 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward the Global Interruption of Wild Poliovirus Type 2 Transmission, 1999Since 1988, when the World Health Assembly resolved to eradicate poliomyelitis globally by 2000 (1), substantial progress has been made in attaining this goal: the Americas, the Pacific Rim, Europe, and central Asia appear to be polio-free. The remaining reservoirs where polio is endemic are confined to India and contiguous countries and to sub-Saharan Africa. In 1999, the recommended polio eradication strategies (i.e., achieving and maintaining high routine vaccination coverage with oral poliovirus vaccine [OPV]; conducting National Immunization Days [NIDs]* to decrease rapid poliovirus circulation; establishing sensitive surveillance systems for polio cases and poliovirus; and carrying out mopping-up vaccination activities** to eliminate poliovirus transmission) have been accelerated in most of the major reservoir countries*** (2,3). This report summarizes progress toward interrupting transmission of wild poliovirus type 2, which appears to be on the threshold of extinction. The goal of the polio eradication initiative is to interrupt all chains of wild poliovirus transmission globally. Most poliovirus genotypes (i.e., a group of polioviruses sharing greater than 85% nucleotide sequence similarity in the capsid genes) found in 1988 have disappeared (4). The genetic diversity of the remaining genotypes has been reduced as chains of transmission are broken and reservoir countries become polio-free. Successive Extinction of Wild Poliovirus Type 2 Genotypes During the prevaccine era, the three poliovirus serotypes were distributed worldwide. Continuous transmission occurred in large population centers, and sporadic outbreaks occurred in isolated communities (4,5). By the mid-1960s, the incidence of cases associated with wild poliovirus type 2 had declined rapidly in areas with high vaccination coverage rates. By the mid-1970s, indigenous wild type 2 polioviruses had disappeared from Australia, Japan, North America, and western Europe (Figure 1). By 1980, type 2 poliovirus had been eliminated in Brazil, Central America, Mexico, and South Africa, and in China and the Soviet Union by 1985. Wild poliovirus type 2 circulation continued until the late 1980s in Colombia, Peru, and Vietnam. The last indigenous wild poliovirus type 2 isolates were found in Egypt in 1990, in Afghanistan and Pakistan in 1997, and in Nigeria in 1998 (Figure 1). Although no wild poliovirus type 2 isolates have been reported from Africa for greater than 1 year, inadequate surveillance in some African countries, particularly Angola, the Democratic Republic of the Congo, and Ethiopia, makes these data difficult to interpret. By 1999, the only known reservoir for wild type 2 polioviruses was in the Ganges valley of India (6). Areas with Wild Poliovirus Type 2 Circulation Endemic circulation of type 2 poliovirus appears to be localized to the northern Indian states of Uttar Pradesh and Bihar (1998 estimated combined population: 250 million). Before accelerated efforts were initiated to eradicate polio in 1995, wild poliovirus type 2 was distributed widely in India, and clinical isolates showed high genetic diversity, indicating multiple independent reservoirs. Isolates from 1998-1999 are closely related to each other, meaning type 2 endemicity is sustained by a few chains of transmission. The states of Uttar Pradesh and Bihar have been at particularly high risk for continued poliovirus transmission (6,7). In these states, the critical risk factors are low vaccination coverage, high population densities, large annual birth cohorts, poor sanitation, and humid subtropical climate. To overcome these challenges to polio control and to interrupt poliovirus transmission, the government of India is planning to conduct four rounds of NIDs from October 1999 through January 2000, followed by two rounds of Sub-National Immunization Days (SNIDs) in Uttar Pradesh, Bihar, and six additional high-risk states during February-March 2000. Reported by: Vaccines and Other Biologicals Dept, World Health Organization, Geneva, Switzerland. Respiratory and Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial Note:The usual order of disappearance of wild polioviruses within a country or region has been type 2, type 3, and type 1 (4,5). The high immunogenicity of type 2 polioviruses in OPV and the efficient spread of type 2 OPV-derived strains to contacts (8) appear to be important factors contributing to the rapid control of this serotype. Continued detection of wild poliovirus type 2 circulation reflects serious deficiencies in vaccination coverage levels. The year of cessation of wild poliovirus type 2 circulation is uncertain in many countries because of inadequate surveillance for cases and because of the imprecision of earlier methods for distinguishing wild from vaccine-derived polioviruses (4). Type 2 polioviruses are the most difficult to detect through polio case surveillance because they have the lowest case:infection ratio (approximately 1:2000) of the three serotypes (5). Consequently, the number of wild poliovirus type 2 isolates available for analysis is smaller than for the other two serotypes. During the prevaccine era, wild poliovirus type 2 genotypes had wide geographic distribution (4), and the early estimates of the years of elimination probably applied to groups of countries (e.g., western Europe or eastern South America) rather than specific countries. These early extinction estimates are conservative, and are based in part on the years when exogenous genotypes were first detected in cases and outbreaks, which suggested that indigenous circulation had ceased already. Wild poliovirus type 2 circulation might persists in the major reservoir countries of Angola, the Democratic Republic of Congo, and Ethiopia (2), where vaccination coverage levels remain low and polio surveillance remains inadequate. However, only poliovirus types 1 and 3 have been detected in these or neighboring countries. Within the next year the only type 2 polioviruses found in nature probably will be OPV-derived. However, intensification of vaccination and surveillance activities will be needed to meet the 2000 goal for the eradication of all wild poliovirus serotypes. References
* Nationwide mass campaigns over a short period (days to weeks), in which two doses of OPV are administered to all children in the target age group (usually aged less than 5 years), regardless of previous vaccination history, with an interval of 4-6 weeks between doses. ** Focal mass campaigns in high-risk areas during a short period (days to weeks) in which two doses of OPV are administered during house-to-house visits to all children in the target age groups, regardless of previous vaccination history, with an interval of 4-6 weeks between doses. *** Countries where polio is endemic that have large populations and that may export poliovirus to neighboring countries and elsewhere. Figure 1 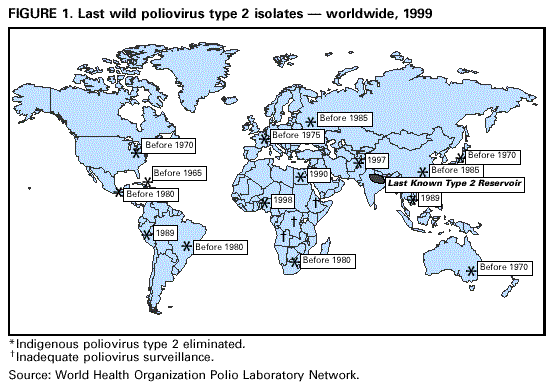 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 8/26/1999 |
|||||||||
This page last reviewed 5/2/01
|