 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hepatitis A Vaccination Coverage Among Children Aged 24--35 Months --- United States, 2003Hepatitis A vaccine was first licensed in the United States in 1995. In 1996, the Advisory Committee on Immunization Practices (ACIP) recommended vaccination of children aged >24 months in populations with the highest incidence of hepatitis A (e.g., American Indian/Alaska Native [AI/AN], Asian/Pacific Islander, and selected Hispanic and religious communities) (1). In 1999, these guidelines were expanded to recommend routine vaccination for children residing in 11 states* where average annual hepatitis A incidence during 1987--1997 was at least 20 per 100,000 population (twice the national average) and to consider routine vaccination for children in six states† where average annual incidence was 10--20 per 100,000 population (2). This report is the first national analysis of hepatitis A vaccination coverage among children. The results indicate that, in 2003, vaccination coverage levels with at least 1 dose of hepatitis A vaccine for children aged 24--35 months varied from 6.4% to 72.7% in areas where routine vaccination is recommended (Table). In addition, hepatitis A vaccination coverage rates for children aged 24--35 months are lower than overall rates for other vaccines recommended for children (3). Sustaining and improving vaccination coverage among young children is needed to ensure continued declines in hepatitis A incidence in the United States. The National Immunization Survey (NIS) provides annual estimates of vaccination coverage as of the time of household interview among children aged 19--35 months for the 50 states and 28 selected urban areas. In 2003, NIS began to collect data regarding hepatitis A vaccination coverage. Hepatitis A vaccine is a 2-dose regimen (administered at least 6 months apart) licensed for use in children aged >24 months. Hepatitis A vaccination coverage data were limited to children aged 24--35 months and calculated by considering children who had received at least 1 vaccine dose. To collect vaccination data for all age-eligible children, NIS uses a quarterly, random-digit--dialing sample of telephone numbers for each of the 78 survey areas and determines vaccination status from health-care provider records (4,5). During 2003, information on vaccination history was collected from telephone interviews for 19,979 children; provider verified vaccination records were available for 13,731 (68.7%). Among children aged 24--35 months residing in the 11 states where routine hepatitis A vaccination is recommended, 50.9% (95% confidence interval [CI] = 47.6%--54.2%; range among states: 6.4%--72.7%) received at least 1 dose of hepatitis A vaccine. Among children aged 24--35 months residing in the six states where routine hepatitis A vaccination should be considered, 25.0% (CI = 21.8%--28.2%; range: 0.6%--32.3%) had received at least 1 dose of hepatitis A vaccine. Among children aged 24--35 months residing in the 33 states without a specific recommendation, 1.4% (CI = 1.0%--1.8%; range: 0.0%--4.3%) had received at least 1 dose of hepatitis A vaccine (Table). Two states (Alaska and Arizona) and four urban areas had coverage estimates >60%. Hispanic and AI/AN children had higher coverage rates than non-Hispanic white or black children in areas where routine vaccination is recommended or should be considered (Figure). Reported by: A Fiore, MD, B Bell, MD, Div of Viral Hepatitis, National Center for Infectious Diseases; L Barker, PhD, N Darling, MPH, National Immunization Program; J Amon, PhD, EIS Officer, CDC. Editorial Note:The national hepatitis A vaccination coverage estimates described in this report indicate that, in 2003, current hepatitis A childhood vaccination recommendations were being implemented in many states. However, coverage varied among areas and populations, likely because of targeted programs within these states. For example, higher coverage in El Paso County, Texas (71%), compared with the overall Texas coverage rate (32%), likely is attributable to vaccination requirements in Texas border counties for all children attending child care programs. Vaccination coverage also varied by race/ethnicity. Higher coverage among Hispanic and AI/AN children than among children of other racial/ethnic populations might be related to greater disease recognition in these populations and local and national vaccination recommendations that have identified these populations as having higher hepatitis A rates (6,7). The findings in this report are subject to at least three limitations. First, NIS is a telephone survey; although statistical weights adjust for nonresponse and households without telephones, some bias might remain. Second, although NIS relies on provider-verified vaccination histories, incomplete records or reporting could result in underestimates of coverage. Finally, although national estimates are reliable, estimates for states and urban areas and for racial/ethnic populations should be interpreted with caution (8). The 1999 ACIP hepatitis A prevention recommendations encouraged state and local immunization programs to analyze their surveillance data and implement vaccination strategies that address the epidemiology of hepatitis A in their areas. The variation by state in coverage among children aged 24--35 months likely reflects the varying vaccination strategies adopted by state and local public health officials in response to the ACIP recommendations. Higher coverage among Hispanic and AI/AN children is one indication that vaccination efforts targeting children at higher risk for illness have been successful. These data do not provide information on why hepatitis A vaccination coverage for children aged 24--35 months remains below that for other childhood vaccinations in most areas where it is recommended. Low coverage rates for young children might be the result of 1) a focus by health-care providers and immunization programs on vaccinating older children, 2) the few areas with hepatitis A vaccine mandates (9), or 3) the lack of a licensed hepatitis A vaccine that can be administered to children aged <24 months. Sustaining and improving vaccination coverage among young children is needed to ensure continued declines in hepatitis A incidence in the United States. References
* Alaska, Arizona, California, Idaho, Nevada, New Mexico, Oklahoma, Oregon, South Dakota, Utah, and Washington. † Arkansas, Colorado, Missouri, Montana, Texas, and Wyoming.
Table 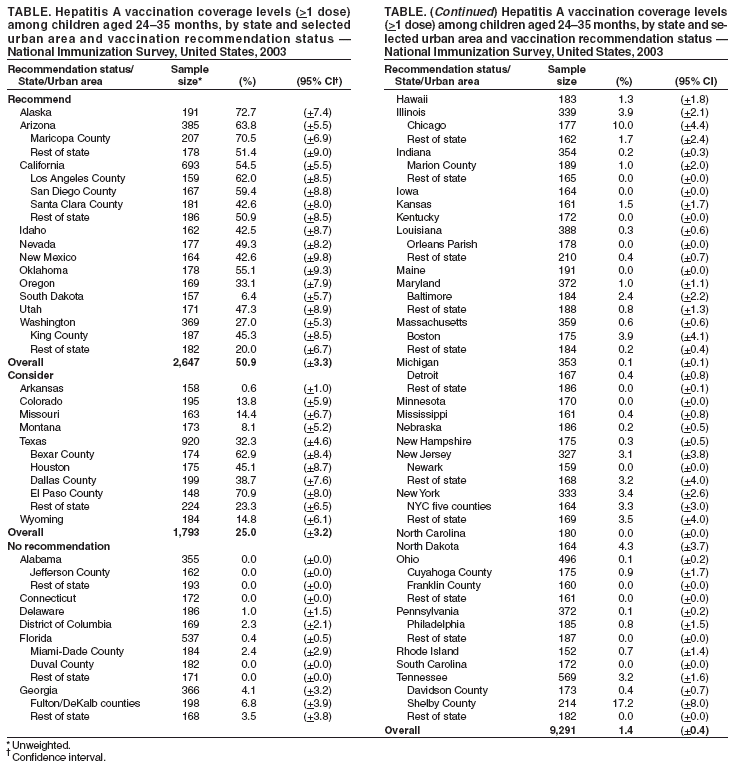 Return to top. Figure 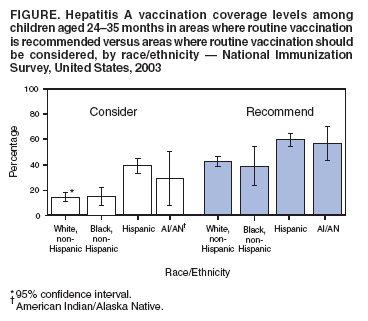 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/17/2005 |
|||||||||
This page last reviewed 2/17/2005
|