 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
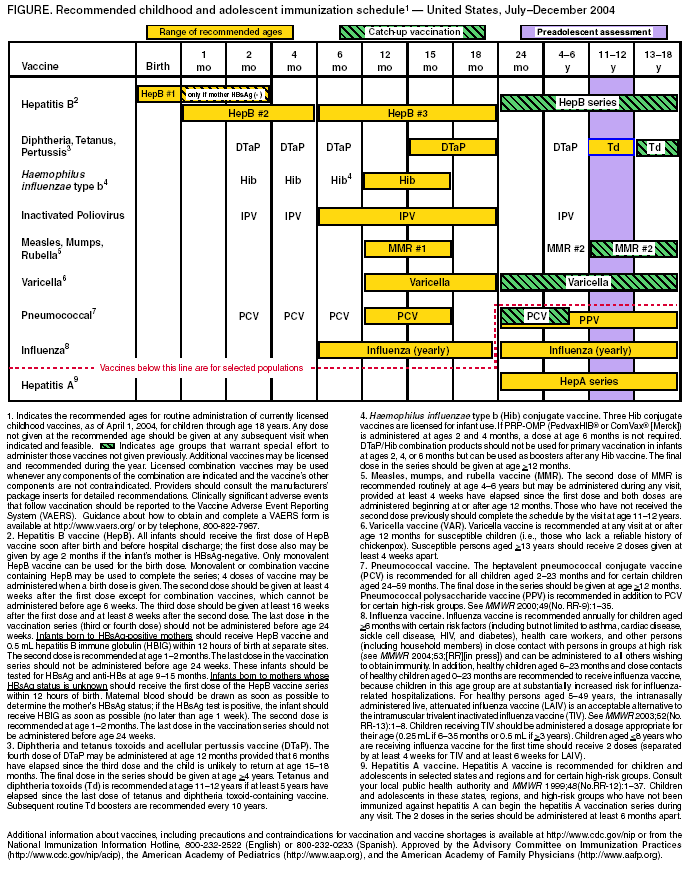
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommended Childhood and Adolescent Immunization Schedule --- United States, July--December 2004CDC’s Advisory Committee on Immunization Practices (ACIP) periodically reviews the recommended childhood and adolescent immunization schedule to ensure that the schedule is current with changes in manufacturers’ vaccine formulations and reflects revised recommendations for the use of licensed vaccines, including those newly licensed. Recommendations and format of the childhood and adolescent immunization schedule for January–June 2004 were approved by ACIP, the American Academy of Family Physicians (AAFP), and the American Academy of Pediatrics (AAP) and published in January 2004 (1). This report updates that schedule with the recommendation that, beginning in fall 2004, children aged 6–23 months, as well as household and out-of-home caregivers for such children, receive annual influenza vaccine (2). This change is reflected in the revised childhood and adolescent immunization schedule for July–December 2004 (Figure). A catch-up immunization schedule for children and adolescents who start late or who are >1 month behind remains unchanged from that published in January 2004 (Table). Changes in the Schedule for July– December 2004The childhood and adolescent immunization schedule for July–December 2004 differs from the previous schedule in the following ways:
Vaccine Information StatementsThe National Childhood Vaccine Injury Act requires that all health-care providers provide parents or patients with copies of Vaccine Information Statements before administering each dose of the vaccines listed in the schedule. Additional information is available from state health departments and at http://www.cdc.gov/nip/publications/vis. Detailed recommendations for using vaccines are available from the manufacturers’ package inserts, ACIP statements on specific vaccines, and the 2003 Red Book (3). ACIP statements for each recommended childhood vaccine can be viewed, downloaded, and printed from CDC’s National Immunization Program website at http://www.cdc.gov/nip/publications/acip-list.htm. Instructions on the use of Vaccine Information Statements are available at http://www.cdc.gov/nip/publications/ vis/vis-instructions.pdf. In addition, guidance on how to obtain and complete a Vaccine Adverse Event Reporting System (VAERS) form is available at http://www.vaers.org or by telephone, 800-822-7967. References
 Return to top. Table 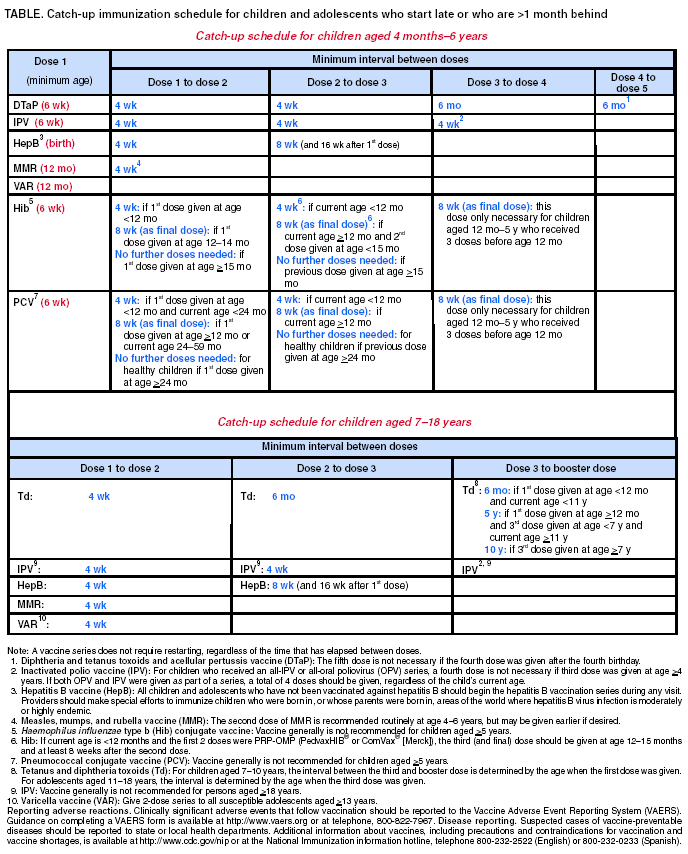 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/29/2004 |
|||||||||
This page last reviewed 4/29/2004
|