 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Intussusception Among Recipients of Rotavirus Vaccine -- United States, 1998-1999On August 31, 1998, a tetravalent rhesus-based rotavirus vaccine (RotaShield[Registered]*, Wyeth Laboratories, Inc., Marietta, Pennsylvania) (RRV-TV) was licensed in the United States for vaccination of infants. The Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics, and the American Academy of Family Physicians have recommended routine use of RRV-TV for vaccination of healthy infants (1,2). During September 1, 1998-July 7, 1999, 15 cases of intussusception (a bowel obstruction in which one segment of bowel becomes enfolded within another segment) among infants who had received RRV-TV were reported to the Vaccine Adverse Event Reporting System (VAERS). This report summarizes the clinical and epidemiologic features of these cases and preliminary data from ongoing studies of intussusception and rotavirus vaccine. VAERS VAERS is a passive surveillance system operated by the Food and Drug Administration (FDA) and CDC (3,4). Vaccine manufacturers are required to report to VAERS any adverse event reported to them, and health-care providers are encouraged to report any adverse event possibly attributable to vaccine. Vaccine recipients and their families also can report adverse events to VAERS. For this report, VAERS case reports of intussusception following rotavirus vaccination were reviewed, and health-care providers, parents, or guardians of patients were contacted by telephone for additional clinical and demographic information. Data on RRV-TV distribution were obtained from the manufacturer. To estimate the expected rate of intussusception among infants aged less than 12 months, hospital discharge data from New York for 1991-1997 were reviewed. Of the 15 infants with intussusception reported to VAERS, 13 (87%) developed intussusception following the first dose of the three-dose RRV-TV series, and 12 (80%) of 15 developed symptoms within 1 week of receiving any dose of RRV-TV (Table 1). Thirteen of the 15 patients received concurrently other vaccines with RRV-TV. Intussusception was confirmed radiographically in all 15 patients. Eight infants required surgical reduction, and one required resection of 7 inches (18 cm) of distal ileum and proximal colon. Histopathologic examination of the distal ileum indicated lymphoid hyperplasia and ischemic necrosis. All infants recovered. Onset dates of reported illness occurred from November 21, 1998, to June 24, 1999 (Figure 1). The median age of patients was 3 months (range: 2-11 months). Ten were boys. Intussusception among RRV-TV recipients was reported from seven states (Table 1). Of the 15 cases reported to VAERS, 14 were spontaneous reports and one was identified through active postlicensure surveillance. The rate of hospitalization for intussusception among infants aged less than 12 months during 1991-1997 (before RRV-TV licensure) was 51 per 100,000 infant-years** in New York (95% confidence interval [CI]=48-54 per 100,000). The manufacturer had distributed approximately 1.8 million doses of RRV-TV as of June 1, 1999, and estimated that 1.5 million doses (83%) had been administered. Given this information, 14-16 intussusception cases among infants would be expected by chance alone during the week following receipt of any dose of RRV-TV. Fourteen of the 15 case-patients were vaccinated before June 1, 1999, and of those, 11 developed intussusception within 1 week of receiving RRV-TV. Postlicensure Studies of Adverse Events Following RRV-TV As part of a preliminary analysis of ongoing postlicensure surveillance of adverse events following vaccination with RRV-TV, cases of intussusception during December 1, 1998-June 10, 1999, were identified among infants aged 2-11 months at Northern California Kaiser Permanente (NCKP) by review of hospital discharge diagnoses, admitting diagnoses for the records for which discharge summaries were not yet complete, and computerized records of all barium enemas performed on children aged less than 1 year. Relative risks were age-adjusted because of differences in the ages of vaccinated and unvaccinated infants, and p values were calculated by Poisson regression. At NCKP, 16,627 doses of RRV-TV were administered to 9802 infants during December 1, 1998-June 10, 1999. Nine cases of intussusception among infants were identified with onset during that same period, all of which were radiographically or surgically confirmed. Three were among vaccinated children, with intervals of 3, 15, and 58 days following vaccination. The rate of intussusception among never-vaccinated children was 45 per 100,000 infant-years, and among children who had received RRV-TV was 125 per 100,000 infant-years (age-adjusted relative risk [RR]=1.9, 95% CI=0.5-7.7, p=0.39). The rate among children who had received RRV-TV during the preceding 3 weeks was 219 per 100,000 infant-years (age-adjusted RR=3.7, 95% CI=0.7-19, p=0.12). Among children who had received RRV-TV during the previous week, the rate was 314 per 100,000 infant-years (age-adjusted RR=5.7, 95% CI= 0.7-50, p=0.11). Minnesota In Minnesota, intussusception cases were identified among infants aged 30 days-11 months who were born after April 1, 1998, and were hospitalized with radiographically or surgically confirmed intussusception with onset during November 1, 1998- June 30, 1999. During October 1, 1998-June 1, 1999, 62,916 doses of vaccine were distributed. Eighteen cases of intussusception were identified, five of which were among infants who had received RRV-TV. Vaccinated children had a median age of 4 months (range: 3-5 months), and unvaccinated children had a median age of 7 months (range: 5-9 months). Four of the five RRV-TV recipients with intussusception required surgical reduction, and five of 13 unvaccinated children required surgical reduction. Intussusception occurred after receipt of dose one (two children), dose two (two children), and dose three (one child). The five RRV-TV recipients developed intussusception within 2 weeks of receipt of vaccine; intervals were 6 days (two children), 7 days, 10 days, and 14 days after receipt of vaccine. Assuming 85% of RRV-TV doses distributed in Minnesota were administered, the observed rate of intussusception within 1 week of receipt of RRV-TV was 292 per 100,000 infant-years. Reported by: K Ehresman, MPH, R Lynfield, MD, R Danila, PhD, Acting State Epidemiologist, Minnesota Dept of Health. S Black, MD, H Shinefield, MD, B Fireman, MS, S Cordova, MS, Kaiser Permanente Vaccine Study Center, Oakland, California. Div of Biostatistics and Epidemiology, Food and Drug Administration. Viral Gastroenteritis Section, Respiratory and Enteric Viruses Br, and Office of the Director, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Safety Datalink Team; Statistical Analysis Br, Data Management Div; Vaccine Safety and Development Activity; Child Vaccine Preventable Diseases Br, Epidemiology and Surveillance Div, National Immunization Program; and EIS officers, CDC. Editorial Note:Rotavirus is the most common cause of severe gastroenteritis in infants and young children aged less than 5 years in the United States, resulting in approximately 500,000 physician visits, 50,000 hospitalizations, and 20 deaths each year. Worldwide, rotavirus is a major cause of childhood death, accounting for an estimated 600,000 deaths annually among children aged less than 5 years. Rotavirus vaccines offer the opportunity to reduce substantially the occurrence of this disease (1). In prelicensure studies, five cases of intussusception occurred among 10,054 vaccine recipients and one of 4633 controls, a difference that was not statistically significant (5). Three of the five cases among vaccinated children occurred within 6-7 days of receiving rotavirus vaccine. On the basis of these data, intussusception was included as a potential adverse reaction on the package insert, and the ACIP recommended postlicensure surveillance for this adverse event following vaccination (1). Because of concerns about intussusception identified in prelicensure trials, VAERS data were analyzed early in the postlicensure period. The number of reported intussusception case-patients with illness onset within 1 week of receiving any dose of vaccine is in the expected range; however, because reporting to VAERS of adverse events following vaccination is incomplete (6), the actual number of intussusception cases among RRV-TV recipients may be substantially greater than that reported. In response to the VAERS reports, a preliminary analysis of data from an ongoing postlicensure study at NCKP was performed, and a multistate investigation was initiated to determine whether an association exists between administration of RRV-TV and intussusception in infants. Preliminary data from Minnesota and from NCKP also suggest an increased risk for intussusception following receipt of RRV-TV. Observed rates of intussusception among recently vaccinated children were similar in both studies. However, the number of cases of intussusception among vaccinated children is small at both NCKP and in Minnesota, and neither study has adequate power to establish a statistically significant difference in incidence of intussusception among vaccinated and unvaccinated children. Available data suggest but do not establish a causal association between receipt of rotavirus vaccine and intussusception, and additional studies are ongoing. Although neither these studies nor the VAERS reports is conclusive, the consistency of findings from these three data sources raises strong concerns. Because more data are anticipated within several months and rotavirus season is still 4-6 months away in most areas of the United States, CDC recommends postponing administration of RRV-TV to children scheduled to receive the vaccine before November 1999, including those who already have begun the RRV-TV series. Parents or caregivers of children who have recently received rotavirus vaccine should promptly contact their health-care provider if the infant develops symptoms consistent with intussusception (e.g., persistent vomiting, bloody stools, black stools, abdominal distention, and/or severe colic pain). Health-care providers should consider intussusception in infants who have recently received RRV-TV and present with a consistent clinical syndrome; early diagnosis may increase the probability that the intussusception can be treated successfully without surgery. Vaccine providers, parents, and caregivers should report to VAERS intussusception and other adverse events following vaccination. Information on reporting to VAERS and case report forms can be requested 24 hours a day by telephone, (800) 822-7967, or the World-Wide Web, http://www.nip.gov/nip/vaers.htm. References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by CDC or the U.S. Department of Health and Human Services. ** An infant-year is a unit of measurement combining infants and time used as a denominator in calculating incidence. In this report, it is the sum of the individual units of time (days, weeks, or months) converted to years that the infants in the study population have been followed. Table 1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Reported cases of intussusception among recipients of tetravalent
rhesus-based rotavirus vaccine (RRV-TV) (RotaShield ®*), by state -- United States,
1998-1999
===========================================================================================
No. doses No. days from
received of dose to
State Age (mos) Sex RRV-TV symptom onset
---------------------------------------------------------------
California 7 M 2 4
California 4 F 2 14
California 3 M 1 3
California 5 M 1 59
Colorado 4 F 1 4
Colorado 3 M 1 5
Kansas 2 F 1 5
Missouri 11 M 1 5
New York 3 F 1 5
New York 2 M 1 3
North Carolina 4 F 1 5
Pennsylvania 6 M 1 3
Pennsylvania 2 M 1 4
Pennsylvania 2 M 1 29
Pennsylvania 3 M 1 7
---------------------------------------------------------------
* Use of trade names and commercial sources is for identification only and does not imply
endorsement by CDC or the U.S. Department of Health and Human Services.
===========================================================================================
Return to top. Figure 1 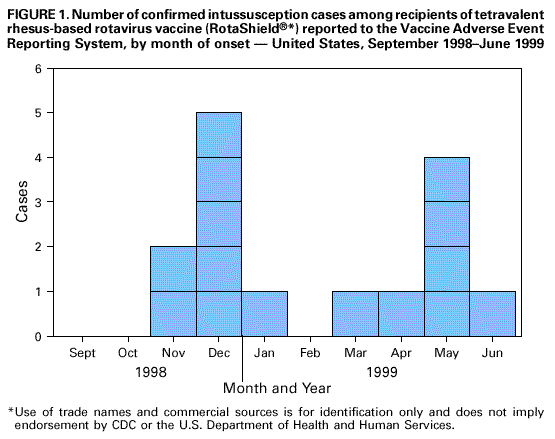 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/15/99 |
|||||||||
This page last reviewed 5/2/01
|