 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Evaluation of Varicella Reporting to the National Notifiable Disease Surveillance System -- United States, 1972-1997Varicella (chickenpox) is a common, highly infectious, vaccine-preventable disease. Before 1995, an estimated 4 million cases of varicella occurred each year in the United States, approximately 100 patients died (1), and approximately 10,000 persons were hospitalized because of varicella and related complications. Approximately 95% of cases (2), 66% of hospitalizations, and 45% of the varicella-related deaths occurred among persons aged less than 20 years (CDC, unpublished data, 1998). In 1972, varicella became nationally notifiable in the United States; subsequently, 46 states * and the District of Columbia (DC) provided weekly reports to CDC's National Notifiable Disease Surveillance System (NNDSS). In 1981, varicella was deleted from the weekly morbidity report, and in 1982, states were encouraged to report varicella to NNDSS annually. In 1995, a live, attenuated varicella vaccine was licensed in the United States for routine use in children. This report describes changes in the annual reported incidence of varicella from 1972 to 1997 and discusses the need for increased surveillance with the availability of a vaccine. Varicella cases reported to NNDSS during 1972-1997 were reviewed. The annual population estimates for the states, DC, and the nation from the Bureau of the Census were used to calculate annual incidence. Because, without a vaccination program, the average annual number of cases of varicella is approximately equal to the size of the birth cohort each year (2), the annual birth cohort was used to estimate the completeness of reporting. In 1972, the reported national incidence of varicella was 78.4 cases per 100,000 population. During 1972-1987, the reported incidence of varicella ranged from 66.3 in 1974 to 94.1 in 1984, peaking every 3-5 years. From 1987 to 1997, the reported national incidence decreased 58%, from 88.0 to 36.9 (Figure_1). The decrease from 1987 to 1997 corresponded with decreases in the number of states reporting to NNDSS and the completeness of reporting. The number of areas reporting varicella weekly to NNDSS declined from 46 states and DC in 1972 to 20 states ** and DC in 1997. In 1972, cases constituting greater than or equal to 3% of the birth cohort were reported in 27 states and DC (range: 0.1%-34.3%); the number of states reporting cases constituting greater than or equal to 3% declined to 21 in 1982 and 17 in 1994. By 1997, of 20 states and DC reporting varicella, 10 states reported cases constituting greater than or equal to 3% of their birth cohorts; three reported greater than or equal to 10% (range: less than 0.1%-20.4%) (Figure_2). During 1972-1997, 14 states maintained continuous reporting to CDC of varicella. In these states, the incidence of reported varicella increased from 107.0 in 1972 to 212.1 in 1987, then decreased to 95.9 in 1996 and 107.1 in 1997. These rates corresponded with levels of reporting, which were 6.2%-9.7% of the birth cohort in the 1970s, 9.1%-14.4% in the 1980s, and 6.6%-11.8% in the 1990s. Reported by: Varicella Activity, Child Vaccine Preventable Diseases Br, and Vaccine Safety and Development Activity, Epidemiology and Surveillance Div, National Immunization Program, CDC. Editorial NoteEditorial Note: The data presented in this report suggest that the decline in the reported national incidence of varicella since 1987 resulted from the changes in state reporting requirements and practices. The low number of reporting states in 1997 and the incomplete reporting from participating states limits the use of NNDSS data to monitor the impact of vaccination against varicella at the national level. Three states have stopped reporting varicella since vaccine licensure. Among the 14 states that have reported continuously, the decline in incidence is due to a decrease in their level of reporting, which will make it difficult to interpret the expected decline in those states resulting from the varicella vaccination program. In these states, annual decreases in incidence that are higher than decreases for previous years might reflect the impact of the vaccination program. Improvements in the quality of varicella reporting are needed to properly monitor vaccine use and its impact on disease trends. Surveillance requirements change depending on the stage of a vaccination program. Aggregate case reporting, preferably by age group, is adequate in the early phase together with outbreak notifications and limited outbreak response. As vaccine uptake increases and disease declines, individual case reporting will be needed with more intensive outbreak control. For measles, reporting of 10% of cases was adequate to monitor disease trends during the introductory phase of the measles vaccination program (3); presumably this level of reporting for varicella would be adequate to monitor the impact of the varicella vaccination program. The varicella vaccine is recommended for susceptible persons aged greater than or equal to 12 months (4,5). In 1997, national varicella vaccine coverage among children aged 19-35 months was 26%, with state estimates ranging from 4% to 40% (6). With low levels of reporting, such coverage levels are too low to have a detectable impact on disease reporting to NNDSS. To better assess the health burden of disease and the impact of national and state varicella vaccination programs, CDC encourages states and local areas to make varicella a reportable disease, to investigate and report varicella deaths to CDC (7), and to establish aggregate case reporting either by enhancing existing surveillance systems or by establishing school, day care, and/or health-care-provider-based reporting of varicella (8). For national varicella surveillance, until all states are reporting varicella cases to CDC with consistent levels of reporting from year to year, complementary surveillance strategies are needed. National surveillance for varicella deaths began in 1998, and deaths became reportable to CDC on January 1, 1999. CDC supports active surveillance for varicella in three geographic areas in the United States and, together with the Council of State and Territorial Epidemiologists, has encouraged and/or supported states in the development of alternative interim methods for surveillance, including monitoring hospitalizations, sentinel surveillance by physicians, schools, and/or child-care centers for varicella, and statewide incidence surveys. Assessing the impact of vaccination at the national level will involve analyzing information from multiple data sources. References
Varicella was not reportable in Louisiana, New Jersey, North Carolina, and Tennessee. ** Arizona, Delaware, Hawaii, Illinois, Kansas, Louisiana, Maine, Massachusetts, Michigan, Missouri, North Dakota, Ohio, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Utah, West Virginia, and Wisconsin. Figure_1 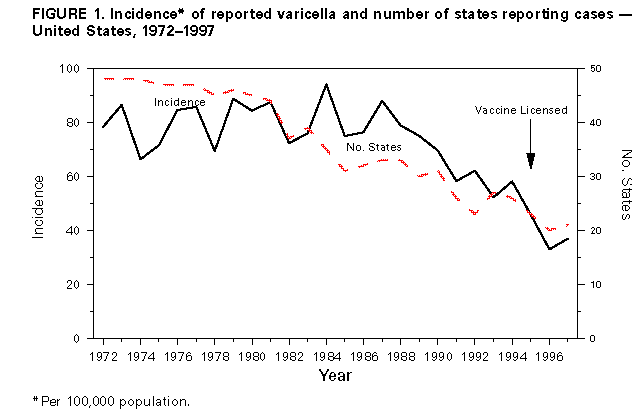 Return to top. Figure_2 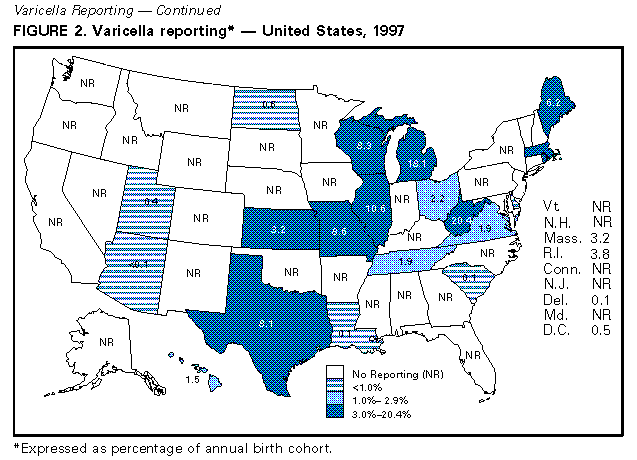 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 01/28/99 |
|||||||||
This page last reviewed 5/2/01
|