

|

|

Volume 1:
No. 4, October 2004
ORIGINAL RESEARCH
A Randomized Controlled
Open Trial of Population-based Disease and Case Management in a Medicare
Plus Choice Health Maintenance Organization
David C. Martin, MD, Marc L. Berger, MD, David T. Anstatt, MBA, Jonathan
Wofford, MPH, DeAnn Warfel, JD, Robin S. Turpin, PhD, Carolyn C. Cannuscio,
ScD, Steven M. Teutsch, MD, MPH, Bernard J. Mansheim, MD
Suggested citation for this article: Martin DC, Berger ML, Anstatt DT,
Wofford J, Warfel D, Turpin RS, et al. A randomized controlled open trial
of population-based disease and case management in a Medicare Plus Choice health
maintenance organization. Prev Chronic Dis [serial
online] 2004 Oct [date cited]. Available from: URL:
http://www.cdc.gov/pcd/issues/2004/
oct/04_0015.htm.
PEER REVIEWED
Abstract
Introduction
The object of this study was to examine the effect of population-based
disease management and case management on resource use,
self-reported health status, and member satisfaction with and retention in a
Medicare Plus Choice health maintenance organization (HMO).
Methods
Study design consisted of a prospective, randomized controlled open trial
of 18 months’ duration. Participants were 8504 Medicare beneficiaries
aged 65 and older who had been continuously enrolled for at least 12 months
in a network model Medicare Plus Choice HMO serving a contiguous nine-county
metropolitan area. Members were care managed with an expert clinical
information system and frequent telephone contact. Main outcomes included
self-reported health status measured by the Medical Outcomes Study 36-Item
Short-Form Health Survey (SF-36), resource use measured by
admission rates and bed-days per thousand per year, member satisfaction, and
costs measured by paid claims.
Results
More favorable outcomes occurred in the intervention group for satisfaction
with the health plan (P < .01) and the social function domain as
measured by SF-36 (P = .04). There was no difference in member
retention or mortality between groups. Use of skilled nursing home services
was significantly lower in the intervention group than in the control (616 vs
747 days per thousand members per year, P = .02). This reduction,
however, did not lead to lower mean total expenditures in the intervention
group compared with the control ($6828 per member for 18 months vs $7001, P
= .61).
Conclusion
Population-based disease management and case management led to improved
self-reported satisfaction and social function but not to a global net
decrease in resource use or improved member retention.
Back to top
Introduction
Although the United States is a wealthy country, our elders suffer from
high rates of chronic disease, social isolation, poor diet, lack of
mobility, and suboptimal function (1,2).
Indeed, chronic disease has now become the greatest challenge to the
health care system, accounting for 76% of direct health care costs (not
including nursing home care) (3). By not adequately addressing
these issues, our elderly population ranks lower than many other
industrialized countries on a number of public health measures (4-6).
To give Medicare recipients the option to enroll in health maintenance
organizations (HMOs), Congress created the Medicare Plus Choice program with
passage of the Balanced Budget Act in 1998. Many HMOs attempt to improve
health outcomes and reduce medical expenditures through disease management
and case management. Several case management demonstration projects have
failed to affect service use, cost, or health outcomes (7). As one senior
health services researcher notes, “The case for case management still
remains to be established definitively” (8). Still, managing chronic disease
through a combination of traditional institutional-based health care and
community-based interventions, supported by clinical information systems,
remains a compelling model (8-11). Several variations of this comprehensive
approach to preventive and chronic care are being tested (12).
A newer paradigm is that of population-based disease management, wherein
subgroups of patients that have modifiable risk factors for adverse medical
outcomes are identified and then entered into a program designed to improve
self-care (13). Because it is not known if disease management programs can
improve health outcomes or produce long-term savings (14), the concept of
testing population-based disease management in fee-for-service Medicare is
under study at the Centers for Medicare & Medicaid Services (13).
Most reported trials have investigated the efficacy of disease management
on single disease states, such as congestive heart failure or chronic
obstructive lung disease. We report a randomized controlled open trial of
case management and population-based disease management that addressed
multiple disease states in a parallel, concurrent, and patient-centric
fashion.
Back to top
Methods
The Senior Life Management program
The Senior Life Management (SLM) program was designed to provide
population-based disease management (helping members with certain disease
states — such as congestive heart failure, identified through analysis of
plan data — to improve self-care) and case management (helping individual
members with complex problems to obtain needed medical and social services).
The program comprised enhanced administrative services rather than new
benefits. SLM was also crafted to be patient-centric (in which one manager
would develop a relationship with the member), rather than disease-specific
(in which a member would be directed to multiple managers for different problems
and disease states). Central to the program were the following components:
creation of an electronic care-management record; comprehensive periodic
member health status assessments; telephonic case management; patient
education materials; community physician education; and coordination with
community services.
SLM services were added to existing services of a Medicare Plus
Choice HMO. The basic benefits included a deductible representing only 10%
of the deductible for traditional Medicare for hospitalization, enhanced
skilled nursing benefits with no three-day hospitalization prerequisite and
no copayments for 100 days, and a limited pharmacy benefit.
SLM also included a drug utilization review program for thirty
medications considered to be relatively contraindicated for use in the
elderly based upon published guidelines (15). The filling of one of these
prescriptions triggered a fax to the prescribing physician asking for
reconsideration of this therapeutic maneuver and soliciting physician
feedback concerning the usefulness of this alert and his or her response.
The Master Console, an electronic health care management record, was
created to deliver just-in-time information to the program’s
administrative and case management staff. This electronic record integrated
historical medical claims, daily updates of current medical claims data,
monthly updates of laboratory test results and prescription information, and
data from assessments of members’ health through a survey and regularly scheduled
phone calls. The Master Console was developed with a Visual Basic client,
which allowed rapid prototyping, revision based on end-user feedback, and
deployment of upgrades. No physician-based medical records were part of
Master Console. Decision support algorithms built into the Master Console
alerted program staff to potential changes in the clinical status of a
patient, need for case management screening (actual assignment to case
management being at the judgment of the nurse care coordinator [NCC] based
at the HMO), or the potential need for a service intervention. For example,
authorization of a joint replacement procedure would trigger a task/reminder
for an outbound phone call by a registered NCC the
following day to determine anticipated therapy needs in addition to a prompt to
perform both a home-safety assessment and a falls-risk assessment. As
another example, the filling of a prescription for flurazepam would trigger
a fax to the prescribing physician about the relative contraindication of
long-acting benzodiazepines in the elderly and asking for reconsideration of
this therapeutic maneuver.
Prior to initiating SLM, 72% of the 8504 study participants completed
either 1) a mailed health assessment (the Medical Outcomes Study 36-Item
Short-Form Health Survey [SF-36]) (16) along with satisfaction questions
from the Medicare Beneficiary Survey or 2) follow-up telephone surveys if
they had not returned their mail-in form. Participant responses were summarized
and entered into the Master Console. Subsequently, periodic 18-item short
assessments of program participants were performed quarterly over the
telephone by program staff, and the data were entered into the database.
Program staff were organized into four teams, each managing 800 to 1000 SLM participants. A team consisted of an NCC, who supervised two personal
service representatives (PSRs). The PSR was a new level of staffing created
specifically for this intervention. PSRs were not required to have clinical
training but were selected based on communication skills and sensitivity to
geriatric issues as ascertained by interviews with experienced nurse
clinicians. PSRs were provided with eight weeks of intensive training
consisting of didactic sessions covering common geriatric issues, in
addition to
direct critiquing and refinement of telephone communication skills by
allowing trainees to handle customer service calls in tandem with
supervisors. After training, PSRs handled outbound phone contacts and
performed periodic health screenings with scripted questionnaires. The NCC
was responsible for outbound contact to all those in complex case management
(50 to 70 participants per team), communicating with treating physicians and
office staff, following up on hospitalizations and ER visits, and arranging
for home health care and durable equipment through the primary care
physician.
A full-time medical director, administrator, and social worker also
staffed the SLM program. Prior to the program’s onset, the medical director
conducted visits with more than 100 of the primary care doctors who cared
for SLM members to brief them on the intervention and to solicit their
cooperation in coordinating services. The medical director and social worker
provided ad hoc consultation to care management teams for any issues related
to their areas of expertise.
SLM members who scored in the lowest quintile of the General Health scale
on the SF-36 were further assessed by phone for possible case management by
NCCs. At least every three months, PSRs contacted all members in the
intervention group not currently in case management to perform an
18-question short assessment. This questionnaire was designed to probe for
significant changes in physical health, mental health, or social supports.
Questions dealt with such domains as stresses and losses, falls, pain,
changes in activities of daily living, incontinence, nutrition, and mood.
Logic built into the short assessment triggered further evaluation by other
clinicians. For example, report of a fall would trigger a home safety
assessment, whereas loss of a loved one or pet would trigger a depression
evaluation by the social worker. PSRs also fulfilled a customer service
role, fielding inbound calls from members. This provided an opportunity to
probe further for changes in health status.
Disease management modules were developed for congestive heart failure,
falls (home safety), nutrition, depression, and diabetes mellitus. These
conditions were chosen because they were prevalent, contributed to
morbidity, and were deemed actionable. Based upon decision support
algorithms, targeted educational materials (selected by program staff and
purchased from Channing Bete Corporation, South Deerfield, Mass) were sent to members. PSRs made
follow-up phone calls within two weeks of patient receipt of such materials.
During phone contact, patients might be referred to a range of care
providers, including primary care physicians or mental health providers, or
connected to community services, such as Meals On Wheels, transportation
services, or adult day care.
Study design
This model was tested as a randomized controlled prospective open trial.
The study protocol was reviewed and approved by the Thomas Beam
Institutional Review Board, New York, NY, and the surveys and promotional
materials were reviewed and approved by the Center for Medicare and Medicaid
Services. Study participants were derived from approximately 13,000 members
of an Medicare Plus Choice HMO who resided in nine contiguous counties
surrounding Pittsburgh, Pa. To be eligible, participants had to be aged 65
or older, have signed consent on their health plan enrollment form to participate, and have been continuously enrolled with the health plan for all of
1999. Members who declined to participate in the study or who opted out
during the study continued to receive their usual benefits.
A subset of members receiving medical care under a special financial risk
arrangement with certain providers was excluded from the study. This was
done because of the potentially different nature of utilization in a
full-risk provider relationship. All other eligible health plan members in
the targeted counties were randomized and included in the study. To minimize
the chances that spouses or neighbors would be split between study and
control groups, members were grouped by zip code and these zip codes were
randomized with a random number generator within SAS statistical software (SAS
Institute Inc, Cary, NC).
Neither subjects nor clinicians could be blinded to the intervention. Aside
from two physician group practices, no providers had more than 5% of their
practice composed of study subjects. This low penetration may have made it
less likely that physician behavior would be influenced much by whether or
not a patient was part of this study. Of the 8504 eligible members who
participated, 4247 were randomly assigned to the control and 4257 to the
intervention group. A summary of enrollment and attrition versus
retention throughout the study is shown in the Figure.
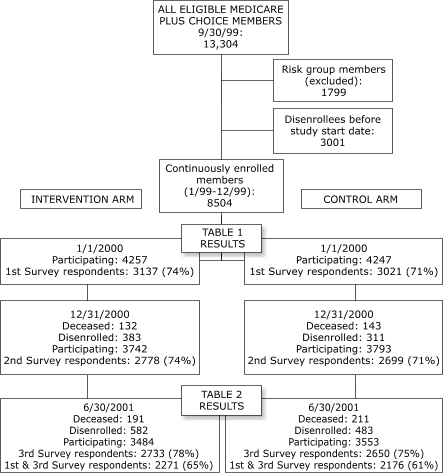
Figure.
Flow chart illustrating participant eligibility, exclusion, enrollment,
retention, survey participation, and attrition, including a comparison of
the intervention and control groups, throughout the randomized controlled
open trial.
(A text description of this chart is also
available.)
Measurements and outcomes
The primary outcomes analyzed included self-reported health status,
member satisfaction, costs measured as paid claims, and use of hospital and
nursing home resources measured as bed-days per thousand per year. Secondary
outcomes included survival and disenrollment from the health plan.
Analyses were based on the intention to treat principle (i.e., for those
who disenrolled from the health plan data were analyzed up to the point of disenrollment). For 151 members who declined to continue to participate in
SLM but remained in the plan, all 18 months of data were analyzed as part of
the intervention group. Health status was measured by administering the
SF-36 at entry, at 12 months, and at 18 months. Surveys were conducted by a
vendor (Geriatric Health Services, San Francisco, Calif) using personnel blinded to
the study objectives. All active and enrolled intervention and control
members were surveyed at each of the three points. Response rates were
nearly identical in both groups. At baseline, 73.7% of intervention and 71.1%
of control subjects responded. For the final survey, response rates for
active participants were 78% for the intervention group and 75% for the
control group. Data collected by mail and by telephone survey
were analyzed together. Comparisons of baseline and 18-month data were used
for the primary analysis. Patient satisfaction was assessed based on the
Medicare Beneficiary Survey.
The cost of the intervention included the salaries and overhead of all
personnel delivering care services (including the medical director and
administrative staff) plus all mailings, educational materials, and vendor
costs specific to the program. This amounted to an additional $10.50 per
member per month.
Statistical analyses
For baseline comparisons, bivariate analyses described the
characteristics of study participants using t-tests to compare the
intervention and control groups. All variables were subjected to two-tailed
tests of significance with statistical significance set at P = .05.
Disenrollments from the study (voluntary disenrollments and death) were
analyzed with Kaplan-Meier and log-rank tests.
Health care resource use and costs were analyzed for the baseline period
(January 1999 through December 1999) and for the study period (January 2000
though June 2001). Cost outcomes were actual paid amounts on claims for
services incurred in the baseline and study periods. Cost data from
outsourced mental health services could not be obtained for use in this
analysis. All primary care services were capitated in both the intervention
and control groups, were therefore essentially identical, and were thus also
not included in the final analysis. Based on plan estimates, claims data
were 98% complete. Claims were identified using a unique program code
assigned at the outset of the study by information services personnel not
involved in the analysis. Costs per member in both baseline and study
periods for the intervention and control groups were not normally
distributed due to small numbers of individuals with very high aggregate
costs. Despite the non-normal distribution, means were reported and t-tests
were used to assess differences in cost outcomes, as has been recommended
for the economic evaluation of health care randomized trials (17,18).
Additional analyses using log transformation yielded identical conclusions.
Resource use is reported for inpatient and skilled
nursing/rehabilitation facility categories and was measured using
actual days incurred on paid claims. To assess differences in rates of use, chi-squared tests were performed using the number of individuals who
did or did not have an admission in each group. Admissions and bed-days were
also reported per 1000 members per year, thus automatically adjusting for
attrition by the change in denominator.
To assess change in self-reported health outcomes, health assessment
baseline scores were subtracted from final scores for individuals who
responded to both surveys, and a matched-pair t-test was used to
compare the first and final surveys and report results for each of the SF-36
domains.
Role of funding sources
Employees of Coventry Health Care, Inc (Bethesda, Md) and Merck & Company, Inc
(West Point, Pa) participated
in the study as coinvestigators. They implemented protocols, coordinated
data collection, and performed statistical analyses. Data interpretation and
decisions about the paper’s content resided with the investigators.
Back to top
Results
At baseline, there were no significant differences between the two groups
in demographic and health status characteristics (Table 1). Data on race and
socioeconomic status were not available. Health care costs were higher in
the intervention group for the one-year preenrollment period (mean $3553 vs
$3417), but this was not significant. During the 18 months of this
study, SLM participants received substantially augmented services in
comparison to the control group. The following data illustrate the scope of
the intervention. PSRs administered more than 24,186 health status short
assessments by telephone. A total of 1640 (38.5%) intervention group
members were evaluated for case management. Two hundred seventy-three home
safety assessments were performed, 419 clinical summaries were mailed to
treating physicians, and more than 800 alerts were faxed to physicians
regarding potential medication safety issues. No similar activities were
performed for the control group.
Over the 18 months of the study, self-reported satisfaction with the
health plan improved significantly in the intervention group (P <
.01) and self-reported social function declined less (P = .04) as measured by
the SF-36 (Table 2). There was a trend toward slower decline in general health in the
intervention group, which did not reach significance (P = .09).
There was no meaningful difference in subject attrition between the two
groups.
There was a 10% difference in mortality rate favoring the intervention
group, but the difference was not statistically significant by Kaplan-Meier
analysis. In the intervention group, there were 191 deaths out of 4257
(4.5%) and in the control group, there were 211 deaths out of 4247 (5.0%) (P
= .18). The study was never powered to detect a difference in survival in
post hoc analysis; however, the power to detect a mortality difference at
α = .05 was 17.8%. Disenrollment rates from the health plan were also
examined with Kaplan-Meier methodology, and again there was no difference
between intervention and control.
Costs were greater during the 18-month study period than during the prior
12 months in both SLM and control groups, reflecting double-digit
medical-cost inflation. There were no significant differences in mean costs
during either the baseline or intervention periods. Pharmacy costs were
nearly identical during the intervention period ($57.20 per member per month
in control and $56.39 per member per month in intervention groups). The cost
of the intervention was $10.50 per member per month or $189 per member per
month over the 18-month period of the study. Hospital inpatient
utilization was not different between the intervention and control groups
during the study (Table 3). Use of nursing homes, however, was lower in the
intervention group during the study period (616 vs 747 days per thousand
members per year, P = .02). Economic savings in the intervention
group offset the costs of the program so that total costs (including the
cost of the intervention) in the two groups were nearly identical (Table 3).
As a check against retention bias, the costs of members lost through death
or disenrollment were examined and found to be the same in both the
intervention and control groups.
Back to top
Discussion
Because a small proportion of Medicare beneficiaries account for a large
fraction of expenditures (approximately 10% of the noninstitutionalized
patients accounting for 70% of expenditures) (20), and because a person with
a chronic disease may be expected to incur costs that are 80% to 300% higher
than average (21), substantial savings might be realized by managing the
high cost of the “sickest of the sick.” This is the rationale for
traditional disease management and case management strategies and has been
recently reiterated as a method of reducing Medicare spending (22).
Historical attempts to target and intervene in the subset of patients who
might use a high volume of health service resources have yielded mixed
results. A program designed to increase access to primary care after
hospital discharge increased rather than decreased rates of
re-hospitalization (23), and a case management program using nurse case
managers increased emergency department visits without favorably influencing
any other measures (24). A review of three Medicare case management
demonstration projects revealed that none improved self care, reduced
symptoms, reduced hospital admission rates, or reduced Medicare spending
(7). These failures were attributed to poor cooperation from clients’
physicians, lack of focus on interventions, and lack of financial incentive
to reduce expenditures. Another review of 16 demonstration projects
concluded that the projects generally failed to meet their goal of overall
cost savings (25). A third review of 36 studies led the authors to conclude
that there was little or no effect on survival, functional health, or use of
hospitals or nursing homes (26). While one of the earlier studies of a
geriatric evaluation and treatment program yielded both cost savings and
lower mortality (27), this encouraging result was not confirmed in larger,
multisite trials (28,29). In a study similar to the one we report,
social-work–directed case management in a Medicare Plus Choice plan did not
reduce cost of health care of high-risk members (30).
Other reports have suggested that targeted interventions may be
worthwhile. For example, early comprehensive discharge planning and home
follow-up with an advance practice nurse lowered readmission rates rates and resulted in
increased time between admissions with attendant cost savings (31). A
case-management study reported significant reduction in costs, fewer
readmissions, and higher quality of life through careful targeting of
patients with congestive heart failure (32), and a nurse case-management
program for chronic heart failure implemented by telephone lowered
inpatient costs by 45.5% at six months (33). An intriguing study of
substituting telephone care for clinic visits not only reduced clinic visits
as expected but also resulted in fewer hospital admissions, lower medication
use, and an estimated 28% lower total expenditure per patient (34).
SLM was an effort to determine if interventions that have been found to
be efficacious in experimental settings would be scalable to broader
implementation. Demonstrating population-level changes with the
interventions described is challenging, because large changes in a small
number of individuals are diluted, and differences are often obscured by
year-to-year variation. The short 18-month intervention period for this
study compounded the challenge of demonstrating improved health outcomes and
cost savings. Some savings were achieved by shifting from higher-cost,
institution-based long-term care to lower-cost, community-based care,
which confirms the findings of others (25). From a global perspective, however,
these savings barely offset the cost of the intervention. Because start-up
costs would be expected to be higher than maintenance costs, and because
economies of scale might be realized in program expansion, adding more
members to the program might enhance its efficiency.
Although there was no difference in mortality, other benefits, such as
improved social well-being and greater satisfaction with care, were
demonstrated. Even though these benefits did not translate into improved member
retention, preservation of social relations is a health goal highly
valued by elderly patients (35). During the time of this study, three
competing Medicare Plus Choice health plans served the nine-county region.
Because voluntary disenrollment rates from Medicare Plus Choice plans can
range from 10% to 15% per year, improved member retention was a hoped-for
effect that did not materialize.
This study had several limitations. Measures of health status and
satisfaction relied on self-reporting, and the study was conducted as an open
trial. Another possible weakness was that primary care physicians caring for
patients in this study were reimbursed under a capitation model. Thus, there
was a financial disincentive for providers to see patients more frequently
in the office to address concerns raised by case managers. Under a
fee-for-service model, physicians might have been more motivated to see
patients early and more frequently to address concerns disclosed by case
mangers. While this might have helped avoid costly hospital admissions, the
more frequent visits would themselves drive up costs, thereby making the
cost-offset analysis less favorable. It is impossible to know which of these
trends would dominate, and one cannot conclude that the model would perform
better under a fee-for-service model.
Although historical plan data were used to flag members for evaluation
for case management, the final selection for case management was based more
on clinical judgment rather than on a highly refined methodology such as risk
modeling. This targeting issue, and difficulties encountered in coordinating
services in the provider community and preprogram sources of care,
represented weaknesses in the intervention.
Strengths of the SLM design were comprehensiveness, proactive
case finding, and attempts to deal with the myriad challenges affecting the
health status of the elderly. Since the study was broadly implemented to
include all members of a community eligible for Medicare Plus Choice, and
because the demographic and health assessment characteristics were similar
to those observed elsewhere (nearly identical to the published norms in the
case of Mental and Physical Summary Scores for the SF-36) (36), the findings
may be generalizable to other Medicare Plus Choice plans. However, this
study reflected one geographic area and only members of one Medicare Plus
Choice plan.
While from a scientific perspective it would have been desirable to
extend the study time, this duration represented a real-world compromise
that acknowledged both business and scientific objectives. It is unknown
whether the effectiveness of SLM might have been greater over a longer time
frame.
Lessons learned from this project point the way to potentially more
rewarding implementations. Interventions could have been better targeted,
perhaps through risk modeling. More cooperation from the provider community
would have greatly enhanced attempts at early intervention. Future studies
should explore better ways to share information and ways to reward provider
efficiency and high-quality care. Finally, the algorithms used in the
electronic management record to identify actionable issues and prompt the
case manager could be improved with higher levels of medical logic and
sophistication.
In summary, this broad implementation of population-based disease
management and case management does not represent a panacea for escalating
medical costs and raises a cautionary note.
Back to top
Acknowledgments
This study was a jointly funded and collaborative effort between the
Government Programs Division of Coventry Health Care, Inc and the Outcomes
Research and Management Department of Merck & Co, Inc. We wish to express
particular appreciation for the unique contributions of several individuals.
Marcia Ferrero and Donna Anderson from Coventry oversaw all aspects of care
management and contributed to educational materials and decision support
algorithms. Keith Blankenship led the Coventry Information Systems team which developed the
Master Console. Nittaya Suppapanya and Pamela Landsman from Merck & Co,
Inc provided valuable statistical support. Mark Beers, MD, and Mary Jane Osmick, MD, served as clinical consultants.
Back to top
Author Information
Corresponding author: David C. Martin, MD, Coventry
Health Care, Inc, Clinical Professor of Medicine, University of Pittsburgh
School of Medicine, Clinical Professor of Behavioral and Community Health
Sciences, University of Pittsburgh Graduate School of Public Health, HealthAmerica, 11 Stanwix St, Suite 2300, Pittsburgh, PA 15222-1344.
Telephone: 412-553-7576. E-mail: dmartin@cvty.com.
Author affiliations: Marc L. Berger, MD, David T. Anstatt, MBA, Robin S.
Turpin, PhD, Carolyn C. Cannuscio, ScD, Steven M. Teutsch, MD, MPH, Merck &
Co, Inc, West Point, Pa; Jonathan Wofford, MPH, DeAnn Warfel, JD, Bernard J. Mansheim,
MD, Coventry Health Care, Inc, Bethesda, Md.
Back to top
References
- Kramarow E, Lentzner H, Rooks R, Weeks J, Saydah S.
Health, United States, 1999 with health and aging chartbook. Hyattsville
(MD):
National Center for Health Statistics; 1999.
- Allen SM, Mor V.
The prevalence and consequences of unmet need:
contrasts between older and younger adults with disability. Med Care 1997;35(11):1132-48.
- Hoffman C, Rice D, Sung H.
Persons with chronic conditions. Their
prevalence and costs. JAMA 1996;276(18):1473-9.
- Starfield B.
Is US health really the best in the world? JAMA 2000;284(4):483-5.
- Morley JE.
International aging: why does the United States do so
poorly? J Am Geriatr Soc 1991;39(8):836-8.
- Musgrove P, Creese A, Preker A, Baeza C, Anell A, Prentice T.
World Health Report 2000. Geneva: World Health Organization; 2000.
ISSN 1020-3311.
- Schore JL, Brown RS, Cheh VA.
Case management for high-cost
Medicare beneficiaries. Health Care Financ Rev 1999;20(4):87-101.
- Kane RL.
Manged care as a vehicle for delivering more effective
chronic care for older persons. J Am Geriatr Soc
1998;46(8):1034-9.
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A.
Improving chronic care illness: translating evidence into action.
Health Aff 2001;20(6):64-78.
- Wagner EH.
The promise and performance of HMOs in improving
outcomes in older adults. J Am Geriatr Soc 1996;44(10):1251-7.
- Boult C, Boult L, Pacala JT.
Systems of care for older populations
of the future. J Am Geriatr Soc 1998;46(4):499-505.
- Boult C, Kane RL, Pacala JT, Wagner EH.
Innovative healthcare for
chronically ill older persons: results of a national survey. Am J Manag
Care 1999;5(9):1162-72.
- Foote SM. Population-based disease management under
fee-for-service Medicare. Health Aff - Web Exclusive
2003;W3:342-356.
- Crippen DL. Disease management in Medicare:
data analysis and
benefit design issues - before the Special Committee on Aging, United
States Senate. Washington (DC): Congressional Budget Office; 2002 Sep 19.
22 p.
- Beers MH.
Explicit criteria for determining potentially
inappropriate medication use by the elderly. An update. Arch Intern Med 1997;157(14):1531-6.
- Tarlov AR, Ware JE, Greenfield S, Nelson EC, Perrin E, Zubkoff M.
The Medical Outcomes Study: an application of methods for monitoring the
results of medical care. JAMA 1989;262(7):925-30.
- Drummond MF, Jefferson TO.
Guidelines for authors and peer
reviewers of economic submissions to the BMJ. BMJ
1996;313(7052):275-83.
- Barber JA, Thompson SG.
Analysis and interpretation of cost data
in randomised controlled trials: review of published studies. BMJ
1998;317(7167):1195-200.
- Ware JE, Snow KK, Kosinski M, Gandek B. The SF-36 Health Survey manual
and interpretation guide. Boston: The Health Institute, New England
Medical Center; 1993.
- Garfinkel SA, Riley GF, Iannacchione VG.
High-cost users of
medical care. Health Care Financ Rev 1988;9(4):41-52.
- Fishman P, Von Korff M, Lozano P, Hecht J.
Chronic care costs in
managed care. Health Aff 1997;16(3):239-47.
- Lieberman SM, Lee J, Anderson T, Crippen DL. Reducing the growth
of Medicare spending: geographic versus patient-based strategies.
Health Aff - Web Exclusive. 2003;W3:603-613.
- Weinberger M, Oddone EZ, Henderson WG.
Does increased access to
primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on
Primary Care and Hospital Readmission.
New Engl J Med 1996;334(22):1441-7.
- Mezey M, Fulmer T, Gagnon A, Schein C, McVey L, Bergman H.
Randomized controlled trial of nurse case management of frail older
people. J Am Geriatr Soc 1999;47(9):1118-25.
- Kemper P, Applebaum R, Harrigan M.
Community care demonstrations:
What have we learned? Health Care Financ Rev 1987;8(4):87-100.
- Weissert W, Hedrick S.
Lessons learned from research on effects of
community-based long-term care. J Am Geriatr Soc
1994;42(3):348-53.
- Rubenstein LZ, Josephson KR, Wieland GD, English PA, Sayre JA,
Kane RL.
Effectiveness of a geriatric evaluation unit: a randomized
clinical trial. New Engl J Med 1984;311(26):1664-70.
- Silverman M, Musa D, Martin DC, Lave JR, Adams J, Ricci EM.
Evaluation of outpatient geriatric assessment: a randomized multi-site
trial. J Am Geriatr Soc 1995;43(7):733-40.
- Cohen HJ, Feussner JR, Weinberger M,
Carnes M, Hamdy RC, Hsieh F, et al.
A controlled trial of
inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002;346(12):905-12.
- Boult C, Rassen J, Rassen A, Moore RJ, Robison S.
The effect of
case management on the costs of health care for enrollees in Medicare Plus
Choice plans: a randomized trial. J Am Geriatr Soc
2000;48(8):996-1001.
- Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly
MV, et al.
Comprehensive discharge
planning and home follow-up of hospitalized elders. A randomized clinical
trial. JAMA 1999;281(7):613-20.
- Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney
RM.
A multidisciplinary intervention to prevent readmission of elderly
patients with congestive heart failure. New Engl J Med
1995;333(18):1190-5.
- Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A.
Effect
of a standardized nurse case-management telephone intervention on resource
use in patients with chronic heart failure. Arch Intern Med
2002;162(6):705-12.
- Wasson J, Gaudette C, Whaley F, Sauvigne A, Baribeau P, Welch
HG.
Telephone care as a subsitute for routine clinic follow-up. JAMA
1992;267(13):1788-93.
- Bradley EH, Bogardus ST Jr, van Doorn C, Williams CS, Cherlin E,
Inouye SK.
Goals in geriatric assessment: are we measuring the right
outcomes? Gerontologist 2000;40(2):191-6.
- Ware JE, Kosinski M, Keller SD. SF-36 Physical and
Mental
health Summary Scales: a user's manual. Boston (MA): The Health
Institute; 1994.
Back to top |
|
