 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Sustained Transmission of Nosocomial Legionnaires Disease -- Arizona and OhioIn 1996, two hospitals reported sustained transmission of nosocomial Legionnaires disease (LD). The hot water distribution systems in each hospital were implicated as the sources of infection. This report summarizes investigations in these two hospitals by hospital personnel, state and local health officials, and CDC and efforts to control transmission. Arizona, 1987-1996 In 1996, eight cases of nosocomial LD were diagnosed among cardiac and bone marrow transplant patients at hospital X. Possible nosocomial LD was first reported at hospital X in 1979, but no source had been identified. Intensified surveillance for nosocomial LD was initiated after the first three case-patients were identified in 1996. A case of definite nosocomial LD in a hospital X patient was defined as respiratory illness with a new infiltrate on chest roentgenogram occurring after greater than or equal to 10 days of continuous hospitalization for a nonpneumonia illness and laboratory confirmation of legionellae infection by at least one of the following: 1) isolation of legionellae from tissue or respiratory secretions, 2) detection of Legionella pneumophila serogroup 1 (Lp-1) antigens in urine by radioimmunoassay or enzyme immunoassay, or 3) a fourfold rise in Legionella serogroup-specific antibody titer to greater than or equal to 128 between acute- and convalescent-phase serum specimens. Possible nosocomial LD was defined as onset of respiratory symptoms of LD after 2-9 days of continuous hospitalization (the incubation period for LD is usually 2-10 days). Through intensified surveillance and examination of infection-control and microbiology laboratory records, 25 cases of LD linked to hospitalization during 1987-1996 were identified; 16 were definite cases, and nine were possible cases (Figure_1). All were diagnosed by culture. The median age of case-patients was 56 years (range: 17-81 years); 13 (52%) were male. Most case-patients had received either heart or heart/lung transplants (11 {44%}) or bone marrow transplants (seven {28%}). Seven (28%) other patients were either immunocompromised (four) or had some form of chronic illness (three). Twelve (48%) patients died during their hospitalization; eight of these patients had LD identified on autopsy. During January-September 1996, cases of nosocomial LD occurred among eight (6%) of the 134 cardiac and bone marrow transplant patients. Based on a case-control study that matched the 25 case-patients with 49 controls (only one appropriate control was available for one case-patient) by age, date of admission to hospital X, and underlying medical condition, no single risk factor for acquisition of disease was identified. However, information about exposure to showers, other aerosol sources, or ingested water for some patients was unavailable. During August 1996, Lp-6, Lp-11, L. anisa, and a Legionella-like organism designated D-1620 were cultured from the hot water distribution system, and Lp-1, Lp-4, Lp-6, and Lp-11 were cultured from swabs and water samples from water softeners. Water obtained from the wellhead of a private well that supplied some areas of the hospital contained Lp-1. Lp-6 was cultured from samples obtained from taps and showers in patients' rooms and a carpet-cleaning unit used on the transplant ward. Air sampling within patient showers identified Lp-6 in respirable (1-5-micron) droplets. Seven patient isolates from 1996 were serogrouped. Of these, one was a serogroup 10; the remaining six were Lp-6, and five of these six were identical to Lp-6 environmental isolates from water softeners, showers, and shower aerosols by pulsed-field gel electrophoresis. Thermal decontamination of the hot water distribution system had been conducted in July 1996, but legionellae were later isolated from the potable water; three cases occurred after thermal decontamination. In response, the hot water distribution system was hyperchlorinated, and the water temperature at the taps was maintained at 120 F (49 C); however, following these measures, Lp-6 was again cultured from the potable water. As a result, additional measures that were implemented included installing chlorine injection devices, removing areas of low flow ("deadlegs") in the potable water plumbing, disconnecting the water softeners, and repeating the hyperchlorination procedures. No new cases of nosocomial LD have been identified at hospital X since September 1996. Although cultures of potable water samples from the distribution system of a unit where transplant patients were not present were positive for L. bozemanni in December 1996, subsequent samples from other areas have been negative. Ohio, 1989-1997 During January-June 1996, nosocomial LD occurred in two patients at hospital Y. Nosocomial LD transmission had occurred at hospital Y in 1977; however, an epidemiologic investigation had not identified the source of transmission. Beginning in 1989, as part of surveillance for nosocomial LD, urine samples from all patients with nosocomial pneumonia were tested for Lp-1 antigen. Cases of nosocomial LD were defined as in the investigation in Arizona. Examination of infection-control and microbiology laboratory records from 1989 through 1996 identified nine patients with definite nosocomial LD and 29 patients with possible nosocomial LD (Figure_2). The median age for these 38 patients was 65 years (range: 36-85 years); 21 (55%) patients were male. Fifteen (39%) had at least one underlying chronic medical illness, and another 13 (34%) were immunocompromised by disease or immunosuppressive medication. Six (16%) were inpatients on the psychiatric ward. Eleven (29%) died during their hospitalization. A case-control study was conducted to assess potential risk factors for infection, matching 36 case-patients (no charts were available for two patients) and 72 controls by age, date of discharge from hospital Y, and underlying condition. Information about exposure to showers, pneumatic nebulizers, other aerosol sources, or ingested water was incomplete for some case-patients and for some controls. However, case-patients were more likely than controls to have had documented exposure to common aerosol-producing devices (showers and/or medication nebulizers) during the 2 weeks before onset (matched odds ratio {MOR}=2.9; 95% confidence interval {CI}=1.2-74.0). Medical (nonpsychiatric) case-patients were more likely than controls to have received at least one medication by nebulizer during the 2 weeks before onset (MOR=3.2; 95% CI=1.1-10.6); however, only 40% of medical case-patients had received nebulized medication. Review of respiratory therapy practices indicated that nebulizer equipment was sometimes rinsed with tap water between doses to reduce clogging. An increased risk for nosocomial LD also was associated with hospitalization in only one of the three inpatient medical/surgical buildings (building 1) (MOR=5.0; 95% CI=1.5-22.5) and within the psychiatric facility (MOR=undefined; 95% CI=3.1-infinity). However, all three medical/surgical inpatient buildings and the inpatient psychiatric facility were implicated as sites of transmission. Lp-1 was isolated from samples obtained from multiple sites in the hot water distribution system during 1994-1996, and the percentage of outflow sites testing positive was consistently highest in building 1 and the psychiatric building. All Lp-1 isolated from potable hot water samples in 1994-1996, as well as Lp-1 isolated from potable water in 1984 and from a hospital Y patient with nosocomial LD in 1985, were identical to the three clinical isolates from 1992, 1994, and 1995 by monoclonal antibody and arbitrarily-primed polymerase chain reaction subtyping. Although L. pneumophila was recovered from cooling tower reservoir water collected from two hospital facilities, these isolates were serogroups other than Lp-1. Periodic culturing of the hot water distribution system at hospital Y since 1994 had been used to guide decontamination efforts. Thermal (heating to 160 F {71 C} at the tap for 5 minutes) and chlorine (maintaining a chlorine level of 1-2 mg/L at the tap for at least 5 minutes) decontamination had been only temporarily effective in reducing the number of sites positive for Lp-1. A copper-silver ionization system installed in 1995 neither reduced the number of positive samples nor terminated transmission. Interventions recommended at the conclusion of this investigation in June 1996 included discontinuing the use of tap water to rinse medical nebulizer equipment, repeating the hyperchlorination procedure as needed in response to positive potable water cultures, increasing the hot water temperature at the point-of-use to at least 120 F (49 C), and identifying deadlegs in the potable water plumbing. Following these interventions, no new cases of nosocomial transmission were identified until February 28, 1997, when a case of possible nosocomial LD occurred in a patient in a critical-care unit. Lp-1 isolates from a sample of the patient's lung tissue and from the potable water supply in his room were identical to all previous isolates by monoclonal antibody subtyping. Hospital personnel discovered a previously undocumented cross-connection between the hot-water tank from an adjacent outpatient-care building and the critical-care unit. This tank was cleaned, and the supply system hyperchlorinated. No new cases have been identified at hospital Y since March 1997. Reported by: C Kioski, MPH, G Cage, B Johnson, C Rosales, B England, MD, State Epidemiologist, Arizona Dept of Health Svcs. TJ Halpin, MD, State Epidemiologist, Div of Preventive Medicine, Ohio Dept of Health. Childhood and Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: The findings in these and other recent investigations (1) indicate the capacity for legionellae to colonize hospital plumbing systems for long periods and, in the absence of effective preventive measures, to represent an ongoing risk for infection. Colonization rates are higher in large hospitals with older, large hot-water tanks in which water is held at lower temperatures (2). Hospital X served a large bone marrow and organ transplant patient population, and nosocomial legionellae transmission resulted in substantial morbidity and mortality during at least a 17-year period in this group of immunocompromised patients. Standard respiratory disease infection-control measures (high efficiency particulate air filtered positive-pressure rooms, patient use of surgical masks while out of the room, and use of sterile water in respiratory therapy equipment) were insufficient to prevent transmission within hospital X. At hospital Y, long-term interruption of transmission had not been achieved since recognition of legionellae transmission in 1977, despite use of a variety of strategies including hyperchlorination, thermal disinfection, and metal ionization. In addition, incomplete plumbing system records resulted in delayed treatment of appropriate potable water systems. Nosocomially acquired LD accounts for a substantial proportion of all reported cases of this disease: during 1980-1989, of 3524 cases reported to CDC, 23% were nosocomial, and mortality was 40%, compared with a mortality of 20% in community-acquired disease (3). Numerous outbreaks of nosocomial LD have been reported since the etiologic agent of LD was first identified in 1977 (4). When a case of nosocomial LD is recognized, additional cases often will be identified (5). Therefore, identification of one patient with definite nosocomial LD or two persons with possible nosocomial LD within 6 months should prompt an epidemiologic investigation. Heightened prospective surveillance for additional cases and a retrospective review of serologic, microbiologic, and postmortem data to identify previously unrecognized cases also should be instituted (4). Diagnosis of nosocomial LD requires heightened clinical suspicion and special laboratory techniques. For disease caused by Lp-1, urinary antigen detection is a rapid and highly specific diagnostic test (6). Infection with non-serogroup 1 L. pneumophila and other legionellae requires culture, paired serum antibody titers, or direct fluorescent antibody testing for diagnosis. The decision to initiate an environmental investigation should consider both the type of patient population exposed and the level of risk for nosocomial transmission. Routine culturing of potable hospital water has been advocated (7) even in the absence of identified LD transmission; however, additional data are needed to define the relation between water culture results and risk for disease. Legionellae are found in natural and man-made aquatic environments, but growth to high concentrations occurs most often at water temperatures of 77 F-108 F (25 C-42 C). Concern about the risk for scalding injuries has prompted some jurisdictions to regulate temperatures in hospital hot water systems at levels conducive to legionellae growth. Additions and alterations to hospital plumbing systems in response to changing hospital facility needs may create areas of stagnation and sediment buildup, factors also shown to enhance legionellae colonization (8,9). These stagnant areas may be resistant to chlorination, thermal disinfection, and ionization. Respiratory therapy equipment should be rinsed only with sterile (not distilled) water. When water distribution systems are suspected as the source of nosocomial LD, patient exposure to potable water aerosols and ingestion of potable water should be minimized. Standard decontamination procedures for potable hot water systems include thermal disinfection and hyperchlorination. Because eradication of legionellae from water distribution systems generally is not possible, a maintenance program to minimize regrowth of legionellae should also be implemented. Raising the water temperature to 120 F-125 F (49 C-52 C) at the fixture (although higher temperatures may increase the risk of scalding) and infusion of chlorine to maintain consistent levels of 1-2 mg/L have been employed to achieve long-term decontamination in many hospitals. Although metal ionization systems may be effective, whether they offer an advantage over conventional methods is unclear; in hospital Y, nosocomial transmission persisted after installation of an ionization system. Detailed guidelines on the prevention of nosocomial LD, including decontamination procedures for contaminated potable water and cooling systems, have been published (4) and are available electronically on the World-Wide Web at http://www.cdc.gov/ncidod/diseases/hip/pneumonia/pneu_mmw.htm. References
Figure_1 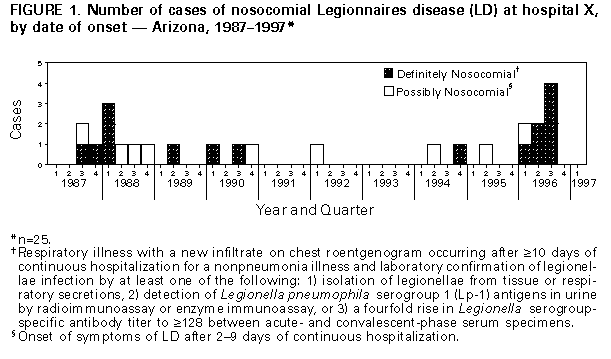 Return to top. Figure_2 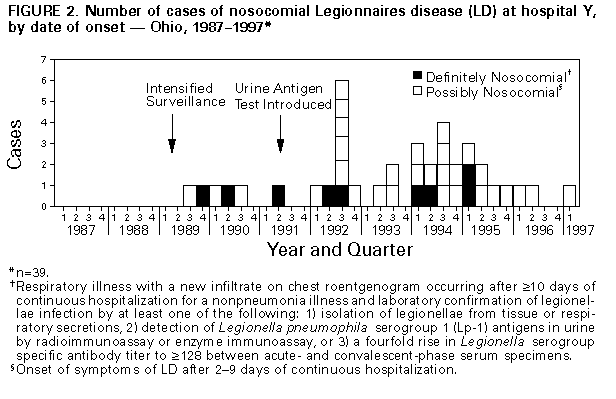 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|