health Organization (PAHO) recommended strategies for rubella control and CRS prevention (26). In 2003, with the success of accelerated rubella control, PAHO member countries voted to establish a goal to eliminate rubella and CRS from the Western Hemisphere by 2010 (27). As of 2004, a total of 43 of 44 countries and territories in the region had included rubella vaccine in their routine immunization programs. For countries reporting rubella cases to PAHO, the number of reported rubella cases dropped from 135,947 in 1998 to fewer than 1,000 cases in 2003. Global ChallengesWith elimination of endemic chains of rubella transmission in the United States, future patterns of rubella will most likely reflect global disease epidemiology. Since 1998, most non-U.S.-born cases of rubella reported in the United States have occurred among persons born in countries where rubella vaccination has not been or was only recently implemented. According to a survey of the member countries in the World Health Organization, the number of countries that have incorporated rubella-containing vaccine into their routine national immunization programs increased from 65 (33%) in 1996 to 110 (57%) in 2003. However, rubella continues to be endemic in many parts of the world. The United States should continue its vigilance against rubella and CRS by 1) maintaining high vaccination rates among children; 2) ensuring vaccination among women of childbearing age, especially women born outside the United States; 3) continuing surveillance of both rubella and CRS; and 4) responding rapidly to any outbreak. Reported by: Epidemiology and Surveillance Div, National Immunization Program; Div of Viral and Rickettsial Disease, National Center for Infectious Diseases, CDC. Immunization Unit, Family and Community Health, Pan American Health Organization/World Health Organization, Washington, DC. References
* The panel included authorities on rubella and representatives of the Advisory Committee on Immunization Practices, American Academy of Pediatrics, American Academy of Family Physicians, Pan American Health Organization, Council of State and Territorial Epidemiologists, March of Dimes, and Mexico.
Figure 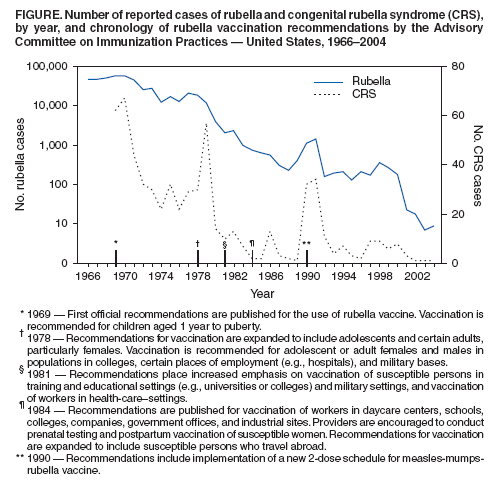 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/24/2005 |
|||||||||
This page last reviewed 3/24/2005
|