 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention of Rotavirus Gastroenteritis Among Infants and ChildrenRecommendations of the Advisory Committee on Immunization Practices (ACIP)
Prepared by The material in this report originated in the National Center for Immunization and Respiratory Diseases (proposed), Anne Schuchat, MD, Director; and the Division of Viral Diseases, Larry Anderson, MD, Director. Corresponding preparer: Umesh D. Parashar, MBBS, National Center for Immunization and Respiratory Diseases, CDC, 1600 Clifton Rd., NE, MS A-34, Atlanta, GA 30333. Telephone: 404-639-4829; Fax: 404-639-1307; E-mail: uap2@cdc.gov. SummaryIn February 2006, a live, oral, human-bovine reassortant rotavirus vaccine (RotaTeq®) was licensed for use among U.S. infants. The Advisory Committee on Immunization Practices recommends routine vaccination of U.S. infants with 3 doses of this rotavirus vaccine administered orally at ages 2, 4, and 6 months. The first dose should be administered between ages 6--12 weeks. Subsequent doses should be administered at 4--10 week intervals, and all 3 doses should be administered by age 32 weeks. Rotavirus vaccine can be co-administered with other childhood vaccines. Rotavirus vaccine is contraindicated for infants with a serious allergic reaction to any vaccine component or to a previous dose of vaccine. IntroductionRotavirus is the most common cause of severe gastroenteritis in infants and young children worldwide. In developing countries, rotavirus gastroenteritis is a major cause of childhood death and is responsible for approximately half a million deaths per year among children aged <5 years (1). Rotavirus gastroenteritis results in relatively few childhood deaths in the United States (approximately 20--60 deaths per year among children aged <5 years) (2). However, nearly every child in the United States is infected with rotavirus by age 5 years, and the majority will have gastroenteritis, resulting in approximately 410,000 physician visits, 205,000--272,000 emergency department (ED) visits, and 55,000--70,000 hospitalizations each year and direct and indirect costs of approximately $1 billion (3--6) (Figure 1). This report presents the Advisory Committee on Immunization Practices (ACIP) recommendations on use of a live, oral, human-bovine reassortant rotavirus vaccine (RotaTeq®, produced by Merck and Company, Whitehouse Station, New Jersey) that was licensed in February 2006 by the Food and Drug Administration (FDA) for use among U.S. infants. BackgroundClinical and Epidemiologic Features of Rotavirus DiseaseRotavirus infects almost all children by age 5 years, but severe, dehydrating gastroenteritis occurs primarily among children aged 3--35 months. The spectrum of rotavirus illness ranges from mild, watery diarrhea of limited duration to severe diarrhea with vomiting and fever that can result in dehydration with shock, electrolyte imbalance, and death (7--11). Following an incubation period of 1--3 days, the illness can begin abruptly, and vomiting often precedes the onset of diarrhea. Up to one third of patients have a temperature of >102°F (>39°C). Gastrointestinal symptoms generally resolve in 3--7 days. Rotaviruses are shed in high concentrations in the stools of infected children and are transmitted primarily by the fecal-oral route, both through close person-to-person contact and through fomites (12). Rotaviruses also are probably transmitted by other modes, such as fecally contaminated food and water and respiratory droplets (13). In the United States, rotaviruses cause winter seasonal peaks of gastroenteritis, with activity usually beginning in the southwest United States during November--December and spreading to the Northeast by April--May (5,14,15). In the United States, rotaviruses are responsible for 5%--10% of all gastroenteritis episodes among children aged <5 years. However, among the various pathogens causing gastroenteritis, rotaviruses lead to the most severe disease and account for a higher proportion of severe episodes leading to clinic or hospital visits (8,16). For example, rotavirus accounts for 30%--50% of all hospitalizations for gastroenteritis among U.S. children aged <5 years and approximately 70% of hospitalizations for gastroenteritis during the seasonal peaks (16--19). Among children aged <5 years in the United States, 17% of rotavirus hospitalizations occur during the first 6 months of life, 40% by age 1 year, and 75% by age 2 years (Figure 2) (5). In the first 5 years of life, four of five children in the United States will have rotavirus gastroenteritis (8,18,20), one in seven will require a clinic or ED visit, one in 70 will be hospitalized, and one in 200,000 will die from this disease (4,9). The risk for rotavirus gastroenteritis and its outcomes does not appear to vary by geographic region within the United States. Limited data suggest that children from disadvantaged socioeconomic backgrounds and premature infants have an increased risk for hospitalization from gastroenteritis, including viral gastroenteritis (21). In addition, children and adults who are immunocompromised because of congenital immunodeficiency, hematopoetic transplantation, or solid organ transplantation sometimes experience severe, prolonged, and even fatal rotavirus gastroenteritis (22--25). Rotavirus also is an important cause of nosocomial gastroenteritis (7,16,17,26--29). Among adults in the United States, rotavirus infection causes gastroenteritis primarily in travelers returning from developing countries, parents and persons caring for children with rotavirus gastroenteritis, immunocompromised persons, and older adults (30). Laboratory Testing for RotavirusBecause the clinical features of rotavirus gastroenteritis do not differ from those of gastroenteritis caused by other pathogens, confirmation of rotavirus infection by laboratory testing of fecal specimens is necessary for reliable rotavirus surveillance and can be useful in clinical settings (e.g., in making decisions about use of antimicrobial agents). Rotavirus is shed in high concentration in the stool of children with gastroenteritis (i.e., 1012 viruses/G), so the most widely available method is antigen detection in the stool by an enzyme immunoassay (EIA) directed at an antigen common to all group A rotaviruses. Several commercial EIA kits are available that are inexpensive, easy to use, rapid, and highly sensitive (approximately 90% compared with detection by electron microscopy), making them suitable for rotavirus surveillance and clinical diagnosis. Latex agglutination and polyacrylamide gel electrophoresis might be less sensitive than EIA but are used in some settings. Other techniques, including electron microscopy, reverse transcription-polymerase chain reaction, nucleic acid hybridization, sequence analysis, and culture, are used primarily in research settings. Rotavirus antigen also has been identified in the serum of patients 3--7 days after disease onset (31,32), but routine diagnostic testing is based primarily on testing of fecal specimens. Serologic methods that detect a rise in serum antibodies, primarily enzyme immunoassay for rotavirus serum immunoglobulin G (IgG) and immunoglobulin A (IgA) antibodies, have been used to confirm recent infections. In vaccine trials, the immunogenicity of rotavirus vaccines has been assessed by measuring rotavirus-specific IgA and neutralizing antibodies to vaccine strains (33,34). Morphology, Antigen Composition, and Immune ResponseRotaviruses are 70-nm nonenveloped RNA viruses in the family Reoviridae. The viral nucleocapsid is composed of three concentric shells that enclose 11 segments of double-stranded RNA. The outermost layer contains two structural viral proteins (VP): VP4, the protease-cleaved protein (P protein) and VP7, the glycoprotein (G protein). These two proteins define the serotype of the virus and are considered critical to vaccine development because they are targets for neutralizing antibodies that might be important for protection (35,36). Because the two gene segments that encode these proteins can segregate independently, a typing system consisting of both P and G types has been developed. Because characterizing the P types by traditional methods of virus neutralization is difficult, molecular methods have been used to define genotype based on sequence analysis. These genotypes correlate well with known serotypes so the genotypes are tentatively designated in brackets (e.g., P1A [8]). In the United States, viruses containing six distinct P and G combinations are most prevalent: P1A[8]G1, P1B[4] G2, P1A[8] G3, P1A[8] G4, P1A[8] G9, and P2A[6] G9 (37,38) (Figure 3); these strains are generally designated by their G serotype specificity (serotypes G1-4, G9). Several animal species (e.g., primates and cows) are susceptible to rotavirus infection and suffer from rotavirus diarrhea, but animal strains of rotavirus differ from those that infect humans. Although human rotavirus strains that possess a high degree of genetic homology with animal strains have been identified (39--41), animal-to-human transmission appears to be uncommon. However, natural reassortant animal-human strains have been identified in humans (41), and two are being investigated as vaccine candidates (42). Although children can be infected with rotavirus several times during their lives, initial infection after age 3 months is most likely to cause severe gastroenteritis and dehydration (43--45). After a single natural infection, 40% of children are protected against subsequent infection with rotavirus, 75% are protected against subsequent rotavirus gastroenteritis, and 88% are protected against severe rotavirus gastroenteritis. Second, third, and fourth infections confer progressively greater protection against severe disease (43). The immune correlates of protection from rotavirus infection and disease are not fully understood. Both serum and mucosal antibodies are probably associated with protection, and in some studies, serum antibodies against VP7 and VP4 have correlated with protection. However, in other studies, including vaccine studies, correlation between serum antibody and protection has been poor (46). The first infection with rotavirus elicits a predominantly homotypic, serum-neutralizing antibody response to the virus, and subsequent infections elicit a broader, heterotypic response (7,47). The influence of cell-mediated immunity is less clearly understood but probably is related both to recovery from infection and to protection against subsequent disease (48,49). Rationale for Rotavirus VaccinationSeveral reasons exist to adopt vaccination of infants as the primary public health measure for prevention of severe rotavirus disease in the United States. First, rates of rotavirus illness among children in industrialized and less-developed countries are similar, indicating that clean water supplies and good hygiene have little effect on virus transmission; therefore, further improvements in water or hygiene are unlikely to have a substantial impact on disease prevention (8,43,50--52). Second, in the United States, a high level of rotavirus morbidity continues to occur despite available therapies. For example, the rate of hospitalizations for gastroenteritis in young children declined only 16% during 1979--1995 (5,6), despite the widespread availability of oral rehydration solutions and recommendations by experts, including the American Academy of Pediatrics and CDC, for the use of oral rehydration solutions in the treatment of dehydrating gastroenteritis (53,54). Third, studies of natural rotavirus infection indicate that initial infection protects against subsequent severe gastroenteritis, although subsequent asymptomatic infections and mild disease might still occur (43,55,56). Therefore, vaccination early in life, which mimics a child's first natural infection, will not prevent all subsequent disease, but should prevent most cases of severe rotavirus disease and their sequelae (e.g., dehydration, physician visits, hospitalizations, and deaths). Rotavirus VaccinesBackgroundThe first rotavirus vaccines were based on monovalent rotavirus strains isolated from either bovine or rhesus hosts, but their development was abandoned because of variable efficacy in clinical trials (57--64). Subsequently, multivalent animal-human reassortant rotavirus vaccines were developed by using gene reassortment (65). In 1998, a rhesus-based tetravalent rotavirus vaccine, RRV-TV (Rotashield®, Wyeth-Lederle Vaccines and Pediatrics) (66), was recommended for routine vaccination of U.S. infants with 3 doses at ages 2, 4, and 6 months (67). However, RRV-TV was withdrawn from the U.S. market within 1 year of its introduction because of its association with intussusception (68). At the time of its withdrawal, RRV-TV had not yet been introduced in any other national vaccination program globally, and the vaccine was not further tested or used in any country. The risk for intussusception was most elevated (>20-fold increase) within 3--14 days after receipt of the first dose of RRV-TV (69), with a smaller (approximately five-fold) increase in risk within 3--14 days after the second dose. Overall, the risk associated with the first dose of RRV-TV was estimated to be approximately 1 case per 10,000 vaccine recipients (70). Certain researchers have reassessed the data on RRV-TV and intussusception and have suggested that the risk for intussusception was age-dependent and that the absolute number of intussusception events, and possibly the relative risk for intussusception associated with the first dose of RRV-TV, increased with increasing age at vaccination (71,72). However, the World Health Organization Global Advisory Committee on Vaccine Safety (GACVS), after reviewing all the available data, concluded that risk for RRV-TV-associated intussusception was high in infants vaccinated after age 60 days and that insufficient evidence was available to conclude that the use of RRV-TV at age <60 days was associated with a lower risk (73). GACVS noted that the possibility of an age-dependent risk for intussusception should be taken into account in assessing rotavirus vaccines. Postlicensure surveillance suggested that, besides intussusception, RRV-TV also was associated with a spectrum of other gastrointestinal symptoms, including gastroenteritis and bloody stools (74). A monovalent vaccine based on an attenuated human rotavirus strain of P1A[8] G1 specificity, RIX 4414 (RotaRix®, GSK Biologicals, Belgium), has shown a clinical efficacy of 85% against severe rotavirus disease in recent trials (75,76). In a trial of approximately 60,000 infants, no increase in intussusception was noted among recipients of the vaccine versus placebo (77). As of June 2006, RotaRix® is licensed in approximately 30 countries in Latin America, Africa, Asia, and in countries of the European Union and has been introduced into national vaccination programs in Brazil, Panama, and Venezuela. A licensure application has not yet been submitted in the United States. Human-Bovine Reassortant Rotavirus Vaccine (RotaTeq®)The 2006 U.S. licensed RotaTeq® is a live, oral vaccine that contains five reassortant rotaviruses developed from human and bovine parent rotavirus strains (Box) (78). Four reassortant rotaviruses express one of the outer capsid proteins (G1, G2, G3, or G4) from the human rotavirus parent strain and the attachment protein (P7[5]) from the bovine rotavirus parent strain. The fifth reassortant virus expresses the attachment protein (P1A[8]) from the human rotavirus parent strain and the outer capsid protein G6 from the bovine rotavirus parent strain. The parent bovine rotavirus strain Wistar Calf 3 (WC3) was isolated from a calf with diarrhea in Chester County, Pennsylvania, in 1981 and was passaged 12 times in African green monkey kidney cells (79). The reassortants are propagated in Vero cells using standard tissue culture techniques in the absence of antifungal agents. RotaTeq® consists of the five human-bovine reassortants suspended in a solution of buffer (sodium citrate and phosphate) and stabilizer (sucrose) that can be stored refrigerated at 36°F--46°F (2°C--8°C) for up to 24 months. Each 2-mL vial of vaccine contains the following minimum concentration of the reassortants: G1 - 2.2 X 106 infectious units; G2 - 2.8 X 106 infectious units; G3 - 2.2 X 106 infectious units; G4 - 2.0 X 106 infectious units; and P1 - 2.3 X 106 infectious units. The vaccine formulation contains sucrose, sodium citrate, sodium phosphate monobasic monohydrate, sodium hydroxide, polysorbate 80, and tissue culture media. Trace amounts of fetal bovine serum might be present. No preservatives or thimerosal are present. RotaTeq® has been tested in three phase III trials, including a large-scale clinical trial of approximately 70,000 infants in 11 countries, with the United States and Finland accounting for approximately 80% of all enrolled persons (80). Phase III trials of RotaTeq® have involved 72,324 infants (36,423 in the RotaTeq® group, 35,825 in the placebo group, and 76 persons who received a mixed regimen). In these trials, 3 doses of RotaTeq® were administered orally beginning at age 6--12 weeks with a 4--10-week interval between doses. Data from these trials on immunogenicity, efficacy, and safety of RotaTeq® are summarized below. ImmunogenicityThe immune correlates of protection from rotavirus infection and disease are not fully understood. In clinical trials, a rise in titer of rotavirus group-specific serum IgA antibodies was used as one of the measures of the immunogenicity of RotaTeq®. Sera were collected before vaccination and approximately 2 weeks after the third dose, and seroconversion was defined as a threefold or greater rise in antibody titer from baseline. Serconversion rates for IgA antibody to rotavirus were 93%--100% among 439 vaccine recipients versus 12%--20% in 397 recipients of the placebo (80). When administered simultaneously, a 3-dose series of RotaTeq® does not diminish the immune response to Haemophilus influenzae type b conjugate (Hib) vaccine, inactivated poliovirus vaccine (IPV), hepatitis B vaccine, pneumococcal conjugate vaccine, and the diphtheria and tetanus antigens in diphtheria, tetanus, and pertussis vaccine (DTaP) (Merck, unpublished data, 2005). Because validation of the pertussis assays is still under review, insufficient immunogenicity data are available to confirm lack of interference when RotaTeq® is administered concomitantly with childhood vaccines to prevent pertussis. EfficacyThe efficacy of the final formulation of RotaTeq® has been evaluated in two phase III trials (80,81). In these trials, the efficacy of RotaTeq® after completion of a 3-dose regimen against rotavirus gastroenteritis of any severity was 74% (95% confidence interval [CI] = 67%--79%) and against severe rotavirus gastroenteritis was 98% (CI = 90%--100%) (Table 1). Severe rotavirus gastroenteritis was defined as a score >16 on an established 24-point severity scoring system based on the intensity and duration of fever, vomiting, diarrhea, and changes in behavior (80). Efficacy was observed against all G1-4 and G9 serotypes (Table 2), but relatively few non-G1 rotavirus cases were reported. In a large study, the efficacy of RotaTeq® against office visits for rotavirus gastroenteritis was evaluated among 5,673 persons and against ED visits and hospitalizations for rotavirus gastroenteritis among 68,038 persons during the first 2 years of life (81). RotaTeq® reduced the incidence of office visits by 86% (CI = 74%--93%), ED visits by 94% (CI = 89%--97%), and hospitalizations for rotavirus gastroenteritis by 96% (CI = 91%--98%) (Table 3). Efficacy against all gastroenteritis hospitalizations of any etiology was 59% (CI = 56%--65%). The efficacy of RotaTeq® in the second rotavirus season postvaccination was 63% (CI = 44%--75%) against rotavirus gastroenteritis of any severity and 88% (CI = 49%--99%) against severe rotavirus gastroenteritis. Data on the efficacy of <3 doses of RotaTeq® are limited. Neither breastfeeding nor concurrent administration of other childhood vaccines appears to diminish the efficacy of a 3-dose series of RotaTeq®. Among 1,566 infants breastfed exclusively, the efficacy of RotaTeq® against rotavirus gastroenteritis of any severity (68%; CI = 54%--78%) was comparable to that among 1,632 infants who were never breastfed (68%; CI = 46%--82%). Among 204 vaccinated infants born prematurely (<37 weeks' gestation), the point estimate of vaccine efficacy against rotavirus gastroenteritis of any severity was comparable to that among nonpremature infants (70%; CI = -15%--95%), but the confidence bounds included zero because of the small sample size. Adverse Events Post-VaccinationIntussusception The risk for intussusception was evaluated in 71,725 persons enrolled in phase III efficacy trials. In a large-scale safety and efficacy trial designed specifically to evaluate the risk for intussusception (80), parents/legal guardians of all persons were contacted by telephone or home visit on approximately day 7, 14, and 42 after each vaccination, and every 6 weeks thereafter for up to 1 year after the first dose. Parents were asked about all serious adverse experiences, including intussusception, among enrolled children. Potential intussusception cases were adjudicated according to a prespecified case definition that included radiographic, surgical, and autopsy criteria. For the prespecified 42-day postvaccination endpoint, six cases of intussusception were observed in the RotaTeq® group versus five cases of intussusception in the placebo group (multiplicity adjusted relative risk = 1.6; CI = 0.4--6.4). No evidence of clustering of cases of intussusception was observed within a 7- or 14-day window post-vaccination for any dose, the period of greatest risk for intussusception associated with the RRV-TV vaccine. For the 1-year follow-up period after administration of the first dose, 13 cases of intussusception were observed in the RotaTeq® group versus 15 cases of intussusception in the placebo group (multiplicity adjusted relative risk: 0.9; CI = 0.4--1.9). Other Adverse Events Serious adverse events and deaths were evaluated in 71,725 infants enrolled in phase III trials. Among RotaTeq® and placebo recipients, the incidence of serious adverse events (2.4% versus 2.6%, respectively), including deaths (<0.1% [n=25] versus <0.1% [n=27], respectively), was similar. No deaths were attributed to vaccination by blinded investigators. The most common cause of death (accounting for 17 of the 52 deaths) was sudden infant death syndrome (SIDS), and deaths from SIDS were equally distributed among RotaTeq® and placebo recipients (n=eight and nine, respectively). A subset of 11,722 persons was studied in detail to assess other potential adverse experiences (e.g., fever, diarrhea, and vomiting). In the 42-day period postvaccination, vaccinees had a small but statistically significantly greater rate of certain symptoms compared with placebo recipients, including 1% excess of vomiting (15% versus 14%, respectively), 3% excess of diarrhea (24% versus 21%, respectively), 1% excess of nasopharyngitis (7% versus 6%, respectively), 2% excess of otitis media (15% versus 13%, respectively), and 0.4% excess of bronchospasm (1.1% versus 0.7%, respectively). Among RotaTeq® and placebo recipients, the incidence of reported episodes of fever (43% versus 43%, respectively) and hematochezia (0.5% versus 0.3%, respectively) was similar. In the 7-day postvaccination period, vaccinees had a small but statistically significantly greater rate of diarrhea, with an excess of 1% after dose 1 (10% versus 9%, respectively), 3% after dose 2 (9% versus 6%, respectively), and 3% after any dose (18% versus 15%, respectively). Similarly, vaccinees had a small but statistically significantly greater rate of vomiting, with an excess of 2% after dose 1 (7% versus 5%, respectively) and 2% after any dose (12% and 10%, respectively). The incidence of fever and irritability during the 7-day period after any vaccine dose was similar among RotaTeq® and placebo recipients. Preterm infants RotaTeq® or placebo was administered to 2,070 preterm infants (25--36 weeks' gestational age; median: 34 weeks) in the phase III trials. All preterm infants were monitored for severe adverse events, and a subset of 308 was monitored in detail for all adverse events. No cases of intussusception were reported among preterm infants. Among preterm infants administered RotaTeq® and placebo, the incidence of serious adverse events (5.5% versus 5.8%, respectively) was similar. Two deaths each were reported among preterm infants administered RotaTeq® (one SIDS and one motor-vehicle accident) and placebo (one SIDS and one unknown cause). Shedding and Transmission of Vaccine VirusFecal shedding of vaccine virus was evaluated by EIA in a subset of persons enrolled in the phase III trials by obtaining a single stool sample during days 4--6 following each vaccination visit and from all children who submitted a rotavirus antigen positive stool specimen at any time. Vaccine virus was shed in 32 of 360 (8.9%; CI = 6.2%--12.3%) persons after dose 1, zero of 249 (0; CI = 0%--1.5%) persons after dose 2, and one of 385 (0.3%; CI = <0.1%--1.4%) after dose 3. In phase III studies, shedding was observed as early as 1 day and as late as 15 days after a dose. The potential for horizontal transmission of vaccine virus was not assessed through epidemiologic studies. Vaccine Administration, Handling, and StorageRotaTeq® is provided in a squeezable plastic dosing tube with a twist-off cap designed to allow for the vaccine to be administered directly to infants by mouth. Each tube contains a single 2-mL dose of the vaccine as a liquid buffered-stabilized solution that is pale yellow in color but might have a pink tint. This formulation protects the vaccine virus from gastric acid and stabilizes the vaccine, allowing for storage at refrigerator temperatures (36°F--46°F [2°C--8°C]) for 24 months. RotaTeq® should be administered as soon as possible after being removed from refrigeration. Additional information on stability under conditions other than those recommended is available by calling 1-800-637-2590. Cost-Effectiveness AnalysisIn an analysis that used estimates of current rotavirus disease burden, vaccine efficacy, vaccine coverage rates, and health costs, investigators estimated that a national rotavirus vaccination program in which 3 doses of RotaTeq® are administered at ages 2, 4, and 6 months would result in 255,000 fewer physician visits, 137,000 fewer ED visits, 44,000 fewer hospitalizations, and 13 fewer deaths per year in children aged <5 years. From the health-care perspective alone, vaccination is likely to be cost-saving at a total cost per child (including administration costs) of up to $66 per child (approximately $12 per vaccine dose). A higher-priced vaccine would be increasingly unlikely to be cost-saving, and at a cost of more than $143 per child (approximately $38 per dose), a rotavirus vaccination program would most likely have a net cost to the health-care system. From the societal perspective, vaccination is likely to be cost-saving at a total cost per child of up to $156 per child (approximately $42 per dose). A higher-priced vaccine would be increasingly unlikely to be cost-saving, and at a cost of more than $268 per child (approximately $79 per dose), a rotavirus vaccination program would most likely have a net cost to society (CDC, unpublished data, 2006). Recommendations for the Use of Rotavirus VaccineRoutine AdministrationACIP recommends routine vaccination of U.S. infants with 3 doses of rotavirus vaccine administered orally at ages 2, 4, and 6 months (Table 4). The first dose should be administered between ages 6--12 weeks. Subsequent doses should be administered at 4--10-week intervals, and all 3 doses of vaccine should be administered by age 32 weeks. Vaccination should not be initiated for infants aged >12 weeks because of insufficient data on safety of the first dose of rotavirus vaccine in older infants. Vaccine should not be administered after age 32 weeks because of insufficient data on the safety and efficacy of rotavirus vaccine in infants after this age. For infants in whom the first dose of rotavirus vaccine is inadvertently administered off label at age >13 weeks, the rest of the rotavirus vaccination series should be completed as per the schedule because timing of the first dose should not affect the safety and efficacy of the second and third dose. Infants who have had rotavirus gastroenteritis before receiving the full course of rotavirus vaccinations should still initiate or complete the 3-dose schedule because the initial infection frequently provides only partial immunity. Infants who are being breastfed can receive rotavirus vaccine. The efficacy of rotavirus vaccine is similar among breastfed and nonbreastfed infants. Like other vaccines, rotavirus vaccine can be administered to infants with transient, mild illnesses, and with or without low-grade fever (82). Simultaneous AdministrationRotavirus vaccine can be administered together with DTaP, Hib vaccine, IPV, hepatitis B vaccine, and pneumococcal conjugate vaccine. Available evidence suggests that the rotavirus vaccine does not interfere with the immune response to the Hib vaccine, IPV, hepatitis B vaccine, and pneumococcal conjugate vaccine, and the diphtheria and tetanus antigens in DTaP. Because validation of the pertussis assays is still under review, insufficient immunogenicity data are available to confirm lack of interference of immune responses when rotavirus vaccine is concomitantly administered with childhood vaccines to prevent pertussis. ContraindicationsRotavirus vaccine should not be administered to infants who have severe hypersensitivity to any component of the vaccine or who have experienced a serious allergic reaction to a previous dose of rotavirus vaccine. PrecautionsAltered Immunocompetence Practitioners should consider the potential risks and benefits of administering rotavirus vaccine to infants with known or suspected altered immunocompetence. Children and adults who are immunocompromised because of congenital immunodeficiency, hematopoetic transplantation, or solid organ transplantation sometimes experience severe, prolonged, and even fatal rotavirus gastroenteritis (22--25). However, no safety or efficacy data are available for the administration of rotavirus vaccine to infants who are potentially immunocompromised, including
Acute Gastroenteritis In usual circumstances, rotavirus vaccine should not be administered to infants with acute, moderate-to-severe gastroenteritis until the condition improves. However, infants with mild acute gastroenteritis can be vaccinated, particularly if the delay in vaccination might be substantial and might make the child ineligible to receive vaccine (e.g., aged >13 weeks before vaccination is initiated). Rotavirus vaccine has not been studied among infants with concurrent acute gastroenteritis. In these infants, the immunogenicity and efficacy of rotavirus vaccine can theoretically be compromised. For example, infants who receive oral poliovirus vaccine (OPV) during an episode of acute gastroenteritis in some instances have diminished poliovirus antibody responses to OPV (83). Moderate-to-Severe Illness Infants with moderate-to-severe illness should be vaccinated as soon as they have recovered from the acute phase of the illness (82). This precaution avoids superimposing adverse effects of the vaccine on the underlying illness or mistakenly attributing a manifestation of the underlying illness to the vaccine. Preexisting Chronic Gastrointestinal Disease Practitioners should consider the potential risks for and benefits of administering rotavirus vaccine to infants with preexisting chronic gastrointestinal disease. Infants with preexisting chronic gastrointestinal conditions who are not undergoing immunosuppressive therapy should benefit from rotavirus vaccine vaccination, and the benefits outweigh the theoretical risks. However, the safety and efficacy of rotavirus vaccine have not been established for infants with these preexisting conditions (e.g., congenital malabsorption syndromes, Hirschsprung's disease, short-gut syndrome, or persistent vomiting of unknown cause). Intussusception Following administration of a previously licensed rotavirus vaccine, RRV-TV, an increased risk for intussusception was observed. Available prelicensure data from a trial of 70,000 infants indicated no evidence of an association between intussusception and the current vaccine. However, additional postlicensure surveillance data are required to confirm that the vaccine is not associated with intussusception at a lower rate than would have been detected in prelicensure trials. In addition, data suggest that infants with a history of intussusception might be at higher risk for a repeat episode than other infants. Therefore, until postlicensure data on safety of rotavirus vaccine are available, the risks for and the benefits of vaccination should be considered when vaccinating infants with a previous episode of intussusception. Special SituationsPremature Infants (<37 weeks' gestation) Practitioners should consider the potential risks for and benefits of vaccinating premature infants against rotavirus. Limited data suggest that premature infants are at increased risk for hospitalization from viral gastroenteritis during their first year of life (21). In clinical trials, the safety and efficacy of rotavirus vaccine appears to be similar for premature and term infants, although a relatively small number of preterm infants have been evaluated. The lower level of maternal antibody to rotaviruses in very low birthweight, premature infants theoretically could increase the risk for adverse reactions from rotavirus vaccine. ACIP supports vaccination of prematurely born infants if they are at least aged 6 weeks, are being or have been discharged from the hospital nursery, and are clinically stable. Until further data are available, ACIP considers that the benefits of rotavirus vaccine vaccination of premature infants outweigh the theoretical risks. Exposure of Immunocompromised Persons to Vaccinated Infants Infants living in households with persons who have or are suspected of having an immunodeficiency disorder or impaired immune status can be vaccinated. The majority of experts believe the protection of the immunocompromised household member afforded by vaccination of young children in the household outweighs the small risk for transmitting vaccine virus to the immunocompromised household member and any subsequent theoretical risk for vaccine virus-associated disease. To minimize potential virus transmission, all members of the household should employ measures such as good hand washing after contact with the feces of the vaccinated infant (e.g., after changing a diaper). Exposure of Pregnant Women to Vaccinated Infants Infants living in households with pregnant women can be vaccinated. The majority of women of childbearing age would have pre-existing immunity to rotavirus and so the risk for infection and disease from potential exposure to the attenuated vaccine virus strain is low. In addition, no evidence exist that rotavirus infection or disease during pregnancy poses any risk to the fetus. Furthermore, vaccination of young children would avoid potential exposure of the pregnant women to wild virus if the unvaccinated infant suffers from rotavirus gastroenteritis. Regurgitation of Vaccine The practitioner should not readminister a dose of rotavirus vaccine to an infant who regurgitates, spits out, or vomits during or after administration of vaccine. The infant can receive the remaining recommended doses of rotavirus vaccine at appropriate intervals. Data are limited regarding the safety of administering a dose of rotavirus vaccine higher than the recommended dose and on the efficacy of administering a partial dose. Additional data on safety and efficacy are needed to evaluate the benefits of and risks for readministration. Hospitalization After Vaccination If a recently vaccinated child is hospitalized for any reason, no precautions other than routine universal precautions need be taken to prevent the spread of vaccine virus in the hospital setting. Reporting of Adverse EventsAny clinically significant or unexpected adverse events that occur after administration of rotavirus vaccine should be reported to the Vaccine Adverse Events Reporting System (VAERS). The National Childhood Vaccine Injury Act requires health-care providers to report to VAERS any event listed by the vaccine manufacturer as a contraindication to subsequent doses of the vaccine or any event listed in the Reportable Events Table (http://vaers.hhs.gov/reportable.htm) that occurs within the specified period after vaccination. Rotavirus vaccine is covered under the general category of rotavirus vaccines in the Reportable Events Table, and no specific conditions are listed for reporting. VAERS reporting forms and information can be requested 24 hours a day at 800-822-7967 or by accessing VAERS at http://vaers.hhs.gov. Enhanced Postlicensure Surveillance for Adverse EventsIn prelicensure clinical trials, rotavirus vaccine has not been associated with any serious adverse events, including intussusception. Nevertheless, continued monitoring for adverse events following introduction of rotavirus vaccine into routine vaccination programs is important, particularly in light of the previous experience with RRV-TV. In addition to manufacturer-sponsored Phase IV studies, postlicensure monitoring will include enhanced review of adverse events reported to VAERS. The Vaccine Safety Datalink (VSD) also will be used to monitor any intussusception risk associated with rotavirus vaccine and to evaluate any other possible associations that might be identified through VAERS or in Phase IV studies. The VSD project includes information on persons enrolled in eight large health maintenance organizations, with an annual birth cohort of >90,000 infants. Data on all vaccines administered within the study population are recorded and linked with diagnoses from medical encounters to determine rates of potential adverse events resulting from vaccination. Recently developed rapid analysis methods allow VSD to conduct near "real time" monitoring for vaccine adverse events (84). Given the background rate of natural intussusception among U.S. infants (25--38 cases per 100,000 infants) and the large number of children that are potentially eligible for vaccination, intussusception cases are expected to occur following vaccination by chance alone that will be unrelated to the vaccine (85). Consequently, intensive postlicensure surveillance will be necessary to assess the safety of this vaccine against this rare event. National Vaccine Injury Compensation ProgramThe National Vaccine Injury Compensation Program (VICP), established by the National Childhood Vaccine Injury Act, is a no-fault system in which persons thought to have suffered an injury or death as a result of administration of a covered vaccine can seek compensation. Persons of all ages who receive a VICP-covered vaccine are eligible to file a claim. The program relies on a vaccine injury table listing the vaccines covered by the program and the injuries, disabilities, illnesses, and conditions (including death) for which compensation can be awarded. Claimants also can prevail for conditions not listed in the table if they can prove causation. To be eligible for compensation, claims must be filed within 3 years after the first symptom of the vaccine injury, or within 2 years of the vaccine-related death and not more than 4 years after the start of the first symptom of the vaccine-related injury from which the death occurred. Rotavirus vaccine is covered by VICP under the general category of rotavirus vaccines in Category XI of the Vaccine Injury Table (http://www.hrsa.gov/osp/vicp/table.htm). This category of vaccines does not include any table injuries. Additional information is available from the following from the National Vaccine Injury Compensation Program, Health Resources and Services Administration, Parklawn Building, Room 11C-26, 5600 Fishers Lane, Rockville, MD 20857 (telephone: 800-338-2382 [24-hour recording] or Internet: http://www.hrsa.gov/vaccinecompensation). Future NeedsSurveillance of Rotavirus Gastroenteritis Rotavirus gastroenteritis is not a reportable disease in the United States, and testing for rotavirus infection is not always performed when a child seeks medical care for acute gastroenteritis. Establishing rotavirus disease surveillance systems that are adequately sensitive and specific to document the effectiveness of vaccination programs will be necessary. National surveillance systems for rotavirus infections include review of national hospital discharge databases for rotavirus-specific or rotavirus-compatible diagnoses and reports of rotavirus isolation from a sentinel system of laboratories and surveillance in three sites that participate in the New Vaccine Surveillance Network. At state and local levels, additional surveillance efforts at sentinel hospitals or by review of hospital discharge databases will be necessary to monitor the impact of the vaccine program. Special studies (e.g., case-control studies and retrospective cohort studies) will be needed to confirm the effectiveness of rotavirus vaccine in routine programmatic use. Detection of Unusual Strains of Rotavirus A national strain surveillance system of sentinel laboratories has been established at CDC to monitor the prevalence of rotavirus strains before and after the introduction of rotavirus vaccines. This system is designed to detect new or unusual strains that might not be effectively prevented by vaccination and might affect the success of the vaccination program. Research Future research should include studies to determine the safety and efficacy of rotavirus vaccine administered to infants born prematurely, infants with immune deficiencies, infants who live in households with immunocompromised persons, and infants with chronic gastrointestinal disease. Postlicensure studies also should be conducted to determine the relative efficacy of <3 doses of vaccine and to address the cost effectiveness of vaccination programs in various settings. Education of Health-Care Providers and Parents The success of a rotavirus vaccination program depends on the acceptance and enthusiasm of physicians and other health-care providers who care for children and caretakers of infants. In light of the experience with the withdrawal of RRV-TV vaccine because of its association with intussusception, some health-care providers and parents might have concerns about vaccination with current rotavirus vaccine. Vaccination program personnel will benefit from education about rotavirus disease and rotavirus vaccine. Parental education on rotavirus gastroenteritis and on the vaccine will be essential to establish and maintain public confidence in this vaccine and to avoid confusion caused by cases of gastroenteritis in early childhood resulting from nonrotaviral etiologies and not preventable by rotavirus vaccine. AcknowledgementThe following persons reviewed and contributed to sections of this report: Jon R. Gentsch, Penina Haber, Frank DeStefano, and Marc-Alain Widdowson, CDC; Rosemary Tiernan, U.S. Food and Drug Administration; Geoff Evans, Health Resources and Services Administration; and Barbara Kuter and Penny Heaton, Merck and Company. References
Advisory Committee on Immunization Practices Rotavirus Vaccine Work Group Chair: John Treanor, MD, Rochester, New York. Members: James Alexander, MD, Atlanta, Georgia; Umesh Parashar, MBBS, Atlanta, Georgia; Marc-Alain Widdowson, MA, Atlanta, Georgia; Rosemary Tiernan, Bethesda, Maryland; Hector Izurieta, MD, Rockville Maryland; Geoffrey Evans, MD, Rockville, Maryland; Roger Glass, MD, Atlanta, Georgia; Edgar Marcuse, MD, Seattle, Washington; Trudy Murphy, MD, Atlanta, Georgia; Paul Gargiullo, PhD, Atlanta, Georgia; Larry Pickering, Atlanta, Georgia; Jane Seward, MD, Atlanta, Georgia; Penina Haber, Atlanta, Georgia; Greg Wallace, MD, Atlanta, Georgia; Penelope Dennehy, MD, Providence, Rhode Island; Samuel Katz, MD, Durham, North Carolina; John Modlin, MD, Lebanon, New Hampshire; and Paul Offit, MD, Philadelphia, Pennsylvania. Advisory Committee on Immunization Practices Membership List, July 2006 Chairman: Jon S. Abramson, MD, Wake Forest University School of Medicine, Winston-Salem, North Carolina. Executive Secretary: Larry K. Pickering, MD, CDC, Atlanta, Georgia. Members: Ban Mishu Allos, MD, Vanderbilt University School of Medicine, Nashville, Tennessee; Robert L. Beck, Palmyra, Virginia; Judith Campbell, MD, Baylor College of Medicine, Houston, Texas; Reginald Finger, MD, Colorado Springs, Colorado; Janet R. Gilsdorf, MD, University of Michigan, Ann Arbor, Michigan; Harry Hull, MD, Minnesota Department of Health, Minneapolis, Minnesota; Tracy Lieu, MD, Harvard Pilgrim Health Care and Harvard Medical School, Boston, Massachusettes; Edgar K. Marcuse, MD, Children's Hospital and Regional Medical Center, Seattle, Washington; Dale L. Morse, MD, New York State Department of Health, Albany, New York; Julia Morita, MD, Chicago Department of Public Health, Chicago, Illinois; Gregory A. Poland, MD, Mayo Clinic and Foundation, Rochester, Minnesota; Patricia Stinchfield, Children's Hospitals and Clinics, St. Paul, Minnesota; John J. Treanor, MD, University of Rochester, Rochester, New York; and Robin J. Womeodu, MD, University of Tennessee Health Science Center, Memphis, Memphis, Tennessee. Ex-Officio Members: James Cheek, MD, Indian Health Service, Albuquerque, New Mexico; Wayne Hachey, DO, Department of Defense, Falls Church, Virginia; Geoffrey S. Evans, MD, Health Resources and Services Administration, Rockville, Maryland; Bruce Gellin, MD, National Vaccine Program Office, Washington, D.C.; Linda Murphy, Centers for Medicare and Medicaid Services, Baltimore, Maryland; George T. Curlin, MD, National Institutes of Health, Bethesda, Maryland; Norman Baylor, PhD, U.S. Food and Drug Administration, Rockville, Maryland; and Kristin Lee Nichol, MD, Department of Veterans Affairs, Minneapolis, Minnesota. Liaison Representatives: Johathan Temte, MD, Madison, Wisconsin, Doug Campos-Outcalt, MD, Phoenix, Arizona, American Academy of Family Physicians; Keith Powell, MD, Akron, Ohio, Carol Baker, MD, Houston, Texas, American Academy of Pediatrics; Andrea Gelzer, MD, Hartford, Connecticut, America's Health Insurance Plans; James C. Turner, MD, Charlottesville, Virginia, American College Health Association; Stanley Gall, MD, Louisville, Kentucky, American College of Obstetricians and Gynecologists; Kathleen M. Neuzil, MD, Seattle, Washington, American College of Physicians; Litjen Tan, PhD, Chicago, Illinois, American Medical Association; Stephan L. Foster, PharmD, Memphis, Tennessee, American Pharmacists Association; Paul W., McKinney, MD, Louisville, Kentucky,Association of Teachers of Preventive Medicine; Clement Lewin, PhD, Orange, Connecticut, Biotechnology Industry Organization; Monica Naus, MD, Vancouver, British Columbia, Canada, Canadian National Advisory Committee on Immunization; Steve Gordon, MD, Cleveland, Ohio, Healthcare Infection Control Practices Advisory Committee; Samuel L. Katz, MD, Durham, North Carolina, Infectious Diseases Society of America; David Salisbury, MD, London, England, United Kingdom, London Department of Health; Nancy Bennett, MD, Rochester, New York, Jeffrey Duchin, MD, Seattle, Washington, National Association of County and City Health Officials; David A. Neumann, PhD, Alexandria, Virginia, National Coalition for Adult Immunization; William Schaffner, MD, Nashville, Tennessee, National Foundation for Infectious Diseases; Romeo S. Rodriquez, Mexico, National Immunization Council and Child Health Program, Mexico; Patricia Whitley-Williams, MD, New Brunswick, New Jersey, Peter Paradiso, PhD, Collegeville, Pennsylvania, National Medical Association; and Amy B. Middleman, MD, Houston, Texas, Society for Adolescent Medicine. Table 1 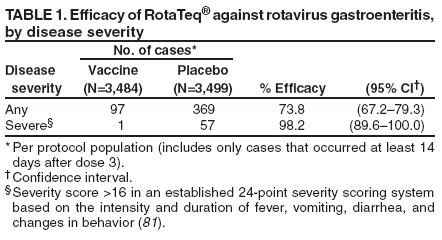 Return to top. Figure 1 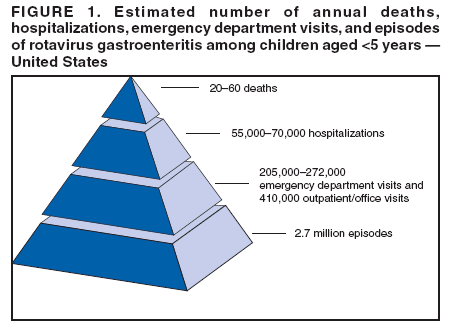 Return to top. Table 2 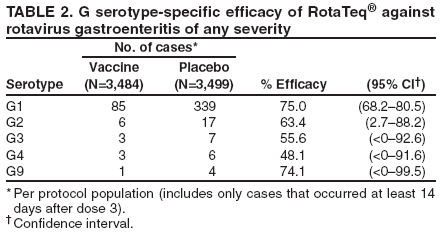 Return to top. Figure 2 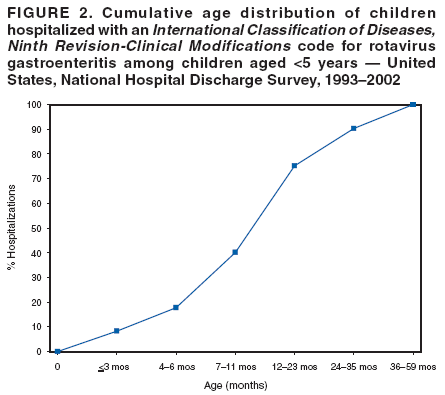 Return to top. Table 3 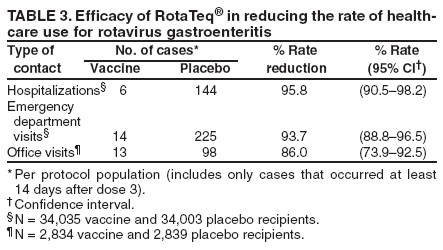 Return to top. Figure 3 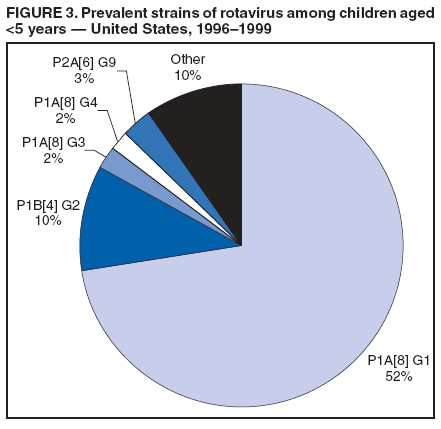 Return to top. Table 4 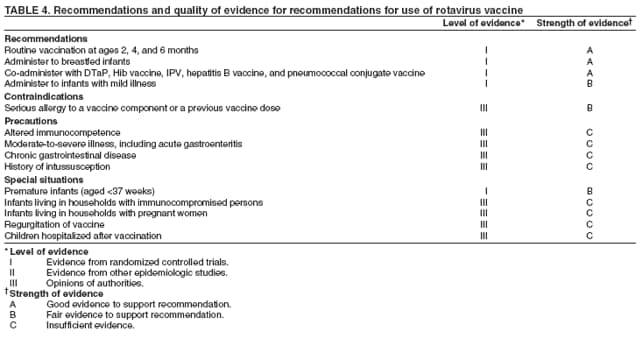 Return to top. Box 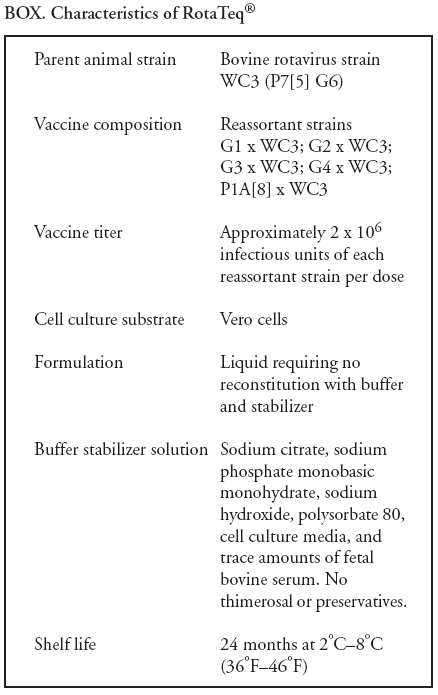 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 7/26/2006 |
|||||||||
|