 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Controlling Tuberculosis in the United StatesRecommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of AmericaPlease note: An erratum has been published for this article. To view the erratum, please click here. Corresponding preparers: Zachary Taylor, MD, National Center for HIV, STD, and TB Prevention, CDC; Charles M. Nolan, MD, Seattle-King County Department of Public Health, Seattle, Washington; Henry M. Blumberg, MD, Emory University School of Medicine, Atlanta, Georgia. SummaryDuring 1993--2003, incidence of tuberculosis (TB) in the United States decreased 44% and is now occurring at a historic low level (14,874 cases in 2003). The Advisory Council for the Elimination of Tuberculosis has called for a renewed commitment to eliminating TB in the United States, and the Institute of Medicine has published a detailed plan for achieving that goal. In this statement, the American Thoracic Society (ATS), CDC, and the Infectious Diseases Society of America (IDSA) propose recommendations to improve the control and prevention of TB in the United States and to progress toward its elimination. This statement is one in a series issued periodically by the sponsoring organizations to guide the diagnosis, treatment, control, and prevention of TB. This statement supersedes the previous statement by ATS and CDC, which was also supported by IDSA and the American Academy of Pediatrics (AAP). This statement was drafted, after an evidence-based review of the subject, by a panel of representatives of the three sponsoring organizations. AAP, the National Tuberculosis Controllers Association, and the Canadian Thoracic Society were also represented on the panel. This statement integrates recent scientific advances with current epidemiologic data, other recent guidelines from this series, and other sources into a coherent and practical approach to the control of TB in the United States. Although drafted to apply to TB control activities in the United States, this statement might be of use in other countries in which persons with TB generally have access to medical and public health services and resources necessary to make a precise diagnosis of the disease; achieve curative medical treatment; and otherwise provide substantial science-based protection of the population against TB. This statement is aimed at all persons who advocate, plan, and work at controlling and preventing TB in the United States, including persons who formulate public health policy and make decisions about allocation of resources for disease control and health maintenance and directors and staff members of state, county, and local public health agencies throughout the United States charged with control of TB. The audience also includes the full range of medical practitioners, organizations, and institutions involved in the health care of persons in the United States who are at risk for TB. IntroductionDuring 1993--2003, incidence of tuberculosis (TB) in the United States decreased 44% and is now occurring at a historic low level (14,874 cases in 2003). The Advisory Council for the Elimination of Tuberculosis (ACET) (1) has called for a renewed commitment to eliminating TB in the United States, and the Institute of Medicine (IOM) (2) has published a detailed plan for achieving that goal. In this statement, the American Thoracic Society (ATS), CDC, and the Infectious Diseases Society of America (IDSA) propose recommendations to improve the control and prevention of TB in the United States and to progress toward its elimination. This statement is one in a series issued periodically by the sponsoring organizations to guide the diagnosis, treatment, control, and prevention of TB (3--5). This statement supersedes one published in 1992 by ATS and CDC, which also was supported by IDSA and the American Academy of Pediatrics (AAP) (6). This statement was drafted, after an evidence-based review of the subject, by a panel of representatives of the three sponsoring organizations. AAP, the National Tuberculosis Controllers Association (NTCA), and the Canadian Thoracic Society were also represented on the panel. The recommendations contained in this statement (see Graded Recommendations for the Control and Prevention of Tuberculosis) were rated for their strength by use of a letter grade and for the quality of the evidence on which they were based by use of a Roman numeral (Table 1) (7). No rating was assigned to recommendations that are considered to be standard practice (i.e., medical or administrative practices conducted routinely by qualified persons who are experienced in their fields). This statement integrates recent scientific advances with current epidemiologic data, other recent guidelines from this series (3--5), and other sources (2,8--10) into a coherent and practical approach to the control of TB in the United States. Although drafted to apply to TB control activities in the United States, this statement might be of use in other countries in which persons with TB generally have access to medical and public health services and resources necessary to make a precise diagnosis of the disease; achieve curative medical treatment; and otherwise provide substantial science-based protection of the population against TB. This statement is aimed at all persons who advocate, plan, and work at controlling and preventing TB in the United States, including persons who formulate public health policy and make decisions about allocation of resources for disease control and health maintenance and directors and staff members of state, county, and local public health agencies throughout the United States charged with control of TB. The audience also includes the full range of medical practitioners, organizations, and institutions involved in the health care of persons in the United States who are at risk for TB. Throughout this document, the terms latent TB infection (LTBI), TB, TB disease, and infectious TB disease are used. LTBI is used to designate a condition in which an individual is infected with Mycobacterium tuberculosis but does not currently have active disease. Such patients are at risk for progressing to tuberculosis disease. Treatment of LTBI (previously called preventive therapy or chemoprophylaxis) is indicated for those at increased risk for progression as described in the text. Persons with LTBI are asymptomatic and have a negative chest radiograph. TB, TB disease, and infectious TB indicate that the disease caused by M. tuberculosis is clinically active; patients with TB are generally symptomatic for disease. Positive culture results for M. tuberculosis complex are an indication of TB disease. Infectious TB refers to TB disease of the lungs or larynx; persons with infectious TB have the potential to transmit M. tuberculosis to other persons. Progress Toward TB EliminationA strategic plan for the elimination of TB in the United States was published in 1989 (11), when the United States was experiencing a resurgence of TB (Figure 1). The TB resurgence was attributable to the expansion of HIV infection, nosocomial transmission of M. tuberculosis, multidrug-resistant TB, and increasing immigration from counties with a high incidence of TB. Decision makers also realized that the U.S. infrastructure for TB control had deteriorated (12); this problem was corrected by a substantial infusion of resources at the national, state, and local levels (13). As a result, the increasing incidence of TB was arrested; during 1993--2003, an uninterrupted 44% decline in incidence occurred, and in 2003, TB incidence reached a historic low level. This success in responding to the first resurgence of TB in decades indicates that a coherent national strategy; coordination of local, state, and federal action; and availability of adequate resources can result in dramatic declines in TB incidence. This success also raised again the possible elimination of TB, and in 1999, ACET reaffirmed the goal of tuberculosis elimination in the United States (1). The prospect of eliminating tuberculosis was critically analyzed in an independent study published by IOM in 2000 (2). The IOM study concluded that TB could ultimately be eliminated but that at the present rate of decline, elimination would take >70 years. Calling for greater levels of effort and resources than were then available, the IOM report proposed a comprehensive plan to 1) adjust control measures to the declining incidence of disease; 2) accelerate the decline in incidence by increasing targeted testing and treatment of LTBI; 3) develop new tools for diagnosis, treatment, and prevention; 4) increase U.S. involvement in global control of TB; and 5) mobilize and sustain public support for TB elimination. The report also noted the cyclical nature of the U.S. response to TB and warned against allowing another "cycle of neglect" to occur, similar to that which caused the 1985--1992 resurgence. As noted, the 44% decrease in incidence of TB in the United States during 1993--2003 (14,15) has been attributed to the development of effective interventions enabled by increased resources at the national, state, and local levels (1,2,16). Whereas institutional resources targeted specific problems such as transmission of TB in health-care facilities, public resources were earmarked largely for public health agencies, which used them to rebuild the TB-control infrastructure (13,17). A primary objective of these efforts was to increase the rate of completion of therapy among persons with TB, which was achieved by innovative case-management strategies, including greater use of directly observed therapy (DOT). During 1993--2000, the percentage of persons with reported TB who received DOT alone or in combination with self-supervised treatment increased from 38% to 78%, and the proportion of persons who completed therapy in <1 year after receiving a diagnosis increased from 63% to 80% (14). Continued progress in the control of TB in the United States will require consolidation of the gains made through improved cure rates and implementation of new strategies to further reduce incidence of TB. Challenges to Progress Toward TB EliminationThe development of optimal strategies to guide continuing efforts in TB control depends on understanding the challenges confronting the effort. The five most important challenges to successful control of TB in the United States are 1) prevalence of TB among foreign-born persons residing in the United States; 2) delays in detecting and reporting cases of pulmonary TB; 3) deficiencies in protecting contacts of persons with infectious TB and in preventing and responding to TB outbreaks; 4) persistence of a substantial population of persons living in the United States with LTBI who are at risk for progression to TB disease; and 5) maintaining clinical and public health expertise in an era of declining TB incidence. These five concerns (Box 1) serve as the focal point for the recommendations made in this statement to control and prevent TB in the United States. Prevalence of TB Among Foreign-Born Persons Residing in the United States Once a disease that predominately affected U.S.-born persons, TB now affects a comparable number of foreign-born persons who reside in the United States permanently or temporarily, although such persons make up only 11% of the U.S. population (14). During 1993--2003, as TB incidence in the United States declined sharply, incidence among foreign-born persons changed little (14). Lack of access to medical services because of cultural, linguistic, financial, or legal barriers results in delays in diagnosis and treatment of TB among foreign-born persons and in ongoing transmission of the disease (18--21). Successful control of TB in the United States and progress toward its elimination depend on the development of effective strategies to control and prevent the disease among foreign-born persons. Delays in Detection and Reporting of Cases of Pulmonary TB New cases of infectious TB should be diagnosed and reported as early as possible in the course of the illness so curative treatment can be initiated, transmission interrupted, and public health responses (e.g., contact investigation and case-management services) promptly arranged. However, delays in case detection and reporting continue to occur; these delays are attributed to medical errors (22--26) and to patient factors (e.g., lack of understanding about TB, fear of the authorities, and lack of access to medical services) (18--20). In addition, genotyping studies have revealed evidence of persistent transmission of M. tuberculosis in communities that have implemented highly successful control measures (27--29), suggesting that such transmission occurred before a diagnosis was received. Improvements in the detection of TB cases, leading to earlier diagnosis and treatment, would bring substantial benefits to affected patients and their contacts, decrease TB among children, and prevent outbreaks. Deficiencies in Protecting Contacts of Person with Infectious TB and in Preventing and Responding to TB Outbreaks Although following up contacts is among the highest public health priorities in responding to a case of TB, problems in conducting contact investigations have been reported (30--32). Approaches to contact investigations vary widely from program to program, and traditional investigative methods are not well adapted to certain populations at high risk. Only half of at-risk contacts complete a course of treatment for LTBI (32). Reducing the risk of TB among contacts through the development of better methods of identification, evaluation, and management would lead to substantial personal and public health benefits and facilitate progress toward eliminating TB in the United States. Delayed detection of TB cases and suboptimal contact investigation can lead to TB outbreaks, which are increasingly reported (26,33--38). Persistent social problems such as crowding in homeless shelters and detention facilities are contributing factors to the upsurge in TB outbreaks. The majority of jurisdictions lack the expertise and resources needed to conduct surveillance for TB outbreaks and to respond effectively when they occur. Outbreaks have become an important element in the epidemiology of TB, and measures to detect, manage, and prevent them are needed. Persistence of a Substantial Population of Persons Living in the United States with LTBI Who Are at Risk for Progression to TB Disease An estimated 9.6--14.9 million persons residing in the United States have LTBI (39). This pool of persons with latent infection is continually supplemented by immigration from areas of the world with a high incidence of TB and by ongoing person-to-person transmission among certain populations at high risk. For TB disease to be prevented among persons with LTBI, those at highest risk must be identified and receive curative treatment (4). Progress toward the elimination of TB in the United States requires the development of new cost-effective strategies for targeted testing and treatment of persons with LTBI (17,40). Maintaining Clinical and Public Health Expertise in an Era of Declining TB Incidence Detecting a TB case, curing a person with TB, and protecting contacts of such persons requires that clinicians and the staff members of public health agencies responsible for TB have specific expertise. However, as TB becomes less common, maintaining such expertise throughout the loosely coordinated TB-control system is challenging. As noted previously, medical errors associated with the detection of TB cases are common, and deficiencies exist in important public health responsibilities such as contact investigations and outbreak response. Errors in the treatment and management of TB patients continue to occur (41,42). Innovative approaches to education of medical practitioners, new models for organizing TB services (2), and a clear understanding and acceptance of roles and responsibilities by an expanded group of participants in TB control will be needed to ensure that the clinical and public health expertise necessary to progress toward the elimination of TB are maintained. Meeting the Challenges to TB EliminationFurther improvements in the control and prevention of TB in the United States will require a continued strong public health infrastructure and involvement of a range of health professionals outside the public health sector. The traditional model of TB control in the United States, in which planning and execution reside almost exclusively with the public health sector (17), is no longer the optimal approach during a sustained drive toward the elimination of TB. This statement emphasizes that success in controlling TB and progressing toward its elimination in the United States will depend on the integrated activities of professionals from different fields in the health sciences. This statement proposes specific measures to enhance TB control so as to meet the most important challenges; affirms the essential role of the public health sector in planning, coordinating, and evaluating the effort (43); proposes roles and responsibilities for the full range of participants; and introduces new approaches to the detection of TB cases, contact investigations, and targeted testing and treatment of persons with LTBI. The plan to reduce the incidence of TB in the United States must be viewed in the larger context of the global effort to control TB. The global TB burden is substantial and increasing. In 2000, an estimated 8.3 million (7.9--9.2 million) new cases of TB occurred, and 1.84 million (1.59--2.22 million) persons died from TB; during 1997--2000, the worldwide TB case rate increased 1.8%/year (44). TB is increasing worldwide as a result of inadequate local resources and the global epidemic of HIV infection. In sub-Saharan Africa, the rate of TB cases is increasing 6.4%/year (44). ACET (1), IOM (2), and other public health authorities (45,46) have acknowledged that TB will not be eliminated in the United States until the global epidemic is brought under control, and they have called for greater U.S. involvement in global control efforts. In response, CDC and ATS have become active participants in a multinational partnership (Stop TB Partnership) that was formed to guide the global efforts against TB. U.S. public and private entities also have provided assistance to countries with a high burden of TB and funding for research to develop new, improved tools for diagnosis, treatment, and prevention, including an effective vaccine. Despite the global TB epidemic, substantial gains can be made toward elimination of TB in the United States by focusing on improvements in existing clinical and public health practices (47--49). However, the drive toward TB elimination in the United States will be resource-intensive (1,12). Public health agencies that plan and coordinate TB- control efforts in states and communities need sufficient strength in terms of personnel, facilities, and training to discharge their responsibilities successfully, and the growing number of nonpublic health contributors to TB control, all pursuing diverse individual and institutional goals, should receive value for their contributions. Continued progress toward TB elimination in the United States will require strengthening the nation's public health infrastructure rather than reducing it (1,50). Basic Principles of TB Control in the United StatesFour prioritized strategies exist to prevent and control TB in the United States (17), as follows:
Structure of this StatementThis statement provides comprehensive guidelines for the full spectrum of activities involved in controlling and preventing TB in the United States. The remainder of this statement is structured in eight sections, as follows:
Scientific Basis of TB Control Transmission of TBM. tuberculosis is nearly always transmitted through an airborne route, with the infecting organisms being carried in droplets of secretions (droplet nuclei) that are expelled into the surrounding air when a person with pulmonary TB coughs, talks, sings, or sneezes. Person-to-person transmission of M. tuberculosis is determined by certain characteristics of the source-case and of the person exposed to the source-person and by the environment in which the exposure takes place (Box 2). The virulence of the infecting strain of M. tuberculosis might also be a determining factor for transmission. Characteristics of the Source-Case By the time persons with pulmonary TB come to medical attention, 30%--40% of persons identified as their close personal contacts have evidence of LTBI (30). The highest rate of infection among contacts follows intense exposure to patients whose sputum smears are positive for acid-fast bacilli (AFB) (31,57--59) (Figure 2). Because patients with cavitary pulmonary TB are more likely than those without pulmonary cavities to be sputum AFB smear-positive (60), patients with cavitary pulmonary disease have greater potential to transmit TB. Such persons also have a greater frequency of cough, so the triad of cavitary pulmonary disease, sputum AFB smear-positivity, and frequency of cough are likely related causal factors for infectivity. AFB smear-negative TB patients also transmit TB, but with lower potential than smear-positive patients. Patients with sputum AFB smear-negative pulmonary TB account for approximately 17% of TB transmission (61). Characteristics of the Exposed Person A study of elderly nursing home residents indicated that persons with initially positive tuberculin skin test results during periods of endemic exposure to TB had a much lower risk for TB than those whose skin test results were initially negative (62,63). This finding suggests that preexisting LTBI confers protection against becoming infected upon subsequent exposure and progression to active disease. Similarly, having prior disease caused by M. tuberculosis had been assumed to confer protection against reinfection with a new strain of M. tuberculosis. However, molecular typing of paired isolates of M. tuberculosis from patients with recurrent episodes of TB disease has demonstrated that reinfection does occur among immunocompetent and immunocompromised persons (64,65). The classic means of protecting persons exposed to infectious diseases is vaccination. Because of its proven efficacy in protecting infants and young children from meningeal and miliary TB (66), vaccination against TB with Mycobacterium bovis bacillus Calmette-Guerín (BCG) is used worldwide (although not in the United States). This protective effect against the disseminated forms of TB in infants and children is likely based on the ability of BCG to prevent progression of the primary infection when administered at that stage of life (67). Epidemiologic evidence suggests that BCG immunization does not protect against the development of infection with M. tuberculosis upon exposure (68), and use of BCG has not had an impact on the global epidemiology of TB. One recent retrospective study found that BCG protective efficacy can persist for 50--60 years, indicating that a single dose might have a long duration of effect (69). A meta-analysis indicated that overall BCG reduced the risk for TB 50% (66); however, another meta-analysis that examined protection over time demonstrated a decrease in efficacy of 5%--14% in seven randomized controlled trials and an increase of 18% in three others (70). An effective vaccine against M. tuberculosis is needed for global TB control to be achieved. Because only 30%--40% of persons with close exposure to a patient with pulmonary TB become infected (30,31), innate immunity might protect certain persons from infection (71). The innate mechanisms that protect against the development of infection are largely uncharacterized (71). Although immunocompromised persons (e.g., those with HIV infection) are at increased risk for progression to TB disease after infection with M. tuberculosis, no definitive evidence exists that immunocompromised persons, including those with HIV infection, have increased susceptibility to infection upon exposure. Observational studies suggest that population-based variability in susceptibility to TB might be related to the length of time a population has lived in the presence of M. tuberculosis and has thus developed resistance to infection through natural selection (72--74). However, the genetic basis for susceptibility or resistance to TB is not well understood (72,75). Characteristics of the Exposure Studies that have stratified contacts of persons with pulmonary TB according to time spent with the infected person indicate that the risk for becoming infected with M. tuberculosis is in part determined by the frequency and duration of exposure (60). In a given environment shared by a patient with pulmonary TB and a contact, the risk for transmitting the infection varies with the density of infectious droplet nuclei in the air and how long the air is inhaled. Indoors, tubercle bacilli are expelled into a finite volume of air, and, unless effective ventilation exists, droplet nuclei containing M. tuberculosis might remain suspended in ambient air (76). Exposures in confined air systems with little or no ventilation pose a major risk for transmission of TB; this has been demonstrated in homes, ships, trains, office buildings, and health-care institutions (77--80). When contact occurs outdoors, TB bacilli expelled from the respiratory tract of an infectious person are rapidly dispersed and are quickly rendered nonviable by sunlight (77). The risk for transmission during such encounters is very limited. Considerable attention has been given to transmission of M. tuberculosis during air travel. Investigations have demonstrated that the risk for transmission from an infectious person to others on an airplane is greater on long flights (>8 hours) and that the risk for contracting M. tuberculosis infection is highest for passengers and flight crew members sitting or working near an infectious person (81,82). However, the overall public health importance of such events is negligible (77,81). Virulence of the Infecting Strain of M. tuberculosis Although much is known about factors that contribute to the risk for transmission of M. tuberculosis from person to person, the role of the organism itself is only beginning to be understood (83). Genetic variability is believed to affect the capability of M. tuberculosis strains to be transmitted or to cause disease once transmitted, or both. The M. tuberculosis W-strain family, a member of the globally spread Beijing family (84), is a group of clonally related multidrug-resistant organisms of M. tuberculosis that caused nosocomial outbreaks involving HIV-infected persons in New York City (NYC) during 1991--1994 (85,86). W-family organisms, which have also been associated with TB outbreaks worldwide, are believed to have evolved from a single strain of M. tuberculosis that developed resistance-conferring mutations in multiple genes. The growth of W-family organisms in human macrophages is four- to eightfold higher than that of strains that cause few or no secondary cases of TB; this enhanced ability to replicate in human macrophages might contribute to the organism's potential for enhanced transmission (87). Whether M. tuberculosis loses pathogenicity as it acquires resistance to drugs is not known. Isoniazid-resistant M. tuberculosis strains are less virulent than drug-susceptible isolates in guinea pigs (88), and genotyping studies from San Francisco, California, and from the Netherlands indicated that isoniazid-resistant strains are much less likely to be associated with clusters of TB cases than drug-susceptible strains (89,90). Nevertheless, because person-to-person spread has been demonstrated repeatedly, persons with TB with drug-resistant isolates should receive the same public health attention at the programmatic level as those with drug-susceptible isolates (91,92). Effect of Chemotherapy on Infectiousness Patients with drug-susceptible pulmonary and other forms of infectious TB rapidly become noninfectious after institution of effective multiple-drug chemotherapy. This principle has been established by studies demonstrating that household contacts of persons with infectious pulmonary TB who were treated at home after a brief period of hospitalization for institution of therapy developed LTBI at a frequency no greater than that of persons with pulmonary TB who were hospitalized for 1 year (93) or until sputum cultures became negative (94). This potent effect of chemotherapy on infectiousness is likely attributable, at least in part, to the rapid elimination of viable M. tuberculosis from sputum (95) and to reduction in cough frequency (96). The ability of chemotherapy to eliminate infectivity is one reason why detecting infectious cases and promptly instituting multiple-drug therapy is the primary means of interrupting the spread of TB in the United States. The effect of chemotherapy to eliminate infectiousness was once thought to occur rapidly, and patients on chemotherapy were thought not to be infectious (97,98). However, no ideal test exists to assess the infective potential of a TB patient on treatment, and infectivity is unlikely to disappear immediately after multidrug therapy is started. Quantitative bacteriologic studies indicate that the concentration of viable M. tuberculosis in sputum of persons with cavitary sputum AFB smear-positive pulmonary TB at the time of diagnosis, which averaged 106--107 organisms/ml, decreased >90% (10-fold) during the first 2 days of treatment, an effect attributable primarily to administration of isoniazid (99), and >99% (100-fold) by day 14--21, an effect attributable primarily to administration of rifampin and pyrazinamide (100). Thus, if no factor other than the elimination of viable M. tuberculosis from sputum were to account for the loss of infectivity during treatment, the majority of patients (at least those with infection attributable to isolates susceptible to isoniazid) who have received treatment for as few as 2 days with the standard regimen (i.e., isoniazid, rifampin, ethambutol, and pyrazinamide) could be assumed to have an infective potential that averages 10% of that at the time of diagnosis. After 14--21 days of treatment, infectiousness averages <1% of the pretreatment level. This statement presents general guidelines on elimination of infectivity with treatment (Box 3). However, decisions about infectiousness of a person on treatment for TB should always be individualized on the basis of 1) the extent of illness; 2) the presence of cavitary pulmonary disease; 3) the degree of positivity of sputum AFB smear results; 4) the frequency and strength of cough; 5) the likelihood of infection with multidrug-resistant organisms; and 6) the nature and circumstances of the contact between the infected person and exposed contacts (101). Patients who remain in hospitals or reside either temporarily or permanently in congregate settings (e.g., shelters and correctional facilities) are subject to different criteria for infectiousness. In such congregate settings, identification and protection of close contacts is not possible during the early phase of treatment, and more stringent criteria for determining absence of infectivity (i.e., three consecutive AFB-negative sputum smears) should be followed (10). All patients with suspected or proven multidrug resistant TB should be subjected to these more stringent criteria for absence of infectivity (10). Progression from LTBI to TB Disease Although the human immune response is highly effective in controlling primary infection resulting from exposure to M. tuberculosis among the majority of immunocompetent persons, all viable organisms might not be eliminated. M. tuberculosis is thus able to establish latency, a period during which the infected person is asymptomatic but harbors M. tuberculosis organisms that might cause disease later (4,71). The mechanisms involved in latency and persistence are not completely understood (71,72). For the majority of persons, the only evidence of LTBI is an immune response against mycobacterial antigens, which is demonstrated by a positive test result, either a tuberculin skin test (3) or, in certain circumstances, a whole blood antigen-stimulated interferon-g release assay result (e.g., QuantiFERON®-TB Gold test [QFT-G] [Cellestis Limited, Carnegie, Victoria, Australia]). The tuberculin skin test measures delayed-type hypersensitivity; QFT-G, an ex vivo test for detecting latent M. tuberculosis infection, measures a component of cell-mediated immune response (102). QFT-G is approved by the Food and Drug Administration (FDA), and CDC will publish guidelines on its use. CDC had previously published guidelines for use of QuantiFERON®-TB, an earlier version of the test that is no longer available (103). T SPOT-TB,® an enzyme-linked immunospot assay for IFN-g, is marketed in Europe along with QFT-G but is not FDA-approved for use in the United States. Although approved by FDA, the Tine Test® is not recommended for the diagnosis of M. tuberculosis infection. Tests available in other countries to diagnose M. tuberculosis infection (e.g., T SPOT-TB and Heaf test) are not recommended for clinical use in the United States. Once a person has contracted LTBI, the risk for progression to TB disease varies. The greatest risk for progression to disease occurs within the first 2 years after infection, when approximately half of the 5%--10% lifetime risk occurs (4,104). Multiple clinical conditions also are associated with increased risk for progression from LTBI to TB disease. HIV infection is the strongest known risk factor (4). Other key risk factors because of their prevalence in the U.S. population are diabetes mellitus (105), acquisition of LTBI in infancy or early childhood, and apical fibro-nodular changes on chest radiograph (106). A recent addition to the known risk factors for progression from LTBI to TB disease is the use of therapeutic agents that antagonize the effect of cytokine tumor necrosis factor alpha (TNF-a) and have been proven to be highly effective treating autoimmune-related conditions (e.g., Crohn's disease and rheumatoid arthritis) (107). Cases of TB have been reported among patients receiving all three licensed TNF-a antagonists (i.e., infliximab, etanercept, and adalimimab) (108). CDC has published interim guidelines for preventing TB when these agents are used (109). Epidemiology of TB in the United StatesSurveillance (i.e., the systematic collection, analysis, and dissemination of data) is a critical component of successful TB control, providing essential information needed to 1) determine patterns and trends of the disease; 2) identify populations and settings at high risk; and 3) establish priorities for control and prevention activities. Surveillance is also essential for quality-assurance purposes, program evaluation, and measurement of progress toward TB elimination. In addition to providing the epidemiologic profile of TB in a given jurisdiction, state and local surveillance are essential to national TB surveillance. CDC's national TB surveillance system publishes epidemiologic analyses of reported TB cases in the United States (110). Data for the national TB surveillance system are reported by state health departments in accordance with standard TB case-definition and case-report formats (110,111). The system tracked the reversal of the declining trend in TB incidence in the United States in the mid-1980s, the peak of the resurgence in 1992 (with a 20% increase in cases reported during 1985--1992), and the subsequent 44% decline to an all-time low number (14,871) and rate (5.1 cases/100,000 population) of TB cases in 2003 (14,15) (Figure 1). Geographic Distribution of TB Wide disparities exist in the geographic distribution of TB cases in the United States. In 2003, six states (California, Florida, Georgia, Illinois, New York, and Texas) each reported >500 cases and accounted for 57% of the national total (14). These states along with New Jersey accounted for approximately 75% of the overall decrease in cases since 1992. The highest rates and numbers of TB cases are reported from urban areas; >75% of cases reported in 2003 were from areas with >500,000 population (14). In 2003, a total of 24 states (48%) had incidence of <3.5 cases of TB/100,000 population, the rate established as the year 2000 interim target for the United States in the 1989 strategic plan for eliminating TB (11). Demographic Distribution of TB In 2003, adults aged 15--64 years accounted for 73.6% of reported TB cases. Incidence of TB was highest (8.4 cases/100,000 population) among adults aged >65 years, who accounted for 20.2% of cases. Children aged <14 years accounted for 6.2% of reported cases and had the lowest incidence of TB; 61.3% of reported cases occurred among men, and case rates among men were at least double those of women in mid- and older-adult age groups. In 2003, the white, non-Hispanic population accounted for only 19% of reported cases of TB, and TB incidence among the four other racial/ethnic populations for which data were available was 5.7--21.0 times that of non-Hispanic whites (Table 2). Foreign-born persons accounted for 94% of TB cases among Asians and 74% of cases among Hispanics, whereas 74% of cases among non-Hispanic blacks occurred among persons born in the United States (15). Distribution of TB by Socioeconomic and Employment Status Socioeconomic status (SES). Low SES is associated with an increased risk for TB. An analysis of national surveillance data that assigned socioeconomic indicator values on the basis of residence zip code indicated that the risk for TB increased with lower SES for six indicators (crowding, education, income, poverty, public assistance, and unemployment), with crowding having the greatest impact (112). Risk for TB increased uniformly between socioeconomic quartile for each indicator, similar to other socioeconomic health gradients for other chronic diseases, except for crowding, for which risk was concentrated in the lowest quartile. Adjusting for SES accounted for approximately half of the increased risk for TB associated with race/ethnicity among U.S.-born blacks, Hispanics, and American Indians (112). Occupation. Increased incidence of TB among persons with certain occupations is attributable to exposure in the work environment and to an increased likelihood that workers will have other risk factors unrelated to occupation, such as foreign birth. A 29-state study of patients with clinically active TB reported during 1984--1985 indicated that increased incidence was independent of occupation. An association between general SES groupings of occupations and risk for TB also was demonstrated in that study (113). Chronically unemployed persons had high incidence of TB; this finding is consistent with surveillance data indicating that >50% of TB patients were unemployed during the 2 years before diagnosis (14). TB among health-care workers (HCWs). Because transmission of M. tuberculosis in health-care institutions was a contributing factor to the resurgence of TB during 1985--1992, recommendations were developed to prevent transmission in these settings (10). In 2003, persons reported to have been HCWs in the 2 years before receiving their diagnoses accounted for 3.1% of reported TB cases nationwide (14). However, the elevated risk among HCWs might be attributable to other factors (e.g., birth in a country with a high incidence of TB) (114). A multistate occupational survey indicated that the majority of HCWs did not have a higher risk for TB than the general population; respiratory therapists, however, did appear to be at greater risk (113). Identification of Populations at High Risk for TB Contacts of infectious persons. A high prevalence of TB disease and LTBI has been documented among close contacts of persons with infectious pulmonary TB (31). A study of approximately 1,000 persons from urban sites with pulmonary AFB sputum smear-positive TB indicated that more than one third of their contacts had positive tuberculin skin tests and that 2% of all close contacts had active TB. Contacts identified with TB disease were more likely to be household members or children aged <6 years (31). Foreign-born persons. The proportion of TB cases in the United States occurring among foreign-born persons increased progressively during the 1990s; in 2003, persons born outside the United States accounted for 53% of reported cases (14) (Figure 3). Although foreign-born persons who received a diagnosis of TB in 2002 were born in >150 countries worldwide, as in each of the 6 previous years, five countries of origin accounted for the greatest number of foreign-born persons with TB: China (5%), India (8%), Mexico (26%), the Philippines (12%), and Vietnam (8%). During 1992--2003, the number of states in which >50% of the total reported cases occurred among foreign-born persons increased from four (8%) in 1992 to 24 (48%) in 2003 (15). Among states and cities, however, this profile can change rapidly, reflecting changes in patterns of immigration and refugee settlement (21). Surveillance data indicate that incidence of TB among foreign-born persons is approximately 23 cases/100,000 population (14). Incidence varied by county of origin, appearing to reflect incidence of TB in the country of birth (21,115,116). In 2003, approximately 47% of foreign-born persons with TB received their diagnoses within 5 years of their arrival in the United States, and 19% received their diagnoses within 1 year of arrival. Among foreign-born persons, TB case rates decreased with longer duration of residence in the United States. TB rates were nearly four times higher among persons residing in the United States for <5 years than in those who were residents for >5 years (115,116). HIV-infected persons. Because reporting of HIV infection among persons with TB is not complete, the exact prevalence of HIV infection among such persons is unknown. During 1993--2001, the prevalence of reported HIV infection occurring among persons also reported with TB decreased from 15% to 8% (14); this decrease has been attributed, in part, to reduced transmission of TB among HIV-infected persons (16). According to a recent worldwide epidemiologic assessment, however, 26% of adult TB cases in the United States are attributable to HIV infection (44). Homeless persons. In 2003, persons known to have been homeless in the year before receiving a diagnosis accounted for 6.3% of cases of TB nationwide. On the basis of available population estimates (117), incidence of TB among homeless persons is approximately 30--40/100,000 population, more than five times the national case rate. However, a prospective study of a cohort of approximately 3,000 homeless persons in San Francisco documented an annual incidence of >250 cases/100,000 population (118). In addition, outbreaks of TB linked to overnight shelters continue to occur among homeless persons and likely contribute to the increased incidence of TB among that population (119,120). Other populations at high risk. In 2003, persons known to have injected drugs in the year before receiving a diagnosis accounted for 2.2% of reported cases of TB, and noninjection drug use was reported by 7.3% of persons with TB. In certain U.S. communities, injection drug use is sufficiently prevalent so as to constitute a high risk for epidemiologic importance rather than simply an individual risk factor, especially when overlap exists between injection drug use and HIV infection (121,122). TB Among Detainees and Prisoners in Correctional Facilities The proportion of cases of TB occurring among inmates of prisons and jails has remained stable at approximately 3%--4% since data began to be collected in 1993; it was 3.2% in 2003 (14). Inmates also have high incidence of TB, with rates often >200/100,000 population (123), and they have a disproportionately greater number of risk factors for TB (e.g., low SES, HIV infection, and substance abuse) compared with the general population (124,125). TB transmission in correctional facilities contributes to the greater risk among those populations, presumably because of the difficulties in detecting cases of infectious TB and in identifying, evaluating, and treating contacts in these settings (37,126). TB outbreaks occur in both prison and jail settings. Dedicated housing units for prison inmates with HIV infection were sites of transmission in California in 1995 (126) and South Carolina in 1999 and in South Carolina in 1999 (37). In the South Carolina outbreak, delayed diagnosis and isolation of an inmate who apparently had active TB after entering the facility led to >15 outbreak cases. Transmission leading to TB infection in the community also was documented in an outbreak that occurred in a jail in Tennessee during 1995--1997 (127,128) that involved approximately 40 inmates; contact investigations were incomplete because of brief jail terms and frequent movement of inmates. During the same period, 43% of patients with TB in the surrounding community had previously been incarcerated in that jail (127), and, after 2 years, the jail outbreak strain was more prevalent in the community than it was during the jail outbreak. Genotyping studies indicated that the outbreak strain accounted for approximately 25% of TB cases in the community, including those among patients with no history of incarceration (128). Contributions of Genotyping of M. tuberculosisM. tuberculosis genotyping refers to procedures developed to identify M. tuberculosis isolates that are identical in specific parts of the genome (83). To date, M. tuberculosis genotyping has been based on polymorphisms in the number and genomic location of mycobacterial repetitive elements. The most widely used genotyping test for M. tuberculosis is restriction fragment length polymorphism (RFLP) analysis of the distribution of the insertion sequence IS6110 (129). However, genotyping tests based on polymorphisms in three additional mycobacterial repetitive elements (i.e., polymorphic guanine cytosine--rich repetitive sequences, direct repeats [e.g., spoligotyping], and mycobacterial interspersed repetitive units [MIRU]) have also been developed (83). M. tuberculosis isolates with identical DNA patterns in an established genotyping test often have been linked through recent transmission among the persons from whom they were isolated. When coupled with traditional epidemiologic investigations, analyses of the genotype of M. tuberculosis strains have confirmed suspected transmission and identified unsuspected transmission of M. tuberculosis. These analyses have also identified risk factors for recent infection with rapid progression to disease, demonstrated exogenous reinfection with different strains, identified weaknesses in conventional contact investigations, and documented the existence of laboratory cross-contamination. Genotyping has become an increasingly useful tool for studying the pathogenesis, epidemiology, and transmission of TB. Epidemiology of TB Among Contacts in Outbreak Settings Conventional contact investigations have used the concentric circles approach to collect information and screen household contacts, coworkers, and increasingly distant contacts for TB infection and disease (17). The concentric circles model has been described previously (130). However, this method might not always be adequate in out-of-household settings. In community-based studies from San Francisco (131), Zurich (132), and Amsterdam (133), only 5%--10% of persons with clustered IS6110-based genotyping patterns were identified as contacts by the source-person in the cluster. This finding indicates that either 1) transmission of M. tuberculosis might occur more commonly than suspected and is not easily detected by conventional contact tracing investigations or 2) genotype clustering does not necessarily represent recent transmission (55). Because genotyping studies discover only missed or mismanaged contacts (i.e., those that subsequently receive a diagnosis of TB), such studies cannot explain the successes of the process or the number of cases that were prevented. Certain populations (e.g., the urban homeless) present specific challenges to conducting conventional contact investigations. Genotyping studies have provided information about chains of transmission in these populations (118,119). In a prospective study of TB transmission in Los Angeles, the degree of homelessness and use of daytime services at three shelters were factors that were independently associated with genotype clustering (119). Additional studies support the idea that specific locations can be associated with recent or ongoing transmission of M. tuberculosis among homeless persons. Two studies among predominantly HIV-infected men have demonstrated evidence of transmission at specific bars in the community (134,135). Genotyping techniques have confirmed TB transmission in HIV residential facilities (136), crack houses (i.e., settings in which crack cocaine is sold or used) (137), hospitals and clinics (54), and prisons (138,139). TB transmission also has been demonstrated among church choirs (140) and renal transplant patients (141) and in association with processing of contaminated medical waste (142) and with bronchoscopy (143,144). Communitywide Epidemiology of TB TB might arise because of rapid progression from a recently acquired M. tuberculosis infection, from progression of LTBI to TB disease, or occasionally from exogenous reinfection (145). The majority of genotyping studies have assumed that clustered isolates in a population-based survey reflect recent transmission of M. tuberculosis. Certain studies have identified epidemiologic links between clustered TB cases, inferring that the clustered cases are part of a chain of transmission from a single common source or from multiple common sources (131,146). The number and proportion of population-based cases of TB that occur in clusters representing recent or ongoing transmission of M. tuberculosis have varied from study to study; frequency of clustering has varied from 17%--18% (in Vancouver, Canada) to 30%--40% (in U.S. urban areas) (131,147,148). Youth, being a member of a racial or ethnic minority population, homelessness, substance abuse, and HIV infection have been associated with clustering (131,133, 148,149). The increasing incidence of TB among foreign-born persons underscores the need to understand transmission dynamics among this population. In San Francisco, two parallel TB epidemics have been described (150,151), one among foreign-born persons that was characterized by a low rate of genotype clustering and the other among U.S-born persons that was characterized by a high rate of genotype clustering. In a recent study from NYC, being born outside the United States, being aged >60 years, and receiving a diagnosis after 1993 were factors independently associated with being infected with a strain not matched with any other, whereas homelessness was associated with genotype clustering and recent transmission (152). Among foreign-born persons, clustered strains were more likely to be found among patients with HIV infection (152). Other Contributions of Genotyping Genotyping can determine whether a patient with a recurrent episode of TB has relapsed with the original strain of M. tuberculosis or has developed exogenous reinfection with a new strain (64,153). In Cape Town, South Africa, where incidence of TB is high and considerable ongoing transmission exists, 16 (2.3%) of 698 patients had more than one episode of TB disease. In 12 (75%) of the 16 recurrent cases, the pairs of M. tuberculosis isolates had different IS6110-based genotyping patterns, indicating exogenous reinfection (154). However, in areas with a low incidence of TB, episodes of exogenous reinfection are uncommon (153). Because TB incidence in the majority of areas of the United States is low and decreasing, reinfection is unlikely to be a major cause of TB recurrence. Genotyping has greatly facilitated the identification of false-positive cultures for M. tuberculosis resulting from laboratory cross-contamination of specimens. Previously, false-positive cultures (which might lead to unnecessary treatment for patients, unnecessary work for public health programs in investigating cases and pseudo-outbreaks, and unnecessary costs to the health-care system) were difficult to substantiate (155). Because of its capability to determine clonality among M. tuberculosis strains, genotyping has been applied extensively to verify suspected false-positive cultures (156--158) and to study the causes and prevalence of laboratory cross-contamination (159,160). The Role of Genotyping of M. tuberculosis in TB-Control Programs In 2004, CDC established the Tuberculosis Genotyping Program (TBGP) to enable rapid genotyping of isolates from every patient in the United States with culture-positive TB (161). State TB programs may submit one M. tuberculosis isolate from each culture-positive case within their jurisdictions to a contracted genotyping laboratory. A detailed manual describing this program, including information on how to interpret genotyping test results and how to integrate genotyping into TB-control activities, has been published (162). Genotyping information is essential to optimal TB control in two settings. First, genotyping is integral to the detection and control of TB outbreaks, including ruling a suspected outbreak in or out and pinpointing involved cases and the site or sites of transmission (54,136--144). Second, genotyping is essential to detect errors in handling and processing of M. tuberculosis isolates (including laboratory cross-contamination) that lead to reports of false-positive cultures for M. tuberculosis (156,158--160,163). More extensive use of M. tuberculosis genotyping for TB control depends on the availability of sufficient program resources to compare results with information from traditional epidemiologic investigative techniques. Time-framed genotyping surveys and good fieldwork can unravel uncertainties in the epidemiology of TB in problematic populations at high risk (150--152,164). Genotyping surveys and epidemiologic investigations also can be used as a program monitoring tool to determine the adequacy of contact investigations (29,119,132--134,164--166) and evaluate the success of control measures designed to interrupt transmission of M. tuberculosis among certain populations or settings (167). Programs that use genotyping for surveillance of all of the jurisdiction's M. tuberculosis isolates should work closely on an ongoing basis with the genotyping laboratory and commit sufficient resources to compare genotyping results with those of traditional epidemiologic investigations. Information from both sources is needed for optimum interpretation of the complex epidemiologic patterns of TB in the United States (84,168). Principles and Practice of TB ControlBasic Principles of TB ControlThe goal of TB control in the United States is to reduce morbidity and mortality caused by TB by 1) preventing transmission of M. tuberculosis from persons with contagious forms of the disease to uninfected persons and 2) preventing progression from LTBI to TB disease among persons who have contracted M. tuberculosis infection. Four fundamental strategies are used to achieve this goal (Box 4) (17,169), as follows:
Vaccination with BCG is not recommended as a means to control TB in the United States because of the unproved efficacy of the vaccine in the U.S. population (174,175), its effect of confounding the results of tuberculin skin testing (176) and the success of other measures in reducing incidence of TB (16). During the 1985--1992 TB resurgence, the documented spread of TB, including multidrug-resistant TB, in health-care institutions and in the community (52--54,177,178) stimulated interest in the potential use of BCG to protect HCWs and others from exposure to M. tuberculosis. In 1996, a statement from ACET and the Advisory Committee on Immunization Practices (179) recommended vaccination with BCG for 1) infants and children with exposure to M. tuberculosis in settings in which other protective measures are either inaccessible or proven to be ineffective and 2) HCWs when likelihood of exposure to multidrug-resistant TB is high and recommended control measures have not been successful. With improved TB control in the United States and the decline of multidrug-resistant TB (13), use of BCG for protection against TB has declined. An improved vaccine, particularly one that protects adults with LTBI against acquiring TB disease, would accelerate progress toward TB elimination in the United States (180). Deficiencies in TB ControlBecause TB control is a complex undertaking that involves multiple participants and processes, mistakes often occur, with adverse consequences. Common errors include 1) delays among persons with active TB obtaining health care; 2) delayed detection and diagnosis of active TB; 3) failed or delayed reporting of TB; 4) failure to complete an effective course of treatment for TB; 5) missed opportunities to prevent TB among children; and 6) deficiencies in conducting contact investigations and in recognizing and responding to outbreaks. Delays in Obtaining Health Care Homeless patients with TB symptoms often delay seeking care or experience delays in gaining access to care (181), and fear of immigration authorities has been associated with patient delay among foreign-born persons (19). Patients who speak languages other than English or who are aged 55--64 years are more likely than others to delay seeking care (20). Cultural factors that might affect health-seeking behavior by foreign-born persons include misinterpretation or minimization of symptoms, self-care by using over-the-counter or folk medicines, and the social stigma associated with TB (18). In certain societies, women with TB are less likely to take advantage of health-care services, perhaps because of stigma associated with the diagnosis, including a lower likelihood of marriage (182,183). Even in areas with open access to public health clinical services, persons at risk for TB might not seek evaluation and treatment because they are not aware that these resources are available for persons with limited financial means (118,184--186). Delayed Detection and Diagnosis of Active TB Delayed detection of a case of TB and resulting delays in initiation of treatment can occur if the clinician does not suspect the diagnosis. A survey conducted in NYC in 1994 found that the median delay within the health-care system (defined as the time from first contact to initiation of treatment for active TB) was 15 days (range: 0--430 days) (20). Asians and homeless persons were more likely to encounter delays in receiving a diagnosis than non-Asians and persons with stable housing. Persons without cough who had AFB smear-negative TB or who did not have a chest radiograph at their initial visit also experienced delays. In London, England, delays in diagnosis occurred among whites and among women of all racial/ethnic populations (187). Regardless of the reason, the consequences of delays in diagnosis and initiation of effective therapy can be serious. In Maine, a shipyard worker aged 32 years who was a TB contact and who was untreated despite having symptoms of active TB, repeated medical visits, and a chest radiograph consistent with active TB did not receive a diagnosis of TB until 8 months after he became ill (188), and 21 additional cases of TB occurred among his contacts. Of 9,898 persons who were investigated as contacts, 697 (7.0%) persons received diagnoses of new LTBIs. A high school student in California was symptomatic for >1 year before TB was diagnosed (177). Subsequently, 12 additional TB cases among fellow students were linked to the source-case, and 292 (23%) of 1,263 students tested had positive tuberculin skin tests. Other instances of delayed or missed diagnoses of TB have been reported that have resulted in extended periods of infectiousness and deaths (22,24,178). These problems reflect the increasing difficulty in maintaining clinical expertise in the recognition of TB in the face of declining disease incidence (41). Recognition of TB among patients with AFB-negative sputum smear results is a challenge for practitioners and has been associated with delays in reporting and treatment (22,189,190). Delayed Reporting of TB Failure to promptly report a new TB case delays public health responses (e.g., institution of a treatment plan, case-management services, and protection of contacts). Although TB cases in the United States rarely remain unreported, timeliness of reporting varies (median: 7--38 days) (190). Failure to Receive and Complete a Standard Course of Treatment for Active TB Failure to receive and complete a standard course of treatment for TB has adverse consequences, including treatment failure, relapse, increased TB transmission, and the emergence of drug-resistant TB (191--193). At least two reasons exist for failure to complete standard treatment. Patients frequently fail to adhere to the lengthy course of treatment (188). Poor adherence to treatment regimens might result from difficulties with access to the health-care system, cultural factors, homelessness, substance abuse, lack of social support, rapid clearing of symptoms, or forgetfulness (18,194). Also, as TB has become less common, clinicians might fail to use current treatment regimens (48). These adverse outcomes are preventable by case-management strategies provided by TB-control programs, including use of DOT (13,195,196). Missed Opportunities To Prevent TB Among Children The absence of TB infection and disease among children is a key indicator of a community's success in interrupting the transmission of TB (197). The 1985--1992 TB resurgence included a reversal of the long-term decline in the incidence of TB among children, which indicated a failure of the public health system to prevent disease transmission (197). A study of 165 children reported with TB in California in 1994 found that for 59 (37%), an adult source-case was identified (198). Factors that contributed to transmission to children included delayed reporting, delayed initiation of contact investigations, and poor management of adult source-cases. Improvements in contact investigations might have prevented 17 (10%) of those cases (198). Deficiencies in Conducting Contact Investigations and in Recognizing and Responding to Outbreaks Deficiencies in contact investigations and failure to recognize and respond to TB outbreaks are among the most important challenges to optimal control of TB in the United States. These topics are discussed in detail in this statement along with the other essential components of TB control. Importance of TB Training and EducationThe 1985--1992 TB resurgence led ACET to call for a renewed focus on training and education as an integral part of strategies for TB control, prevention, and elimination (1). Factors indicating a need for this focus include the following:
Educating Patients and Communities at High Risk Education of patients by clinicians, TB program staff, and trusted community members promotes acceptance and adherence to authoritative advice about controlling and preventing TB. Such education can influence patients' decision-making about whether to accept and complete treatment for LTBI (202). Because cultural and health beliefs might act as barriers to effective control of TB (18,19), an increasing need exists for education targeted at populations at high risk (19). TB-control programs should enlist community-based organizations and other key informants to discover the health beliefs, norms, and values of communities at high risk in their jurisdictions (202,203). Professional associations and academic institutions (including schools of medicine, public health, and nursing) will be valuable partners in developing an understanding of the health perceptions of these populations. Education materials should be developed with input from the target audience to ensure that they are culturally and linguistically appropriate (203,204). The Strategic Plan for TB Training and Education In 1997, CDC funded a project to develop a Strategic Plan for Tuberculosis Training and Education (the Strategic Plan) that provided guidance to agencies and organizations in the United States that offer TB training and education for public- and private-sector providers. The Strategic Plan specified critical areas requiring attention, including 1) the need for culturally competent programs and materials, 2) effective methods and technologies, 3) collaboration and cooperation among training and education partners outside TB-control programs, and 4) adequate funding for training and education efforts. Other Resources for TB Training and Education Substantial progress has been made in developing and disseminating resources for TB training and education. CDC and national TB centers, NTCA, regional controllers associations (e.g., the Northeast Tuberculosis Training Consortium), state and local health departments, and the National Laboratory Training Network have all conducted education programs or developed training and education materials. In 2001, as stipulated by the Strategic Plan, the Tuberculosis Education and Training Network was established. The network is coordinated by CDC and includes educators in local, state, and territorial health agencies. CDC has also developed the Tuberculosis Information CD-ROM, Version 3, and the Tuberculosis Education and Training Resource Guide; these products are designed to enhance awareness and accessibility of resources (available at http://www.cdc.gov/nchstp/tb/default.htm) for TB education and training. The establishment in 2004 of the National Tuberculosis Curriculum Coordinating Center at the University of California at San Diego by the National Heart Lung and Blood Institute signals a commitment by the National Institutes of Health (NIH) to provide basic TB education for health-care students and providers. Professional societies and specialty boards are means for reaching private medical providers. Including TB as a subject in state medical society programs, hospital grand rounds, and medical specialty board examinations would be a valuable resource for providers serving populations at low risk. New linkages should be established to reach providers serving populations at high risk (e.g., foreign-born, homeless, and HIV-infected persons). For example, the AIDS Education and Training Centers funded by the Health Resources and Services Administration are a resource for reaching HIV/AIDS providers, and foreign physicians' associations and community-based organizations are potential partners for reaching international medical graduates and health-care providers of foreign-born persons. Laboratory Services for Optimal TB ControlThe diagnosis of TB, management of patients with the disease, and public health control services rely on accurate laboratory tests. Laboratory services are an essential component of effective TB control, providing key information to clinicians (for patient care) and public health agencies (for control services). Up to 80% of all initial TB-related laboratory work (e.g., smear and culture inoculation) is performed in hospitals, clinics, and independent laboratories outside the public health system, whereas >50% of species identification and drug susceptibility testing is performed in public health laboratories (205). Thus, effective TB control requires a network of public and private laboratories to optimize laboratory testing and the flow of information. Public health laboratorians, as a component of the public health sector with a mandate for TB control, should take a leadership role in developing laboratory networks and in facilitating communication among laboratorians, clinicians, and TB controllers. Role of Public Health Laboratories Public health laboratories should ensure that clinicians and public health agencies within their jurisdictions have ready access to reliable laboratory tests for diagnosis and treatment of TB (206). Specific tasks to ensure the availability, accessibility, and quality of essential laboratory services are 1) assessment of the cost and availability of TB laboratory services and 2) development of strategic plans to implement and maintain a systems approach to TB testing (207). In this process, public health laboratories should assess and monitor the competence of laboratories that perform any testing related to the diagnosis, management, and control of TB within their jurisdictions; develop guidelines for reporting and tracking of laboratory results; and educate laboratory staff members, health-care providers, and public health officials about available laboratory tests, new technologies, and indications for their use. For example, public health laboratories should lead the discussion on the costs, logistics requirements (e.g., collection and transport of clinical specimens within the required time), and quality assurance issues associated with the use of QFT-G, the new test for latent M. tuberculosis infection (103). The process of coordinating TB laboratory services is usually best organized at the state level (208), and the Association of Public Health Laboratories has compiled descriptions of successful organizational models for integrated laboratory services (207). Role of Clinical Laboratories Because the majority of initial TB laboratory work related to diagnosis of TB is conducted in hospitals, clinics, and independent laboratories (205), clinicians and public health agencies are increasingly dependent on the laboratory sector for the confirmation of reported cases, and public health laboratories are similarly dependent for referral of specimens for confirmatory testing and archiving. However, as a result of laboratory consolidation at the regional or national level (206), private laboratories are experiencing more difficulties in fulfilling this function. In certain instances, consolidation has resulted in poor communication among laboratory personnel, clinicians, and public health agencies (206,209). Problems also have been identified in specimen transport, test result reporting, and quality control (206,209,210). In response, certain states (e.g., Wisconsin*) have adopted laws and regulations that mandate essential clinical laboratory services for TB control (e.g., drug susceptibility testing and reporting of the first M. tuberculosis isolate from each patient and submission of isolates to the state public health laboratory). The clinical laboratory sector should accept the responsibilities that accompany its emergence as a provider of essential TB testing (209). This statement provides recommendations to guide turnaround times for essential tests, reporting to clinicians and jurisdictional public health agencies, and referral of specimens to public health laboratories or their designees. Essential Laboratory Tests Six tests performed in clinical microbiologic laboratories are recommended for optimal TB control services (Table 3). These laboratory tests should be available to every clinician involved in TB diagnosis and management and to jurisdictional public health agencies charged with TB control. In addition, other tests that are useful in the diagnosis and management of selected patients and for specific TB control activities include M. tuberculosis genotyping, serum drug levels, tests used for monitoring for drug toxicity, and QFT-G for diagnosis of latent M. tuberculosis infection (5,103,162). Access to these specialized tests should be provided as needed. For suspected cases of pulmonary TB, sputum smears for AFB provide a reliable indication of potential infectiousness; and for AFB smear-positive pulmonary cases, a nucleic acid amplification assay (NAA) provides rapid confirmation that the infecting mycobacteria are from the M. tuberculosis complex. These two tests, which should be available with rapid turnaround times from specimen collection, facilitate decisions about initiating treatment for TB or a non-TB pulmonary infection, and, if TB is diagnosed, for reporting the case and establishing priority to the contact investigation. Growth detection and identification of M. tuberculosis by culture of sputum and other affected tissue is essential for confirmation of the identity of the organism and for subsequent drug susceptibility testing, which is recommended on all initial isolates for each patient. Cultures also remain the cornerstone for the diagnosis of TB in smear-negative pulmonary and extrapulmonary cases and, along with sputum smears for AFB, provide the basis for monitoring a patient's response to treatment, for release from isolation, and for diagnosing treatment failure and relapse (5). The use of liquid media systems, which can provide information in less time than solid media (in certain cases, 7 days), should be available in all laboratories that perform culture for mycobacteria. Detailed descriptions of these recommended laboratory tests; recommendations for their correct use; and methods for collecting, handling, and transporting specimens have been published (3,211). Recommended Roles and Responsibilities for TB ControlThis section delineates organizational and operational responsibilities of the public health sector that are essential to achieve the goals of TB control in the United States. However, a central premise of this statement is that continuing progress toward elimination of TB in the United States will require the collaborative efforts of a broad range of persons, organizations, and institutions in addition to the public health sector, which has responsibility for the enterprise. For example, clinicians who provide primary health care and other specialized health services to patients at high risk for TB, academic medical centers that educate and train them, hospitals in which they practice, and professional organizations that serve their interests can all make meaningful contributions to improve the detection of TB cases, one of the most important obstacles to continuing progress (Box 1). Similarly, important roles exist for such entities as community-based organizations representing populations at risk for TB and the pharmaceutical industry, which takes academic advances and develops the tools for diagnosis, treatment, and prevention of TB. This section discusses the importance to the TB elimination effort of participants outside the public health sector and proposes specific roles and responsibilities that each could fulfill toward that goal. The sponsoring organizations intend for these proposals to serve as the basis for discussion and consensus building on the important roles and responsibilities of the nonpublic health sector in continuing progress toward the elimination of TB in the United States. Public Health SectorThe infrastructure for TB control has been discussed extensively in recent years. An analysis of contributing factors to the rise in the number of TB cases during 1985--1992 concluded that the resurgence never would have occurred had the public health infrastructure been left in place and supported appropriately (212). The need to maintain the TB-control infrastructure has been expressed repeatedly (1,2,13,213,214). Public health activities have been described as consisting of four interrelated components: mission/purpose, structural capacity, processes, and outcomes (215). Among these four components, structural capacity (i.e., persons who do the work of public health, their skills and capacities, the places where they work, the way they are organized, the equipment and systems available to them, and the fiscal resources they command) represents the public health infrastructure for TB control. The responsibility for TB control and prevention in the United States rests with the public health system through federal, state, county, and local public health agencies. Programs conducted by these agencies were critical to the progress that has been made in TB control, and the deterioration of those programs following the loss of categoric federal funding contributed to the resurgence of TB in the United States during 1985--1992 (1,2,13,212--214). Since 1992, as a result of increased funding for TB-control programs, national incidence of TB disease has declined. In 2004, $147 million in federal funds were dedicated to domestic TB control, compared with $6.6 million in 1989, during the resurgence. These funds have been used to rebuild public health--based TB-control systems, and the success achieved highlights the critical role of the public health system in TB control. TB control in the United States has traditionally been conducted through categoric programs established to address the medical aspects of the disease and the specific interventions required for its successful prevention and management (17,216). CDC's Division of TB Elimination, in partnership with other CDC entities that conduct TB-related work, provides guidance and oversight to state and local jurisdictions by conducting nationwide surveillance; developing national policies, priorities, and guidelines; and providing funding, direct assistance, education, and program evaluation. Setting the national agenda for support of basic and clinical research is also a critical function of federal health agencies, including NIH and CDC, with support from nongovernment organizations such as ATS and IDSA. To meet the priorities of basic TB control (Box 4), state and local public health agencies with responsibility for TB control should provide or ensure the provision of a core group of functions (Box 5). Jurisdictional public health agencies should ensure that competent services providing these core elements function adequately within their jurisdictions and are available with minimal barriers to all residents. How the core components of TB control are organized differs among jurisdictions, depending on the local burden of disease, the overall approach to public health services within the jurisdiction, budgetary considerations, the availability of services within and outside the public health sector, and the relationships among potential participants. Certain jurisdictions provide core program components themselves, whereas other jurisdictions contract with others to provide them. In the majority of cases, the organization includes a mix in which the public health agency provides certain services, contracts for others, and works collaboratively with partners and stakeholders to accomplish the remainder (48). Sharing of direct services, including patient management, increases the importance of the public health sector, which retains responsibility for success of the process. This evolving role of the public health sector in TB control is consistent with the widely accepted concept of the three core functions of public health that IOM proposed in 1988: assessment, policy development, and assurance (43). Health Insurance Portability and Accountability ActThe Health Insurance Portability and Accountability Act (HIPAA) of 1996 included provisions to protect the privacy of individually identifiable health information. To implement these privacy protections, the U.S. Department of Health and Human Services has issued a ruling on how health-care providers may use and disclose personally identifiable health information about their patients; these regulations provide the first national standards for requirements regarding the privacy of health information (217). HIPAA also recognizes the legitimate need for public health authorities and others responsible for ensuring the public's health and safety to have access to personal health information to conduct their missions and the importance of public health disease reporting by health-care providers. HIPAA permits disclosure of personal health information to public health authorities legally authorized to collect and receive the information for specified public health purposes. Such information may be disclosed without written authorization from the patient. Disclosures required by state and local public health or other laws are also permitted. Thus, HIPAA should not be a barrier to the reporting of suspected and verified TB cases by health-care providers, including health-care institutions. Additional information about HIPAA is available at http://www.hhs.gov/ocr/hipaa. Roles and Responsibilities of Federal Public Health Agencies
Roles and Responsibilities of Jurisdictional Public Health Agencies Planning and policy development. The blueprint for TB control for a given area is a responsibility of the jurisdictional public health agency. Policies and plans should be based on a thorough understanding of local epidemiologic data and on the capabilities and capacities of clinical and support services for clients, the fiscal resources available for TB control, and ongoing indicators of program performance. Open collaboration is essential among public health officials and community stakeholders, experts in medical and nonmedical TB management, laboratory directors, and professional organizations, all of whom provide practical perspectives to the content of state and local TB-control policy. Policies and procedures should reflect national and local standards of care and should offer guidance in the management of TB disease and LTBI. A written TB control plan that is updated regularly should be distributed widely to all interested and involved parties. The plan should assign specific roles and responsibilities; define essential pathways of communication between providers, laboratories, and the public health system; and assign sufficient resources, both human and financial, to ensure its implementation, including a responsible case manager for each suspected and verified case of TB. The plan should include the provision of expert consultation and oversight for TB-related matters to clinicians, institutions, and communities. It should provide special guidance to local laboratories that process TB-related samples, assist local authorities in conducting contact or outbreak investigations and DOT, and provide culturally appropriate information to the community. Systems to minimize or eliminate financial and cultural barriers to TB control should be integral to the plan, and persons with TB and persons at high risk with TB infection should receive culturally appropriate education about TB and clinical services, including treatment, with no consideration for their ability to pay. Finally, the plan should be consistent with current legal statutes related to TB control. Relevant laws and regulations should be reviewed periodically and updated as necessary to ensure consistency with currently recommended clinical and public health practice (e.g., mandatory reporting laws, institutional infection-control procedures, hospital and correctional system discharge planning, and involuntary confinement laws) (218). Collection and analysis of epidemiologic and other data. The development of policies and plans for the control of TB within a jurisdiction requires a detailed understanding of the epidemiology of TB within the jurisdiction. Mandatory and timely case reporting from community sources (e.g., providers, laboratories, hospitals, and pharmacies) should be enforced and evaluated regularly. To facilitate the reporting process and data analyses, jurisdictions should modify systems as necessary to accommodate local needs and evolving technologies. State and local TB-control programs should have the capability to monitor trends in TB disease and LTBI in populations at high risk and to detect new patterns of disease and possible outbreaks. Populations at high risk should be identified and targeted for active surveillance and prevention, including targeted testing and treatment of LTBI (4). Timely and accurate reporting of suspected and confirmed TB cases is essential for public health planning and assessment at all levels. Analyses of these data should be performed at least annually to determine morbidity, demographic characteristics, and trends so that opportunities for targeted screening for disease or infection can be identified. Regular reviews of clinical data (e.g., collaborative formal case presentations and cohort analyses of treatment outcomes; completeness, timeliness and effectiveness of contact investigations; and treatment of LTBI) may be used as indicators of program performance. Data should be collected and maintained in a secure, computerized data system that contains up-to-date clinical information on persons with suspected and confirmed cases and on other persons at high risk. Each case should be reviewed at least once monthly by the case manager and by field or outreach staff to identify problems that require attention. The TB-case registry should ensure that laboratory data, including data on sputum culture conversion and drug susceptibility testing of clinical isolates, are promptly reported, if applicable, to the health-care provider so any needed modifications in management can be made. This requires a communications protocol for case managers, providers, and the public health and private laboratory systems that will transmit information in a timely fashion. Aggregate program data should be available to the health-care community and to community groups and organizations with specific interests in public health to support education and advocacy and to facilitate their collaboration in the planning process. Clinical and diagnostic services for patients with TB and their contacts. TB-control programs should ensure that patients with suspected or confirmed TB have ready access to diagnostic and treatment services that meet national standards (3,5). These services are often provided by state- or city-supported TB specialty clinics and staffed by health department personnel or by contracted service providers; however, persons may seek medical care for TB infection or disease in the private medical sector. Regardless of where a person receives medical care, the primary responsibility for ensuring the quality and completeness of all TB-related services rests with the jurisdictional health agency, and health departments should develop and maintain close working relations with local laboratories, pharmacies, and health-care providers to ensure that standards of care, including those for reporting, are met. Clinical services provided by the health department, contracted vendors, or private clinicians should be competent, accessible, and acceptable to members of the community served by the jurisdiction. Hours of clinic operation should be convenient, and waiting intervals between referral and appointments should be kept to a minimum. Persons with symptoms of TB should be accommodated immediately (i.e., on a walk-in basis). Staff, including providers, should reflect the cultural and ethnic composition of the community to the extent that this is possible, and competent clinical interpreter services should be available to those patients who do not speak English. All clinical services, including diagnostic evaluation, medications, clinical monitoring, and transportation, should be available without consideration of the patient's ability to pay and without placing undue stress on the patient that might impair completion of treatment. Clinical facilities should provide diagnostic, monitoring, and screening tests, including radiology services. Health-care providers, including nurses, clinicians, pharmacists, laboratory staff members, and public health officials, should be educated about the use and interpretation of diagnostic tests for TB infection and disease. Clinics and providers should monitor patients receiving TB medications at least monthly for drug toxicity and for treatment response, according to prevailing standards of care (5). Counseling and voluntary testing for HIV infection should be offered to all persons with suspected and proven TB and to certain persons with LTBI, with referral for HIV treatment services when necessary. A case manager, usually a health department employee, should be assigned to each patient suspected or proven to have TB to ensure that adequate education is provided about TB and its management, standard therapy is administered continuously, and identified contacts are evaluated for infection and disease. A treatment plan for persons with TB should be developed immediately on report of the case. This plan should be reviewed periodically by the case manager and the treating clinician and modified as necessary as new data become available (219). The treatment plan should include details about the medical regimen used, how and where treatment is to be administered, monitoring of adherence, drug toxicity, and clinical and bacteriologic responses. Social and behavioral factors that might interfere with successful completion of treatment also should be addressed. Patient-specific strategies for promoting adherence to treatment should take into account each patient's clinical and social circumstances and needs (5). Such strategies might include the provision of incentives or enablers (e.g., monetary payment, public transportation passes, food, housing, child care, or transportation to the clinic for visits). Whether the patient's care is managed by a public health clinic or in the private sector, the initial strategy used should emphasize direct observation of medication ingestion by an HCW. Patient input into this process (e.g., regarding medications to be taken or the location of DOT) is often useful as it can minimize the burden of treatment and provide the patient a degree of control over an anticipated lengthy course of therapy. Expert medical consultation in TB should be available to the health-care community, especially for patients who have drug-resistant disease or medical diagnoses that might affect the course or the outcome of treatment. Consultants may be employees of the health department or clinicians with expertise who are under contract with the health department. Inpatient care should be available to all persons with suspected or proven TB, regardless of the person's ability to pay. Hospitalized patients with suspected proven TB should have access to expert medical and nursing care, essential diagnostic services, medications, and clinical monitoring to ensure that diagnostic and treatment standards are met. Inpatient facilities that manage persons who are at risk for TB should have infection-control policies and procedures in place to minimize the risk for nosocomial spread of infection. Facilities should report persons with suspected or confirmed TB to the health department and arrange for discharge planning as required by statute. Public health agencies should have legal authority and adequate facilities to ensure that patients with infectious TB are isolated from the community until they are no longer infectious. This authority should include the ability to enforce legal confinement of patients who are unwilling or unable to adhere to medical advice (218,220). This authority also should apply to nonadherent patients who no longer are infectious but who are at risk for becoming infectious again or becoming drug resistant. TB-control programs should serve as sources of information and expert consultation to the health-care community regarding airborne infection and appropriate infection-control practice. A TB program's presence raises overall provider awareness of TB and facilitates timely diagnosis, reporting, and treatment. Collaboration with local health-care facilities to design and assist in periodic staff education and screening is often a health department function. Expertise in airborne infections by TB-control personnel may be shared with biologic terrorism programs to assist in the design and implementation of local protocols. Contact investigation, including education and evaluation of contacts of persons with infectious TB, is a key component of the public health mandate for TB control. Often the primary responsibility of the case manager, contact investigation should proceed as quickly and as thoroughly as indicated by the characteristics of the specific case and by those of the exposed contact (e.g., young children or immunocompromised persons). This statement includes recommendations on organizing and conducting contact investigations. TB-control programs that are prepared to implement enhanced TB-control strategies should initiate or facilitate implementation by other medical providers of programs for targeted testing and treatment of persons with LTBI on the basis of local epidemiologic data that identify populations at high risk. A public health approach to this activity is presented in this statement (see Essential Components of TB Control in the United States). Liaison with communities at high risk is critical to the success of TB control in any jurisdiction. TB-control programs should develop strong lines of communication with local community groups and organizations and their health-care providers to understand local priorities and beliefs about TB. Trusted community members can facilitate the design and implementation of strategies to improve TB diagnosis and prevention. Community-based clinical services that use local providers who are educated in TB treatment and prevention and who have a connection with the TB-control program can improve community acceptance of prevention and treatment of TB (221). Training and education. TB-control programs should provide education and training in the clinical and public health aspects of TB to all program staff. Staff members should receive appropriate education at regular intervals on the basis of their particular responsibilities in the program and should demonstrate proficiency in those areas when tested. Public health TB programs also should educate health-care providers (both public and private), community members, public health officials, and policy makers on the basis of local epidemiology and needs. To ensure the availability of a competent workforce for TB that understands and meets the needs of its community, state TB programs should use resources from CDC-funded national TB centers, NIH-supported TB curriculum centers, NTCA, and other national and local agencies to create and implement education activities in coordination with schools of medicine, nursing, pharmacy, dentistry, and public health; community-based organizations and their constituents; local health-care providers; and health-care institutions (222). A Strategic Plan for Public Health Work Force Development (223) and a Strategic Plan for Tuberculosis Training and Education have been developed. Information management. Information-management systems are key factors in medical safety and quality improvement (224,225) and should be prioritized by all TB-control programs. Information technology can improve care of patients with TB through standardized collection of data; tracking of test results and details of treatment, including administration of DOT; and prediction of interactions among medications. Information technology can also facilitate analysis and rapid distribution of epidemiologic data and the management of individualized treatment plans (5) and support ongoing program performance analyses. Barriers to successful implementation of information technology include costs and resistance to change. Monitoring and evaluation. The systematic monitoring and analysis of program activities is a critical factor in enhancing program performance. Evaluation techniques provide TB programs with an evidence-based approach to assess and improve their TB-control strategies by understanding what causes good or bad program performance. Evaluation can also be used for program advocacy, assessing staffing needs, training and capacity building, directing limited resources to the most productive activities, accounting for available resources, generating additional resources, and recognizing achievement (226). Each public health agency should develop its own priorities for program evaluation on the basis of the nature and dimensions of the TB problem in its jurisdiction and the way that services are organized. In general, the first priority for evaluation efforts should be to focus on those activities and outcomes that relate most directly to the key strategies of TB control: detecting patients with infectious TB and administering a complete course of treatment; finding contacts and other persons at high risk with LTBI and treating them; and interrupting transmission of M. tuberculosis in high-risk settings (Box 4). Targets for program performance have been established by CDC (227) to assist public health agencies in treating TB patients, protecting their contacts, and improving the quality of case reporting for national surveillance (Table 4). These national objectives for program performance provide a starting point for state and local TB-control programs to use for program evaluation, but each TB-control program should establish methods to evaluate its performance. TB case management has typically been evaluated by reviewing individual charts and case conferences. However, cohort analysis, a systematic evaluation of the treatment outcomes of all TB cases during a stipulated period of time, is the preferred means of determining the number and percentage of cases that complete a course of treatment in <12 months. Cohort analyses should be a cornerstone of evaluation by all TB-control programs. A guide to cohort analysis and other evaluation tools has been published (228). National objectives have been set for completing treatment for LTBI among contacts of infectious cases of TB (Table 4). Other program areas that should be monitored through formal evaluation methods include timeliness and completeness of reporting of TB cases and suspected cases, frequency of use of a recommended treatment regimen for patients with TB and LTBI, and quality of the program's databases for surveillance and case management. To respond to the need for improved and standardized program evaluation activities, CDC and six state TB-control programs have established an Evaluation Working Group whose goal is to improve the capacity of TB-control programs to routinely conduct self-evaluations and use the findings to improve and enhance their programs. The group is developing indicators for program performance and an inventory of evaluation tools, including data collection instruments, data analysis methods, and evaluation training materials. During the next 2 years, a draft set of these materials will be tested in three TB-control programs for utility, feasibility, and accuracy. Ultimately, this package of evaluation materials and resources will be made available to all TB-control programs. Public Health Workforce No single model exists for staffing public health TB-control programs. Approaches to TB control should be flexible and adaptable to local needs and circumstances. Two components of the public health workforce, public health nurses and community outreach workers, merit specific attention. Public health nurses. Public health nurses are registered nurses with a Bachelor of Science degree who are employed or whose services are contracted for by health departments. Certain states require certification for additional competencies before being hired as a public health nurse. Public health nurses traditionally have played a prominent role in TB control in the United States. Their training, including that in nonmedical aspects of disease, has provided nurses with the special skills needed to manage or coordinate the medical and the social-behavioral concerns associated with the prevention and treatment of TB (229). Their training includes 1) designing contact and source-case investigations; 2) educating patients, contacts, and families; 3) identifying ineffective drug therapy regimens and drug toxicities; 4) recognizing patient behaviors that might lead to poor adherence; and 5) developing strategies to encourage completion of therapy. As health departments adapt to changing health-care environments, the role of public health nurses working to control TB also is evolving to accommodate the varied mechanisms by which services are delivered. Standards of practice for TB nursing are being updated by the National Tuberculosis Nurse Consultant Coalition, a section of NTCA, to guide jurisdictions in creating and maintaining a specialized nursing resource for TB control and prevention. Community outreach workers. Community outreach workers are staff members who provide services, such as DOT, to patients outside of the clinic. They may also be classified as disease investigation specialists or community health educators. Because TB has become concentrated in specific populations (e.g., foreign-born and homeless persons) in the United States, outreach workers have assumed a key role in TB control. Often members of the communities they serve, outreach workers connect the health-care system with populations at high risk, ensuring that the principles and processes of TB control are communicated to and understood by those populations. Outreach workers' functions include facilitating treatment for patients and contacts; providing DOT; educating patients, their families, workplace personnel, and communities; and participating in contact investigations. In each case, outreach workers form a bridge between patients and health-care providers to achieve common understandings and acceptance of plans for diagnoses and treatment. Clinicians with specialized expertise, including nurse-case managers, should supervise outreach workers. CliniciansClinicians in medical practice in the nonpublic health sector play a vital role in TB control throughout the United States. Hospital- or clinic-based medical practitioners, including those working in emergency departments (EDs), are usually the first source of medical care for persons with TB (230--232); they also may provide ongoing management for TB patients (48). The role of medical practitioners in TB control will increase as TB morbidity in the United States decreases and jurisdictions reduce or even eliminate public health clinical services for TB. Medical practitioners are often not sufficiently knowledgeable about TB (233), and clinicians in private practice frequently do not follow recommended guidelines and make errors in prescribing anti-TB therapy (231,234,235). The failure of public health and private practitioners to interact effectively is a weak link in global TB control (236). Successful models exist for acknowledging and facilitating the work of private medical practitioners in the complex process of diagnosing and treating persons with TB. For example, for each reported TB case in New Mexico, a collaborative case-management strategy is used that includes treating clinicians and pharmacists from the private sector in addition to public health case managers (48). Another model of effective private-public partnerships was employed in NYC during the 1985--1992 TB resurgence, with health department case management and DOT for patients under private care (13). As TB elimination efforts continue, the role of medical practitioners will further expand because they provide access to populations that have been targeted for testing and treatment of LTBI. Greater participation by the nonpublic health sector in preventive intervention has been advocated (2,51), and clinical standards have been published to guide medical practitioners in managing patients with TB disease and LTBI (8). Roles and Responsibilities of Clinicians
Civil surgeons are licensed physicians who are certified by the U.S. Citizenship and Immigration Service (CIS) to conduct a required health screening examination, including testing for LTBI and active TB disease, on foreign-born persons living in the United States who apply for permanent residency. In 2002, approximately 679,000 foreign-born persons applied for permanent residency and were screened by civil surgeons, compared with 245,000 such persons in 1995 (238). CDC has responsibility for providing guidance on screening and treatment but has no regulatory role in monitoring the quality or outcomes of these examinations. Because of their access to foreign-born persons at high risk, civil surgeons are a critical component of TB control. U.S.-based immigration screening can identify foreign-born persons with LTBI for whom treatment is indicated (239). Although civil surgeons receive immigration-focused training, little information is available on the knowledge, attitudes, and practices of civil surgeons. A recent survey indicated that among 491 physicians serving as civil surgeons in California, Massachusetts, and New York, the majority were graduates of U.S. medical schools; 75% were primary care practitioners; and 47% were board certified in their specialty. Among 5,739 foreign-born applicants examined by these civil surgeons, 1,449 (25%) received nonstandard screening (240). As a result of these findings, efforts are under way to develop guidance documents and training materials for physicians who screen immigrants for TB infection and disease. Roles and Responsibilities of Civil Surgeons
Community health centers typically provide primary health-care services to populations that encounter barriers to receiving those services at other sites in the health-care system, such as low-income working persons and their families, immigrants and refugees, uninsured persons, homeless persons, the frail elderly, and poor women and children. Patients at high risk for TB often receive primary and emergency health care in community health centers (51). For example, community health centers in certain inner-city areas might serve primarily a clientele of homeless persons, whereas centers in neighborhoods in which certain racial and ethnic populations are concentrated might become predominant health-care providers for immigrants and refugees. Newly arriving refugee families are frequently directed to community health centers to receive federally supported health-screening services, which might include targeted testing and treatment for LTBI. Persons with symptoms of TB might go first for evaluation and care to a community health center. For these reasons, community health centers are a critical part of efforts to control and prevent TB. Roles and Responsibilities of Community Health Centers
Hospitals provide multiple services that are instrumental to the diagnosis, treatment, and control of TB. Hospitals with active outpatient and EDs often serve as sites of acute and primary medical care for homeless persons, inner-city residents, immigrants and refugees, and other persons at high risk for TB. Also, hospital staff members often provide medical consultation services for the diagnosis and management of TB by public health and community clinicians. Laboratory services provided by hospitals for community-based medical care providers might include key diagnostic tests for TB. TB cases often are detected during hospitalization at acute-care hospitals (230,242). In a prospective cohort study at 10 sites in the United States, 678 (45%) of 1,493 patients reported with TB received their diagnosis during hospitalization (230). Hospital-based health professionals evaluate patients for TB, establish the diagnosis, and initiate treatment regimens and reporting of cases to public health departments. Instances of delayed recognition, diagnosis, and treatment for TB among hospitalized patients subsequently found to have TB have been reported (24,178), indicating a need for more effective training and education of hospital medical staff members. Because 25%--45% of patients with TB receive their diagnostic evaluation while in a hospital (230,242), hospitals have an opportunity to provide patient-based teaching on TB for their own staff members and for health professionals from the community served by the hospital. Venues such as staff conferences and medical grand rounds, conducted regularly by hospitals, can be sources of training and education on clinical, laboratory, and public health concerns that arise during evaluation and initial medical management of hospitalized patients with TB. Hospitals should protect their patients, staff, and visitors from exposure to M. tuberculosis. The importance of effective TB infection control was emphasized during the 1985--1992 TB resurgence in the United States, when hospitals were identified as sites of transmission of multidrug-resistant TB (243). Implementation of effective infection-control guidelines has been effective in reducing transmission of TB in hospitals (56,244,245). Roles and Responsibilities of Hospitals
Academic institutions (including schools of medicine, public health, and nursing) have an opportunity to contribute to TB control in the United States and worldwide. Students from diverse disciplines, including the clinical and laboratory sciences, nursing, epidemiology, and health services should be introduced to applicable concepts of public health in general and, because TB is a major cause of preventable illness and death in developing countries (44), to TB in particular. During the resurgence of TB in the United States during 1985--1992, expertise in TB was limited. Federal funding for programs (e.g., the NIH National Heart Lung Blood Institute's Tuberculosis Academic Award program) helped provide funding to incorporate teaching of TB more fully into medical school curricula. Researchers at academic institutions are critical to efforts to improve the prevention, management, and control of TB because of their efforts to develop new tools, including new diagnostic tests, new drugs, better means of identifying and treating LTBI, and basic research to create a vaccine for TB (180,246,247). As with hospitals, academic institutions can provide benefits to other participants in TB control. Conferences, grand rounds, and other presentations are a source of continuing education for private medical practitioners and other community-based HCWs. As well-trained specialists, researchers at academic institutions can provide clinical, radiographic, and epidemiologic consultation to medical practitioners and public health agencies. A majority of academic institutions manage university-based hospitals, which often serve populations at high risk. University hospitals can become models for TB risk assessment of patients, inpatient care, and infection-control practice, and they can serve as tertiary care sites for an entire community or region. Partnerships between academic institutions and public health agencies are mutually beneficial (248). In certain cases, health departments and public health TB clinics are staffed or managed by faculty physicians from academic institutions. This partnership facilitates use of these clinics for graduate medical training for physicians in subspecialty areas (e.g., pulmonary and infectious diseases), enhances training for clinic staff, and provides opportunities for clinical and operational research. Roles and Responsibilities of Academic Institutions
Because they are involved with medical practice, research, education, advocacy, and public health, medical professional organizations are critical partners in TB control efforts. Greater participation of the nonpublic health medical sector is needed to maintain clinical expertise in the diagnosis and management of TB in an era of declining incidence. Organizations whose memberships include primary care medical practitioners can make significant contributions to the control, prevention, and elimination of TB by including TB in their training and education agendas. ATS and IDSA both support TB control efforts in the United States. With a membership of approximately 14,000 health professionals, including clinicians trained in pulmonary diseases, ATS conducts research, education, patient care, and advocacy to prevent respiratory diseases worldwide. IDSA promotes and recognizes excellence in patient care, education, research, public health, and the prevention of infectious diseases. In recent years, IDSA has joined ATS in focusing education and advocacy activities on TB through its annual meetings, publications, and sponsorship of this series of statements. Other medical professional organizations also can support TB control efforts. Medical professional organizations can 1) provide TB education to their members through meetings, symposia, statements, and web sites; 2) serve as venues for better communication between the private medical and public health sectors; 3) promote the TB research agenda locally and nationally; and 4) advocate for resources for strong TB control globally and in the United States. Roles and Responsibilities of Medical Professional Organizations
Involvement of community groups in TB control has long been encouraged (17). The critical importance of such involvement is underscored by the trend in the United States for TB to be limited to certain populations at high risk (e.g., contacts of persons with active cases, persons born outside the United States, homeless persons, incarcerated persons, and persons with HIV infection). Programs for education and targeted testing and treatment of LTBI should be organized for these populations. The public health sector frequently experiences difficulty in gaining access to persons in populations of high risk (51). Such persons might be socially marginalized, as in the case of new refugees, or they might be suspicious of persons representing government agencies, as in the case of undocumented aliens. Furthermore, the target population's own view of its health-care priorities, often best articulated by community-based organizations that represent them, should be considered in the design of public health interventions (249). Social, political, religious, and health-related organizations that have arisen from grassroots efforts to meet community needs often can facilitate access to public health programs (221). Community-based organizations can be particularly effective in providing information and education on TB to their constituencies. As part of the communities they serve, such organizations are often highly regarded in their communities, and their messages might be accepted more positively than those delivered by the jurisdictional health department. Roles and Responsibilities of Community-Based Organizations
Correctional facilities are common sites of TB transmission and propagation (250,251). Incidence of TB and of LTBI are substantially higher in prisons and jails than in the general population (252,253). TB is believed to be the leading cause of death for prisoners worldwide (254). Targeted testing for and treatment of LTBI in correctional facilities have been demonstrated to have a substantial public health impact (124). Testing and treatment for LTBI is carried out more easily in prisons (255) because the length of stay is generally sufficient to permit completion of a course of treatment. Jails have proved convenient sites for targeted testing, but subsequent treatment of LTBI has proved challenging (256). Innovative methods for assuring completion of treatment for LTBI in jail detainees have been proposed (257). Because of their communal living arrangements, correctional facilities, like health-care facilities, have the responsibility to limit the transmission of TB within the institution and to protect their inhabitants and staff from exposure. This is a particular challenge in jails because of the short lengths of stay for the majority of detainees. Even in prison systems, abrupt and unexpected transfers of detainees among institutions might occur, with little consideration for health issues. Prisons and jails frequently house HIV-infected persons in separate facilities to ensure adequate health care. However, recent publications describing outbreaks of TB in such settings have emphasized the hazard of this strategy (35,126). Roles and Responsibilities of Correctional Facilities
Because of their essential role in developing new diagnostics, drugs, and vaccines, the pharmaceutical and biotechnology industries are partners in TB control. Although development of new tools for diagnosis, treatment, and prevention of TB has been deemed essential to the effort to combat the disease globally and to continue to make progress toward its elimination in the United States and other developed countries (1,2,45,259), progress in these fields has been slow. Slow progress in this field has been attributed to private industry's perception that such products are not needed in developed countries and do not offer profit opportunities in resource-poor countries (246,260). However, new public-private partnerships are emerging to facilitate the development of essential new tools (261), including three partnerships established with support from the Bill and Melinda Gates Foundation: the Global Alliance for Tuberculosis Drug Development (http://www.tballiance.org), the Aeras Global Tuberculosis Vaccine Foundation (http://www.aeras.org), and the Foundation for Innovative New Diagnostics (http://www.finddiagnostics.org). These organizations have provided venues to identify and address obstacles to developing new tools for TB among private industry, public and academic researchers, and philanthropic organizations. These organizations also receive support from the private sector. The pharmaceutical industry has also contributed to the global TB control effort by assisting in making drugs for TB, including second-line drugs for patients with multidrug-resistant TB, more affordable (262,263). Such actions can enable pharmaceutical companies to become leaders in efforts to improve TB control and prevention. Roles and Responsibilities of the Pharmaceutical and Biotechnology Industries
Essential Components of TB Control in the United StatesCase Detection and ManagementCase detection and case management include the range of activities that begin when a diagnosis of TB is first suspected and end with the completion of a course of treatment for the illness. TB case management describes the activities undertaken by the jurisdictional public health agency and its partners to ensure successful completion of TB treatment and cure of the patient. The rationale and methodology of TB case management have been described previously (5). Organizational aspects of case management from the viewpoint of the jurisdictional public health agency are also discussed in this statement. Case detection includes the processes that lead to the presentation, evaluation, receipt of diagnosis, and reporting of persons with active TB. Case detection involves patients with active TB who seek medical care for symptoms associated with TB, their access to health care, their health-care providers, the consultants and clinical laboratories used by those health-care providers, and the responsible public health agency. Although steadily increasing treatment completion rates (14) indicate that progress has been made in the management of TB patients, TB case detection is still problematic. Delays in diagnosis and report of TB cases continue to be common. Also, despite the 44% reduction in TB incidence in the United States since 1992, the proportion of pulmonary cases that are sputum smear-positive at diagnosis has changed little, accounting for >60% of all reported cases (14). The majority of pulmonary TB cases continue to be diagnosed at an advanced stage. Earlier diagnosis would result in less individual morbidity and death, greater success in treatment, less transmission to contacts, and fewer outbreaks of TB. Improvement in the detection of TB cases is essential to progress toward elimination of TB in the United States (Box 1). The first step in improving TB case detection is to remove barriers in access to medical services that are often encountered by persons in high-risk categories. Such barriers might be patient-related, such as cultural stigmas associated with the diagnosis of TB, which might lead foreign-born persons to deny or hide symptoms (264,265), or fear of being reported to immigration authorities if medical care is accessed (19). Foreign-born persons, particularly recently arrived immigrants, refugees, and other persons of low SES might not have access to primary health services because they do not have health insurance or they are not familiar with the U.S. medical care system (20,118,266). Removing patient-related barriers to health care is particularly difficult. Improved patient education about TB is needed (18). Continuing immigration from countries at high risk, often including persons with strong cultural views about TB, underscores the need for patient education. As with other interventions to enhance TB control and prevention, local public health action should be based on the local pattern of disease. In developing education messages and outreach strategies, public health authorities should work with organizations that serve communities at high risk to gain community input (203). This statement provides recommendations on working with community-based organizations, key informants, and academic institutions to gain ethnographic information, learn about the health beliefs and values of populations at high risk within the community, and develop targeted interventions that will be most effective. The majority of TB cases are detected during the medical evaluation of symptomatic illnesses (19,267). Persons experiencing symptoms ultimately attributable to TB usually seek care not at a public health TB clinic but rather from other medical practitioners and health-care settings. In 18 California counties with the highest TB morbidity of persons during 1996--1997, initial points of entry into the health-care system for persons who received a diagnosis of TB were hospital inpatient evaluations (45%), private outpatient offices or clinic evaluations (32%), TB clinic evaluations (12%), and other sites (11%), including a non-TB clinic in a health department and correctional facilities (California Tuberculosis Controllers Association, unpublished data, 2003). A similar pattern was observed in Washington state. In Seattle and its suburban areas in 1997, primary care practitioners or clinics reported 48% of TB cases during evaluations of outpatients with symptoms and 32% during hospital evaluations; only 2% of cases were diagnosed during a public health TB clinic evaluation for a symptomatic illness (Seattle-King County Department of Public Health, unpublished data, 1998). These data indicate that the professionals in the primary health-care sector, including hospital and ED clinicians, should be trained to recognize patients with symptoms consistent with TB. Dramatic reductions in TB were recorded in NYC (13) and Baltimore (195) in association with extensive education campaigns for health-care providers in the community. These studies indicate the need to maintain clinical expertise for the diagnosis and treatment of TB (24,41). Because pulmonary disease among adults is most frequently associated with the spread of TB, the following discussion and recommendations regarding TB case detection are limited to considerations of pulmonary TB among adults. A classic set of historic features, signs, symptoms, and radiographic findings occurring among adults should raise a suspicion of pulmonary TB and prompt a diagnostic investigation (3,189,267--271). Historic features include exposure to TB, a positive test result for M. tuberculosis infection, and the presence of risk factors such as immigration from a high-prevalence area, HIV infection, homelessness, or previous incarceration. Signs and symptoms typical of TB include prolonged coughing with production of sputum that might be bloody, fever, night sweats, and weight loss. On a chest radiograph, the classical findings of TB in immunocompetent patients are upper-lobe infiltrates, frequently with evidence of contraction fibrosis and cavitation (270). However, these features are not specific for TB, and, for every person in whom pulmonary TB is diagnosed, an estimated 10--100 persons are suspected on the basis of clinical criteria and must be evaluated (272,273). The clinical presentation of TB varies considerably as a result of the extent of disease and the host response. In addition, variation in clinical symptoms and signs of TB is associated with underlying illnesses (e.g., HIV infection, chronic renal failure, alcoholism, drug abuse, and diabetes mellitus). The signs of TB are also associated with race and ethnicity and are attributed to unknown factors (3,267,270). The chest radiograph among persons with advanced HIV infection and pulmonary TB, for example, might have lower-lobe and lobar infiltrates, hilar adenopathy, or interstitial infiltrates (274). TB should be suspected in any patient who has persistent cough for >2--3 weeks or other compatible signs and symptoms as noted previously (10,267,275). In the drive toward TB elimination in the United States, effective TB case detection is essential, and medical practitioners should recognize patients in their practice who are at increased risk for TB and be aware of the possibility of diagnosing TB if they observe compatible symptoms. Guidelines have been provided for the initial steps of TB case detection in five clinical scenarios encountered by providers of primary health care, including those serving in medical EDs (Table 5). In these settings, evidence exists to support proceeding with a diagnostic evaluation for pulmonary TB. The subsequent management of suspected cases in these scenarios depends on the judgment of the medical practitioner, in consultation with specialists in internal medicine, pulmonary diseases, or infectious diseases if necessary (5). These recommendations do not cover the spectrum of clinical presentations of pulmonary TB in adults and are not meant to substitute for sound clinical judgment. Cases of pulmonary TB also are detected through directed public health activities designed to detect TB disease among certain persons who have not sought medical care. Compared with persons whose cases were detected passively by medical practitioners among patients who have sought medical care, persons whose cases are detected actively are usually in a less advanced stage of pulmonary disease, as manifested by the absence of symptoms and by negative sputum AFB smear results. Although no supporting literature exists, cases detected in that stage of disease might be less advanced and easier to cure. Active efforts to detect cases of TB among persons who have not sought medical care are routinely made during evaluation of contacts of patients with pulmonary TB (30,31,276) and of other persons with newly diagnosed infection with M. tuberculosis (4). Screening for TB also is performed during evaluation of immigrants and refugees with Class B1 or Class B2 TB notification status (277--279), during evaluations of persons involved in TB outbreaks (34,35,136,172,280,281), and occasionally in working with populations with a known high incidence of TB (167,185). Screening for TB disease is indicated when the risk for TB in the population is high and when the consequences of an undiagnosed case of TB are severe (282), such as in jails and prisons (253,283). Screening for TB disease (i.e., active case finding) might contribute substantially to overall TB case detection. A population-based study from Los Angeles indicated that 30% of reported TB cases during the period of study were detected through screening activities (267). During 1998--2001, of 356 TB cases reported by the Seattle-King County TB Program, 40 (11%) were detected through active case detection in contact investigation and evaluations of immigrants and refugees with Class B1 and B2 TB notification status. The clinical settings in which TB has been effectively detected among persons without symptoms, the methodology of testing, and outcomes of the screening process have been described (Table 6). On the basis of its very high yield of detecting TB cases, domestic follow-up evaluation of immigrants and refugees with Class B1 and B2 TB notification status should be given highest priority by all TB-control programs. The yield of detecting TB cases during screening at homeless shelters increased sharply in an outbreak setting (Table 6). Although prevalence data from individual studies are not available, investigations undertaken to control TB outbreaks that involved diverse settings and groups of immunocompetent and immunocompromised persons have consistently been productive in detecting TB cases and high rates of LTBI among exposed persons (34,35,136,173,280,281). Outbreak investigations should be counted among the settings in which screening for active TB is recommended. Contact Investigation and Outbreak ControlContact investigation is an essential function of TB control in the United States (Box 4) (1,17). The investigation of a case of TB results in identifying approximately 10 contacts (284). Among close contacts, approximately 30% have LTBI, and 1%--3% have progressed to TB disease (30,284). Without intervention, approximately 5% of contacts with newly acquired LTBI progress to TB disease within 2 years of the exposure (285). The prevalence of TB among close contacts is approximately 1,000/100,000 population (>100-fold higher than in the general population) (285). Examination of contacts is therefore one of the most important activities for identifying persons with disease and those with LTBI who have a high risk for acquiring TB disease. Transmission of M. tuberculosis has occurred in health-care facilities (286,287), bars (134,288), doctors' offices (289), airplanes (290), crack houses (291), respite facilities that provide care for HIV-infected persons (136), drug rehabilitation methadone centers (36), navy ships (292), homeless shelters (120), schools (173), church choirs (140), and renal transplant units (141). The utility and importance of contact investigations in those settings and also for populations at high risk (e.g., foreign-born persons [293], children [294--297], and persons exposed to multidrug-resistant TB cases [91,298]) has also been documented. In the United States, state and local public health agencies perform 90% of contact investigations as part of the public health mandate for TB control (Box 5) (2). Public health TB-control programs are responsible for ensuring that contact investigations are conducted effectively and that all exposed contacts are identified, provided with access to adequate care, and followed to completion of therapy. For health agencies to fully discharge this responsibility, adequate funding and political commitment are required. Health agencies use a general epidemiologic framework for contact investigations (299). However, this approach alone might have limited effectiveness because of factors such as initial diagnostic delays and failure to ensure completion of therapy for LTBI. Consequently, programs have recognized the necessity of widening traditional contact investigation sites to include nonhousehold locations (e.g., homeless shelters, correctional facilities, nursing homes, and hospices that serve HIV-infected persons) and households. Genotyping studies have documented that traditional contact investigation methods have failed to identify contacts or detect transmission of M. tuberculosis (28,33,34,151,172). As a result, IOM (2) and ACET (1) have called for the development and implementation of enhanced techniques for contact investigation. The primary goal of a contact investigation is to identify persons who were exposed to infectious M. tuberculosis and ensure that they are tested for M. tuberculosis infection, screened for TB disease, are followed up, and complete a standard course of treatment, if indicated. Secondary goals are to stop transmission of M. tuberculosis by identifying undetected patients with infectious TB and to determine whether a TB outbreak has occurred. In that case, an expanded outbreak investigation should ensue. Steps of a Contact Investigation State and local public health agencies, often represented by TB-control programs, are responsible for initiating and conducting contact investigations and evaluating their outcomes to ensure their effectiveness. A contact investigation has 14 steps, as follows:
Outbreak Investigations Failure to recognize an increase in the occurrence of TB (162) or to expand a contact investigation when needed can result in continued transmission of TB. Missed epidemiologic links among patients with TB can have severe consequences as evidenced in an outbreak associated with a floating card game in the rural south (172) and an outbreak in Kansas among exotic dancers and their close contacts that occurred during a 7-year period (38). When TB occurs with high incidence, clusters of cases that have epidemiologic links likely occur constantly but tend to blend into the generally high morbidity (306). In a low-incidence setting, however, clusters of linked TB cases can be identified more readily. Three criteria have been established to determine that a TB outbreak is occurring (162): 1) an increase has occurred above the expected number of TB cases; 2) transmission is continuing despite adequate control efforts by the TB-control program; and 3) contact investigations associated with the increased cases require additional outside help. TB outbreaks have occurred in low-incidence areas in which expertise and experience in dealing with such outbreaks might be lacking. Such outbreaks have occurred among different populations and settings, including a young foreign-born child in North Dakota (25); exotic dancers and their contacts in Kansas (38), homeless persons in Syracuse, New York (120); factory workers in Maine (188); and limited, seemingly unrelated clusters of cases that were the cause over time of perpetuating transmission in Alabama (307). For an increase in the expected number of TB cases (the first criterion of an outbreak) to be identified, the local epidemiology of TB should be understood. Detection of a TB outbreak in an area in which prevalence is low might depend on a combination of factors, including recognition of sentinel events, routine genotype cluster analysis of surveillance data, and analysis of M. tuberculosis drug-resistance and genotyping patterns. When an outbreak is identified, short-term investigation activities should follow the same principles as those for the epidemiologic part of the contact investigation (i.e., defining the infectious period, settings, risk groups, mode of transmission, contact identification, and follow-up). However, long-term activities require continued active surveillance, M. tuberculosis genotyping, additional contact investigations and related follow-up for additional cases, and continuing education of providers, staff, and patients. Consequently, a plan for long-term support should exist from the outset of the investigation. A written protocol should be developed. At a minimum, the protocol should outline the outbreak response plan, including indications for initiating the plan, notification procedures, composition of the response team, sources of staffing, plan for follow-up and treatment of contacts, indications for requesting CDC assistance, and a process for evaluation of the outbreak response. The outbreak response plan should also include information on how to work strategically with the media during the public health emergency. CDC offers training packages to assist public HCWs in media communications, including emergency and crisis communication. This training emphasizes prevent planning, event response activities, and post-event follow-up. Information on public health communication programs is available at http://www.cdc.gov/communication/cdcynergy.htm. Targeted Testing and Treatment of LTBIAn estimated 9.5--14.7 million persons in the United States have LTBI (39). Continued progress toward eliminating TB in the United States and reducing TB among foreign-born persons will be impossible without devising effective strategies to meet this challenge. Guidelines on targeted testing and treatment of LTBI have been published (4) and revised (308). Those guidelines include recommendations for diagnosing LTBI and treating infected persons, limiting the possibility of treatment-associated hepatotoxicity, and identifying persons and populations to target for testing. A new diagnostic test for LTBI, QFT-G, has been approved by FDA, and guidelines for its use will be published by CDC. This section outlines a recommended approach to planning and implementing programs for targeted testing and treatment of LTBI to create an effective public health tool for communitywide prevention of TB. Targeted testing and treatment of persons with LTBI is not a new concept for the prevention of TB in the United States (309). The effectiveness of treating LTBI among populations at high risk has been established in clinical trials (285), but this intervention has not been proven to have an impact on the incidence of TB in the United States. Theoretically, the epidemiologic impact would be considerable if cases of TB in a population were largely the result of progression of LTBI and if all persons at high risk with latent infection could be identified and treated successfully. Practically, those circumstances rarely exist. In the United States, the effectiveness of targeted testing and treatment of LTBI as a public health measure has been limited by concern for the side effects of treatment (notably hepatotoxocity) (310), poor acceptance of the intervention among health professionals (311), and poor adherence among patients to the lengthy course of treatment (45,312). Two approaches exist to increasing targeted testing and treatment of LTBI. One is to promote clinic-based testing of persons who are under a clinician's care for a medical condition (e.g., HIV infection or diabetes mellitus) that also confers a risk for acquiring TB. This approach, which depends on a person's risk profile for TB and not on the local epidemiology of the disease, requires education of health-care providers and depends ultimately on their initiative. Although difficulties exist in quantifying and evaluating its effectiveness, this approach could conceivably become a useful tool to reduce the incidence of TB among foreign-born and other persons at high risk because they can be accessed conveniently where they receive primary health-care services. The other approach is to establish specific programs that target a subpopulation of persons who have a high prevalence of LTBI or who are at high risk for acquiring TB disease if they have LTBI, or both. This approach presumes that the jurisdictional TB-control agency has identified the pockets of high TB risk within its jurisdiction through epidemiologic analysis and profiling (313--316). Those high-risk pockets might consist of foreign-born, homeless, or HIV-infected persons, or they might be geographic regions (e.g., a neighborhood within a city or town) or specific sites (e.g., a homeless shelter or an HIV-housing facility). The epidemiologic profile should include an assessment of the risk for TB in the population or at the site, the ease of access to the population or site, and the likelihood of acceptance of and adherence to targeted testing and treatment. For this assessment to be facilitated, populations at high risk may be separated into three tiers (Box 6). Assignment of groups to these three tiers is based on six criteria: 1) incidence of TB; 2) prevalence of LTBI; 3) risk for acquiring TB disease if the person is infected with M. tuberculosis; 4) likelihood of accepting treatment for LTBI and adhering to it; 5) ease of access to the population; and 6) in a congregate setting, the consequence of transmission of M. tuberculosis. Tier 1 is made up of well-defined populations at high risk that can also be conveniently accessed and followed, either in locations such as clinics or community health centers, prisons, or other congregate living sites or through mandatory registration. Persons in this tier often have a high prevalence of TB and LTBI (immigrants and refugees with Class B TB notification status), an increased risk for TB disease if infected with M. tuberculosis (persons with HIV infection), or both (certain homeless and detained populations). The consequences of the spread of TB in congregate settings increase the necessity of preventive action. Location-based, high-risk communities in Tier 1 are, for the most part, readily identifiable and easily accessible; often have their own resources; and generally include the probability of access for a long enough period to permit completion of treatment for LTBI. These populations should be the first priority for targeted testing programs. Persons enrolled in substance-abuse treatment centers may be considered transitional between Tier 1 and Tier 2, depending on local epidemiologic and demographic factors. Substance abusers might have a high prevalence of LTBI. Injection drug users also might have an increased risk for acquiring TB if they are infected with M. tuberculosis and at increased risk for HIV infection (317). Access and factors related to acceptance and completion of therapy also might vary by location. Typically, substance abuse treatment centers that include long-term inpatient treatment or regularly scheduled appointments (e.g., methadone treatment centers) are the best choices for intervention because ease of ongoing access allows sufficient time for completion of therapy. Voluntary HIV counseling and testing should be offered routinely as part of any targeted testing program among this population. Populations in Tier 2 also include identifiable and accessible populations made up of persons at high risk, but the distinguishing characteristic is that obtaining satisfactory rates of completion of treatment for LTBI might be difficult because of dispersal of the population throughout a larger community or a brief duration of residency in congregate settings. For example, in Atlanta, Georgia, after local epidemiology of TB was analyzed, community sites for targeted testing and treatment of LTBI of residents of high-risk inner-city areas were identified (184). Sites of access included outpatient areas of the public hospital, the city jail, clinics serving homeless persons, and neighborhoods frequented by substance abusers. Although 65% of the targeted population that had a tuberculin skin test placed returned to have the skin test read, only 20% of those with an indication for treatment of LTBI completed a course of therapy; this represented 1% of persons who underwent targeted testing. Tier 3 consists of persons born in countries with a high incidence of TB or U.S.-born persons in racial/ethnic minority populations with high prevalence of LTBI who do not necessarily have an increased risk for progressing to TB disease. Eventually, the control of TB among foreign-born persons and progress toward elimination of TB in the United States depends on achieving greater success in preventing TB among populations at high risk by widespread targeted testing and treatment of LTBI in the public and private medical sectors. However, establishing successful targeted testing and treatment programs for foreign-born persons who are not found in Tier 1 or Tier 2 settings is challenging. Obstacles include the limitations of the tuberculin skin test to differentiate between reactions attributable to BCG or infection with M. tuberculosis, the prevalent belief among a substantial number of foreign-born persons that BCG vaccination is the cause of a positive test for M. tuberculosis infection and is also protective against TB disease, language and cultural barriers, barriers in access to medical care, and difficulties in providing outreach and education. Typical Tier 3 populations are new refugee and immigrant groups that are not yet assimilated into U.S. society. Such populations might be ignorant of their TB risk, usually lack ready access to health-care services, and might have strong cultural understandings about TB that are at variance with those that guide TB-control activities in the United States. TB-prevention activities in this kind of community are highly cost-intensive (221). Engaging such communities is a challenging task. Community-based TB prevention for Tier 3 populations requires a partnership between the jurisdictional health department and the affected community. The community should gain an understanding of the TB problem as it relates to them and should participate in the design of the intervention. Community education is essential for this approach to succeed. The target population should be involved in the design and implementation phases of the intervention, interventions should be developed within the cultural context of the targeted population, and intermediate goals or benchmarks should show the population that program activities are achieving success. For example, in Los Angeles, California, the public health TB program contracted with community-based organizations to screen and provide treatment for LTBI to persons at risk in Latino and Asian neighborhoods and at schools teaching English as a second language (249). In Cambridge, Massachusetts, a coalition of Haitian community groups identified TB education as an issue for their community; strategies to achieve this goal included development of a videotape written and produced for viewing in Haitian barbershops and beauty salons in the community, a lottery, and measures for evaluation in terms of knowledge and future access to care (S. Etkind, Massachusetts Department of Health, personal communication, 2002). For communities in Tier 3, TB is only one (and often not the most important) of multiple medical and public health needs. A broad approach should be adopted that includes TB prevention with other activities to improve health status. Certain Tier 3 populations have achieved sufficient self-identity and development to establish access to health care through a community health center, individual medical providers, or clinics. Those communities that have an already established route of access to health care have an infrastructure in place to establish programs for targeted testing and treatment of LTBI. Obstacles to overcome often include lack of medications and chest radiographs, the need for a system to track patients who do not return for monthly appointments, and the capacity to evaluate the program. Programs for population-based targeted testing and treatment for LTBI often have been conducted by public health agencies through TB-control programs. However, recent studies have also described the establishment of such programs in nonpublic health venues. Promising results, in terms of access to persons at high risk and completion of treatment of LTBI, have been achieved from nontraditional sites, including syringe exchanges (318), jails (256), neighborhood health clinics (319), homeless shelters (320), and schools (321,322). This trend indicates a widening interest in this means of preventing TB and is possibly influenced by the emergence of community-oriented primary care (241,323), which places primacy on interventions for specific patients that help prevent disease and preserve the health of the entire population from which these patients are drawn. As programs move from Tier 1 to Tier 2 and Tier 3 populations, the complexity of the effort and the cost of the program will increase. Also, because persons in Tier 3 populations generally have a lower risk for progression from LTBI to TB disease, the effectiveness and impact of a program will be less than efforts directed to Tier 1 and Tier 2 populations. Whatever population is selected or strategy is employed for the targeted testing project, programs should systematically evaluate the activity to ensure the efficient use of resources. Process, outcome, and impact indicators should be selected and routinely monitored by the program. For purposes of monitoring and evaluation, activities associated with targeted testing and treatment for M. tuberculosis infection can be divided into three phases: the testing itself, the medical evaluation of persons with positive test results, and the treatment of those persons with LTBI. Performance indicators should be selected for each phase. For the testing phase, indicators include the number of persons at high risk identified and the number and proportion of those that were actually tested. Among those tested, the number and proportion that had a positive result for M. tuberculosis infection should be tracked. Useful indicators for the medical evaluation phase include the proportion of persons with a positive test result who completed a medical evaluation and the number and proportion that were determined to have TB disease. Indicators for the treatment phase include the proportion of eligible persons starting treatment for LTBI and the number and proportion that completed treatment. Reasons for failure to complete treatment (e.g., adverse drug effects, loss of interest, and loss to follow-up) should be monitored. Costs should be measured for each phase of the project. The cost per person with LTBI completing treatment provides a measure of the relative efficiency of the program. Finally, the impact of the program can be estimated by estimating the number of cases of TB prevented, which is dependent on the number of persons completing treatment and the estimated risk for progressing to TB disease. Surveillance of persons with LTBI does not routinely occur in the United States. However, CDC has recently developed a national surveillance system to record serious adverse events (i.e., hospitalization or death) associated with treatment of LTBI. Surveillance of these events will provide data to evaluate the safety of treatment regimens recommended in current guidelines (4,324). Control of TB Among Populations at RiskThis section contains recommendations for measures to control and prevent TB in five populations (children, foreign-born persons, HIV-infected persons, homeless persons, and detainees and prisoners in correctional facilities). Each of these populations occupies an important niche in the epidemiology of TB in the United States. Individual members of each population have been demonstrated, on the basis of their membership in the population, to be at higher risk for exposure to M. tuberculosis or for progression from exposure to disease, or both. Furthermore, nationwide surveillance and surveys (27,118--120,127,136,139,150,198,295,315,325,326 ) indicate that the epidemiology of TB in these populations is similar from community to community, which suggests that the recommended control measures are subject to generalization and can be applied more or less uniformly throughout the United States. Children, foreign-born persons, HIV-infected persons, homeless persons, and detainees and prisoners should not be assumed to be the only populations at high risk for TB, nor are homeless shelters and detention facilities the only settings in need of enhanced TB-control strategies. Local surveillance and surveys frequently have identified populations and settings of high TB risk and transmission that required the formulation of specific control measures (122,137,152,313,315,316,327,328). This is the primary reason why state and local surveillance should be conducted to develop a clear understanding of the epidemiology of TB at the jurisdictional level. Most important, the concept of identifying and targeting populations and settings at high risk should be viewed as a dynamic rather than as a static process. Such populations emerge and recede in importance at the local, state, and national levels. For example, foreign-born persons received little attention in the 1992 edition of this statement (6). A population whose risk for TB is now being recognized and delineated is U.S.-born non-Hispanic blacks, who account for approximately 25% of TB morbidity in the United States and who have TB rates approximately eight times those of whites (329,330) (Table 2). CDC and collaborating public health agencies in Chicago, Illinois and the states of Georgia and South Carolina are exploring new strategies to address this problem (331). Control of TB Among Children and AdolescentsThe occurrence of TB among infants and young children indicates recent transmission of M. tuberculosis and often the presence in the community of an unidentified adult with infectious TB. Thus, a case of TB in a child is a sentinel health event that signals a public health breakdown (197). Also, certain features of TB among children mandate special considerations in case detection and case management, contact investigations, and targeted testing and treatment of LTBI. For example, if LTBI results from exposure to TB in infancy and early childhood, a substantial risk exists for rapid progression to TB disease, including the development of potentially lethal forms of TB (198,294,325). The recommendations in this statement for control of TB among children and adolescents should receive high priority in all state and community TB-control plans. Basis for Recommendations for TB Control Among Children and Adolescents Case detection and primary prevention strategy: contact investigation of adults with pulmonary TB. The majority of infants and children who acquire TB disease do so within 3--12 months of contracting M. tuberculosis infection. Infants and toddlers aged <3 years are especially prone to the rapid progression from infection to disease, and they often acquire severe forms of TB, including meningitis and disseminated disease. The most important step to detect and prevent TB among children is the timely identification and effective treatment of adults with active TB. The cornerstone of TB prevention among children is high-quality contact investigations of suspected cases of pulmonary TB in adults, because 20%--40% of pediatric cases of TB could have been prevented if contact investigation had been more timely and thorough (198,293,325). Contact investigation of adult pulmonary TB cases is crucial to the detection, control, and prevention of pediatric TB and its complications (332,333). The yield of detection of TB and LTBI is high, with an average of 50% of childhood household contacts having LTBI or TB disease (31,60). Because <50% of cases of TB among children are asymptomatic despite abnormal radiographic findings, contact investigation leads to earlier discovery of TB among children, better treatment outcomes, and fewer complications (326). Also, children with LTBI or TB disease identified through contact investigation are more likely to receive DOT at the same time as the source-case, which increases adherence to therapy. Another benefit of contact investigations is the ability to identify and treat infants and young children who have been exposed to a person with a contagious case of TB and who might be infected but nevertheless have a negative tuberculin skin test (the role of QFT-G for diagnosis of LTBI in children aged <17 years has not been determined). A tuberculin skin test might take 2--3 months after infection to become positive in an infant or toddler. However, the incubation period for severe TB, including meningitis and disseminated disease, might be only 4--6 weeks. Failure to give empiric treatment for LTBI to exposed infants and young children with negative tuberculin skin test results, particularly those aged <3 years, might therefore result in rapid acquisition of disease (295,325). Case management. The record for adherence to treatment for TB is no better for children than it is for adults (333). Children with TB might live in socially disorganized or disadvantaged homes and receive care from multiple adults. A chaotic environment can lead to a poor understanding of TB and its treatment and decreased adherence. DOT is effective in TB treatment for children and adolescents. However, almost 10% of children receiving DOT experience gaps in treatment that require extensions of therapy (326). Intensive-case management, including use of incentives and enablers, is a crucial element of a TB-treatment plan for children. Contact investigation of cases of TB among children and adolescents. Contact investigations for children with suspected TB are generally conducted to identify the adult source-case. Identifying a source-case serves to establish the diagnosis of TB in the majority of children and, if the source-case is culture-positive for M. tuberculosis, to determine the likely drug susceptibility pattern of the infecting strain of M. tuberculosis in the child. Even with optimal medical evaluation, M. tuberculosis can be isolated from <50% of children with clinically suspected TB. While microbiologic testing determines the diagnosis of TB for the majority of adults, positive culture results often are lacking for children. In the majority of cases, the diagnosis of pediatric TB is established by the triad of 1) a positive tuberculin skin test result, 2) either an abnormal chest radiograph or physical examination or both, and 3) discovery of a link to a known or suspected case of contagious pulmonary TB. Because culture yields from children with TB are low, determining the drug susceptibility pattern from the source-case isolate often is the only way to determine optimal treatment for children with either LTBI or TB disease (334,335). Because TB among infants and young children usually occurs within weeks to months of contracting infection with M. tuberculosis, having a child with disease is a marker of recent transmission from someone in the child's environment. The source-case, often a parent or other caregiver (336--338), might not have been identified as having TB by the time the child becomes ill. Consequently, parents and other adults who are close contacts of children hospitalized with TB should be evaluated themselves for TB disease as soon as possible to serve as a case-detection tool and to prevent nosocomial transmission of M. tuberculosis (339). A chest radiograph should be performed on these family members to exclude pulmonary TB; certain centers have implemented this recommendation by requiring that adults who accompany a child have a chest radiograph performed and interpreted immediately while at the health-care facility (339). Other adult family members or friends also should be required to show evidence of a normal chest radiograph, performed by the health department or other provider, before being allowed to visit the child. Because TB in the child, not LTBI, is the reliable marker of recent infection, chest radiograph screening of accompanying adults is not necessary if the child has LTBI without TB disease. Associate investigations (i.e., efforts to identify and evaluate household contacts of a child with LTBI to identify the infectious person responsible for the child's infection) are often performed as part of the evaluation of a child with LTBI (5,17,340--343). The usefulness of this approach depends on the criteria for placing skin tests on children. If testing of children at low risk is undertaken, associate investigations will be costly, have a low yield, and divert TB-control resources from more important activities. Associate investigations of children at high risk, however, usually detect a limited number of persons with TB but do identify substantial numbers of other persons with LTBI who are candidates for treatment (341--343). Targeted testing and treatment of LTBI. In the 1950s and 1960s, child-centered TB control activities were based on periodic testing of all children for LTBI (344). However, as the number of TB cases dropped, the disease became concentrated among persons at high risk in particular subpopulations. Consequently, the majority of U.S. children have negligible risk for acquiring LTBI. Among children at low risk, the majority of positive tuberculin skin test results are false positives caused by nonspecific reactivity or exposure to nontuberculous mycobacteria in the environment (344). False-positive results lead to unnecessary health-care expenditures and anxiety for the child, family, school, and HCWs (345). Thus, while the testing of children with an expected high prevalence of LTBI is desirable, mass testing of children with a low prevalence of LTBI is counterproductive and should not be undertaken. The optimal approach is to perform tuberculin skin testing only on those children with specific risk factors for LTBI. A questionnaire that assesses risk factors for TB can be used successfully in clinics and private offices to identify children at risk for LTBI (237,346--348); this approach can also be used to identify at-risk college students (349). The screening tool is the questionnaire; only those children whose answers indicate that they are at risk for LTBI should receive a tuberculin skin test. Use of a questionnaire can also address issues related to discrimination; all children in a setting such as a school or child-care center can be screened easily, but only those with identified risk factors for LTBI should receive a tuberculin skin test, thereby diminishing the number of false-positive results. No single questionnaire has been validated for use in all settings and for all ages of children. Factors that have correlated highly with risk for LTBI among children in more than one study include 1) previous positive tuberculin skin test result; 2) birth in a foreign country with high prevalence; 3) nontourist travel to a high-prevalence country for >1 week; 4) contact with person with TB; and 5) presence in the household of another person with LTBI. Questions pertaining to a locally identified population with a high rate of TB should be included in a questionnaire, but validation of these questions is difficult. In certain treatment programs for LTBI among children in the United States, the completion rate associated with 6--9 months of self-supervised isoniazid therapy is only 30%--50%. As LTBI among young children might progress rapidly to TB disease, DOT is recommended. Children with LTBI, who are most likely to benefit from DOT because of their high risk for rapid progression of infection to disease, include contacts of persons with recently diagnosed cases of pulmonary TB, infants and young children, and children with immunologic deficiencies, especially HIV infection. Control of TB Among Foreign-Born PersonsTB among foreign-born persons is of increasing importance. During 1992--2003, the number of TB cases decreased 64% among U.S.-born persons but increased 8% among persons born outside the United States (14,15). During 1992--2003, the percentage of TB cases in the United States that occurred among foreign-born persons increased from 27% in 1992 to 53.3% in 2003 (15), and the number of states in which >50% of reported cases of TB occurred among foreign-born persons increased from four (8%) in 1992 to 25 (50%) in 2003 (15). In 2003, eight states (California, Florida, Illinois, Massachusetts, New Jersey, New York, Texas, and Virginia) accounted for 71% of cases among foreign-born persons. Foreign-born persons with TB have been more likely than U.S.-born persons to harbor drug-resistant strains of M. tuberculosis; in 2003, 10.6% of foreign-born persons with TB had TB with primary isoniazid resistance, compared with 4.6% of U.S.-born persons with TB (14). The increase in cases of TB among foreign-born persons has been attributed to at least three factors (350). First, the number of persons entering the United States from other countries in which TB occurs with high incidence (44) now accounts for >75% of the immigrant flow (116,278); during 1994--2003, an estimated 80%--86% of immigrants admitted to the United States came from high-incidence countries (351). Second, foreign-born persons are subject to cultural and linguistic barriers that might affect health-seeking behavior and access to medical care, resulting in delays in diagnosis and difficulty in understanding and completing treatment (18,19,194,325). Third, these barriers, which have implications for the treatment, control, and prevention of TB among foreign-born persons, have not been sufficiently appreciated and addressed in TB-control program planning in the United States. Precise information is lacking to assist in the identification of foreign-born persons who have an elevated risk for acquiring TB during residence in the United States. Immigrants entering either Canada or the United States have a risk for TB during their early years of residence that approximates that of residents of the country of birth (115,352,353). Over time, the risk declines and approaches that of residents of the host country. Consequently, recent guidelines have designated immigrants from countries with a high prevalence of TB who have resided in the United States <5 years as foreign-born persons at high risk (4). Criteria for characterizing countries as having a high prevalence of TB have not been developed, and no consensus exists on which countries should be designated as having a high prevalence of TB. In rank order, the 14 countries listed most frequently as country of origin of foreign-born persons with reported TB in the United States are Mexico, the Philippines, Vietnam, India, China, Haiti, South Korea, Somalia, Guatemala, Ecuador, Ethiopia, Peru, El Salvador, and Honduras), and these 14 countries accounted for 76% of cases among foreign-born persons during 1999--2002 (14). Estimated incidence rates of TB in these countries in 2002 ranged from 33/100,000 population (Mexico) to 406/100,000 population (Somalia) (354). However, the country of origin of foreign-born persons with TB can vary substantially among localities within a state and between states and regions across the United States. State and local TB control programs should develop their own profiles of risk for TB among foreign-born persons as part of the jurisdiction's overall epidemiologic analysis of TB and then define which immigrant and foreign-born populations in their areas should be considered as being at high risk for TB. Data sources for TB programs to use in making this determination include 1) WHO data on the estimated incidence of TB in countries of origin (354); 2) local epidemiologic and surveillance data (151,152,313--316,355); 3) published guidelines (4,279), and other sources of data (115,116); 4) qualitative information on refugee and immigrant movement into the jurisdiction; and 5) availability of resources to establish control and prevention measures targeted toward the foreign-born population. The principles and priorities of TB control among foreign-born persons at high risk are not different from those for control of TB among U.S.-born persons (Box 4). However, for the reasons given previously, TB control among foreign-born persons at high risk might present challenges requiring targeted strategies specific to that population (152,356). How Foreign-Born Persons Enter the United States Foreign-born persons enter the United States legally through different official channels (Table 7). As a condition of entry, persons migrating as immigrants, refugees, and asylees are required to be screened for diseases of public health significance, including TB. Persons entering in the nonimmigrant category do not require preentry screening. Persons who enter the country without legal documentation are referred to as unauthorized aliens. During 1992--2002, an estimated 380,000--536,000 persons entered the United States annually as immigrants, refugees, or asylees (Table 8). In 2002, among the estimated 516,000 persons in those categories, 86.6% were from countries with high incidence of TB. Immigrants, refugees, and asylees constitute only a fraction of foreign-born persons who enter the United States each year; the majority (20--35 million persons) enter in one of the nonimmigrant subcategories (Table 8). The majority of entering nonimmigrants are tourists or business travelers who spend only a short time in the United States. However, an estimated 850,000--1.9 million workers, students, and other visitors and their families might reside in the United States for multiple years (Table 8). A nonimmigrant, refugee, or asylee residing in the United States who meets the eligibility requirements and applies for a change in visa status to that of a lawful permanent resident should undergo required health screening assessment by a civil surgeon. During 2002, of the 679,305 persons who adjusted their immigration status under this program, 536,995 (79%) were from countries with high incidence of TB (238). In addition, an estimated 7 million unauthorized aliens resided in the United States in January 2000, and during 1990--1999, the unauthorized alien population increased annually by approximately 350,000 persons (357). Current Requirements for TB Screening of Immigrants U.S. immigration law mandates screening outside the United States for applicants designated as immigrants who are applying for permanent residence status and for applicants designated as refugees or asylees (Table 7). The purpose of mandated screening is to deny entry to persons who have either communicable diseases of public health import or physical or mental disorders associated with harmful behavior, abuse drugs or are addicted to drugs, or are likely to become wards of the state. The current list of infectious diseases of public health significance that are grounds for exclusion include infectious TB, HIV infection, leprosy, and certain sexually transmitted diseases (358). Worldwide, approximately 400 licensed local physicians, designated as "panel physicians," perform these medical examinations. Panel physicians are appointed by U.S. embassies and consulates that issue visas. CDC is responsible for monitoring the quality of these examinations and for providing technical guidance and consultation for TB diagnosis and treatment. The TB screening process is a program for active TB case detection designed to deny entry to persons with infectious pulmonary TB (identified by positive sputum AFB smear results). For persons aged >15 years, a brief medical history and a chest radiograph are obtained (Figure 4). If the chest radiograph is considered compatible with pulmonary TB, three sputum specimens are obtained and examined for AFB. Although procedures vary from site to site, smears are usually performed by Ziehl-Neelsen staining and examined with light microscopy. Cultures for M. tuberculosis are not required and are not routinely performed. Persons aged <15 years are evaluated only if they have symptoms that are consistent with TB or are a contact of person with infectious TB. A test for M. tuberculosis infection is performed, and a chest radiograph is obtained if the test is positive or if the child is suspected to have TB. Persons with abnormal chest radiographs suggestive of TB and with AFB-positive sputum smear results are classified as having Class A TB, which is an excludable condition for entry into the United States (358). Persons so designated have two options: 1) to complete a course of treatment for TB, including documented negative sputum AFB smears at the end of treatment, at which point they are classified according to their chest radiograph results and may enter the United States; or 2) to receive TB treatment until sputum smear results for AFB convert from positive to negative and then apply for an immigration waiver. A U.S. health-care provider who agrees to assume responsibility for the completion of TB treatment after a person's arrival in the United States should sign the waiver. The waiver is countersigned by a representative of the jurisdictional public health agency of the person's intended U.S. destination. An applicant whose chest radiograph is compatible with active TB but whose sputum AFB smear results are negative is classified as having Class B1 status and may enter the United States. If the chest radiograph is compatible with inactive TB, no sputum specimens are required, and the applicant enters the country with Class B2 status (358). Immigrants with a Class A waiver or with Class B1 or B2 status are identified at ports of entry to the United States by CIS on entry to the United States and reported to CDC's Division of Global Migration and Quarantine (DGMQ). DGMQ notifies state and local health departments of refugees and immigrants with TB classifications who are moving to their jurisdiction and need follow-up evaluations. Persons with a Class A waiver are required to report to the jurisdictional public health agency for evaluation or risk deportation. For persons with Class B1 and B2 status, however, the stipulated evaluation visits to the health agency are voluntary. Persons Seeking Adjustment of Status After Arrival Persons seeking to adjust their immigration status after residing in the United States with nonimmigrant visa status should undergo a medical evaluation by one of the approximately 3,000 U.S. medical practitioners designated by DGMQ as civil surgeons. TB screening by civil surgeons is based on tuberculin skin testing; QFT-G is also approved for detecting LTBI. If an applicant seeking adjustment of status has a tuberculin skin test reading of >5 mm, a chest radiograph is required. If the radiograph is compatible with active TB, the person is referred to the jurisdictional public health agency for further evaluation (358). Civil surgeons are also advised that persons with a positive tuberculin test result and no signs or symptoms of TB disease should be referred to public health agencies for evaluation for treatment of LTBI, following ATS/CDC/IDSA guidelines (4,324). Because data on the outcomes of TB screening of persons seeking to adjust their immigration status are not aggregated or analyzed, only limited information is available. In an evaluation of the screening practices in five U.S. Immigration and Naturalization Service jurisdictions, among 5,739 applicants eligible for screening through tuberculin skin testing, 4,290 (75%) were considered to have been screened appropriately (240). In Denver, Colorado, where health department physicians serve as civil surgeons, 7,573 persons were evaluated for adjustment of status during May 1987--December 1988 (239). Applicants were screened with tuberculin skin testing, chest radiographs, or both. Among 4,840 applicants that had a tuberculin skin test placed, 2,039 (42%) had a reaction >10 mm. Sixteen persons (0.7%) were sputum culture-positive for M. tuberculosis. Therapy with isoniazid was recommended for 1,029 applicants, of whom 716 (70%) completed 6 months of treatment. Immigration Status of Foreign-Born Persons with TB Studies have sought to identify the initial immigration status of foreign-born persons with reported TB. During 1992--1993 in Hawaii, 78% of TB cases occurred among immigrants, 4% among student nonimmigrants, and 4% among nonimmigrant tourists (350); in 14% of cases, the immigration status could not be determined. During 1992--1994 in Seattle, Washington, 58% of TB cases among foreign-born persons who had resided in the United States for <1 year occurred among immigrants or refugees (293); immigration status was not determined among the remaining foreign-born persons. During 1998--2000, a total of 59% of foreign-born persons with TB in Tarrant County, Texas, were immigrants or refugees, 24% were unauthorized immigrants, and 17% were nonimmigrant students and workers (316). Assessment of TB Screening Requirements for Immigrants The priority for immigration screening efforts is to detect cases of pulmonary TB among persons applying for permanent residence in the United States and to prevent the most infectious persons from entering the United States. However, requirements for screening outside the United States do not apply to the majority of foreign-born persons entering the United States because those classified as nonimmigrants and unauthorized immigrants do not undergo screening (Table 7) (277). Furthermore, a significant proportion of immigrants with Class B1 (4%--14%) and B2 (0.4%--4%) status allowed to enter the United States with abnormal chest radiographs because of having AFB-negative sputum smears on screening outside the United States are later discovered (on the basis of follow-up evaluations by U.S. public health agencies) to have active TB at the time of entry (350). This finding has great importance for TB-control activities in certain U.S. jurisdictions. IOM, NTCA, and CDC have suggested changes in the screening procedures for immigrants, as follows:
TB Control at the U.S.-Mexican Border The U.S.-Mexican border presents specific challenges to TB control. Four U.S. states (California, Arizona, New Mexico, and Texas) and six Mexican states (Baja California Norte, Sonora, Chihuahua, Coahuila, Nuevo León, and Tamaulipas) comprise the U.S.-Mexican border, and an estimated 1 million persons cross the border daily. In the six Mexican border states, estimated annual TB incidence is 27.1 cases/100,000 population, compared with 5.1 cases/100,000 population in the United States (359). In 1999, Mexico was the country of origin for 23% of foreign-born persons in the United States with reported TB, and 75% of those cases were reported from the four U.S. border states. In 1996, those same states reported 83% of TB cases among foreign-born Hispanics (360). The high rate of TB at the border, the substantial number of border crossings, the substantial geographic area involved, and the prevalent cultural and linguistic barriers make TB control a challenge in this region. Recommendations to improve TB control at the U.S.-Mexican border have been published (361). These recommendations include use of a binational case definition and development of a binational registry of TB cases, improvements in clinical care of binational TB patients and close contacts by cross-border case-management strategies, development of performance indicators for these activities, and setting research priorities (361). Basis for Recommendations on TB Control Among Foreign-Born Persons Surveillance. The inability to distinguish imported TB present at the time of entry of foreign-born persons into the United States from domestically occurring disease obscures the progress that certain states and cities have made in TB control. Standardized reporting of new TB cases does not allow separating TB among foreign-born persons that is present at the time of entry from cases that arise during residence in the United States. This is more than a semantic distinction because cases of TB that occur among short-term visitors and workers, students, and unauthorized aliens are counted as U.S. incident cases even though a substantial number are imported (115). Surveys using sputum cultures indicate that 4%--13% of immigrants and refugees with Class B1 status have TB disease at the time of entry (279). TB present at the time of entry is likely to contribute to the higher incidence rates of TB noted among foreign-born persons in the first 2 years after arrival (115). The importance of imported cases and the need to distinguish them from domestic cases has also been demonstrated in the smallpox, polio, and measles eradication efforts in North America. Case detection. Multiple factors common to the experience of foreign-born persons in the United States might lead to delays in the detection of TB. Preexisting culturally derived beliefs about TB might serve as a disincentive to seek health care when symptoms of TB are experienced (18,279). Also, foreign-born persons wishing to receive a medical evaluation might encounter financial, linguistic, or other barriers to access (19). Once medical services are sought, foreign-born persons are likely to receive their evaluation from certain kinds of health-care providers (e.g., foreign-born physicians or those working in community health centers or hospital EDs) rather than from TB clinics conducted by public health agencies. These challenges to optimal case detection among foreign-born persons will require 1) targeted public education for foreign-born populations at high risk to explain that TB is a treatable, curable disease; 2) better access to medical services, especially for recently arrived immigrants and refugees; and 3) maintenance of clinical expertise in the diagnosis and management of TB among medical practitioners (Box 1). The TB-screening process for visa applicants (i.e., identification of persons with abnormal chest radiographs) has provided opportunities for active case detection in follow-up evaluations in the United States. Data derived from programs that have sought to identify active TB cases on the basis of positive sputum cultures for M. tuberculosis among immigrants with Class B notification status indicate that 3%--14% of the approximately 6,000 immigrants with Class B1 status who enter the United States each year and 0.4%--4.5% of the 12,000 immigrants with Class B2 status have TB at the time of entry (279). In San Francisco, California, during July 1992--December 1993, of 182 immigrants with Class B1 status who received follow-up evaluations, 27 (14.8%) had active TB, and 134 (73.3%) had inactive TB (362). Among 547 immigrants with Class B2 status, 24 (4.3%) had active TB, and 301 (54.5%) had inactive TB. In California, 3.5% of all persons with a Class B notification status who arrived during January 1992--September 1995 were reported to have active TB <1 year of arrival (277). Recent arrivals with Class B notification status accounted for 38% of all foreign-born persons with TB reported <1 year of arrival. Among 124 immigrants and refugees in Hawaii who were reported during 1992--1993 to have TB <1 year of arrival, 78 (63%) had been classified as having Class B1 status and 17 (14%) as having Class B2 status (350). However, a study from Los Angeles suggested that the visa application process was more effective in identifying cases among persons recently arrived from Southeast Asia than among those from Mexico and Central America (363). An active Class B1/B2 follow-up program can be relatively cost effective. During October 1995--June 1996, in Santa Clara County, California, 87% of immigrants with Class B status responded to letters inviting them to receive a follow-up evaluation, resulting in a cost of $9.90 to locate one immigrant with Class B1/B2 status and $175.88 to locate one person with TB (364). Case management. As with case detection, cultural and linguistic differences might impede successful treatment outcomes among foreign-born persons. Case management of persons whose primary language is not English depends on reliable and competent medical translation. Providers and agencies that work with foreign-born patients at high risk should ensure that adequate translation and interpretation services are available. In jurisdictions in which the majority of the cases occur among foreign-born person, providing these services can be costly. For example, in 2000, the Tarrant County Health Department TB Program (Fort Worth, Texas), spent approximately $24,000 on professional translation services (365). Ideally, professional services should be used for translation rather than relatives or family friends (365). Culturally derived attitudes and beliefs about TB and its treatment can also be impediments to the management of TB among foreign-born persons. Each culture has its own knowledge, attitudes, and beliefs about TB and how it should be treated. For example, in a study that used focus groups to evaluate attitudes regarding TB among Filipino immigrants, participants expressed a belief that TB was extremely contagious (264) and mentioned the associated social stigma and isolation. Although all participants agreed that medical therapy was necessary, participants also trusted the effectiveness of traditional treatments. As more of the burden of TB in the United States is borne by foreign-born persons, the need for health-care providers to understand cultural attitudes toward TB will increase. Case management is particularly difficult at the U.S.-Mexico border where, until recently, tracking systems for persons who migrated between the two countries were not in place. A new binational system has been established to ensure continuity of care and completion of TB treatment for patients who migrate between the United States and Mexico and to coordinate the referral of patients between the health systems of both countries. The project is now being tested in four U.S.-Mexican jurisdictions (San Diego, California, and Tijuana, Baja California; El Paso, Texas--Las Cruces, New Mexico, and Ciudad Juarez, Chihuahua; Webb and Cameron Counties, Texas, and Matamoros, Tamaulipas; and Arizona and Sonora). If the pilot project proves successful, this binational TB patient referral and information system will likely be expanded to other parts of the United States and Mexico. Contact investigation. Contact investigations have a particularly high yield when conducted on foreign-born patients. In Seattle, for example, contacts of foreign-born persons with TB were more numerous (6.0 versus 3.4 per case) and substantially more likely to be have positive tuberculin skin test results (50% versus 18%) and to be started on treatment for LTBI (40% versus 23%) than were contacts of U.S.-born persons with TB (293). A multicenter survey from around the United States demonstrated that the tuberculin skin test was positive among 71% of foreign-born contacts compared with 32% of all close contacts (31). Although not all foreign-born contacts identified during a contact investigation are recently infected, the majority would nevertheless be considered candidates for treatment of LTBI under current guidelines (4). In addition, a Canadian study indicated that contact investigations were more cost effective than preimmigration screening and postarrival surveillance (276). Targeted testing and treatment of LTBI. Surveys using molecular epidemiologic methods have consistently demonstrated that less clustering of M. tuberculosis isolates occurs from foreign-born patients than from U.S.-born patients; this has been interpreted as evidence that less person-to-person spread of TB occurs among foreign-born persons in the United States and that the majority of cases of TB among foreign-born persons occur as a result of activation of a latent infection (150--152,356). In fact, one reason for the lack of progress in reducing TB among foreign-born persons might be that insufficient attention has been given to targeted testing and treatment (152), which should be the most applicable prevention strategy for this population, in which TB disease occurs mainly by progression from LTBI. The success of programs for targeted testing and treatment of LTBI among populations at high risk in the United States has been thwarted by poor interest in the intervention on the part of medical practitioners and poor adherence by patients (51). Among foreign-born persons, these problems are magnified by the lack of access to care and by cultural and linguistic obstacles. Successful models for administering targeted testing and treatment of LTBI among refugees have been published; these models are resource-intensive and require a commitment to working within the population's cultural contexts (202,221). In addition, the use of DOT increases treatment completion rates (366). Other opportunities to conveniently access foreign-born persons for targeted testing programs include school-based testing of foreign-born students. The majority of persons residing as students in the United States remain long enough to receive targeted testing for LTBI and, if TB is diagnosed, to complete a course of treatment. Screening for TB is required by 61% of colleges and universities: for all students in 26%, for all international students in 8%, and for students in specific academic programs in 47% (367). School-based screening also has been evaluated among younger students (150,322,345). In California, widespread TB screening of kindergarten and high school students yielded a low prevalence of skin test reactors and a limited number of cases of TB, but foreign-born students were >30 times more likely than U.S.-born students to have the infection (345). In a cost-benefit analysis, screening all students would be expected to prevent 14.9 cases/10,000 children screened, whereas targeted testing would prevent 84.9 cases/10,000 screened and would be less costly (345). Control of TB Among Persons with HIV InfectionHIV and M. tuberculosis interact in ways that tend to worsen both diseases among coinfected persons (368). When a person with HIV infection is exposed to a patient with infectious TB, the risk for acquiring TB disease soon after that exposure is markedly increased (369). In outbreaks in which the start of exposure could be determined, HIV-infected persons acquired active TB in as little as a month after exposure to a person with infectious TB (136). HIV coinfection is also a highly potent risk factor for progression from LTBI to TB (44,46,370). Persons with LTBI and HIV coinfection have a risk for progressing to TB disease of approximately 10%/year (317,371,372), which is 113--170 times greater than the risk for a person with LTBI who is HIV-seronegative and has no other risk factors (4,44). On a global level, HIV infection has had a substantial effect on the epidemiology of TB. Areas of the world most heavily affected by the global epidemic of HIV/AIDS (e.g., sub-Saharan Africa) have also sustained increases in the incidence of TB (44,46,373). TB is the most common infectious complication and the most common cause of death among persons with HIV/AIDS in places where the incidence of both diseases is high (374). In the United States, HIV infection has been associated with TB outbreaks in institutional settings, including health-care facilities (53), correctional facilities (37), and homeless shelters (33). Before the advent of highly active antiretroviral therapy (HAART) in the early 1990s, HIV infection caused a progressive decline in immune competence and death. However, the use of HAART using combination therapy plus protease inhibitors has prolonged the survival among persons with HIV infection (375--377). The introduction of HAART has also decreased the incidence of TB among HIV-infected persons: an 80% decrease in risk for TB has been demonstrated among HIV-infected persons receiving HAART (378). With the declining incidence of TB in the United States since 1992, the incidence of HIV infection among persons with TB also has decreased. This is likely attributable to increased understanding of the biologic interactions between the two pathogens, leading to more targeted TB-control efforts and to the introduction of HAART. Another factor is improved TB infection control in health-care facilities, because HIV- infected persons were particularly affected by health-care--associated transmission of M. tuberculosis (53). HIV infection was a prominent cause of the 1985--1992 TB resurgence in the United States, especially the incursion of health-care--associated TB (including multidrug-resistant disease). That fact, along with the knowledge that the global epidemics of HIV infection and TB are continuing unabated (44), dictates a high degree of respect and vigilance for the adverse consequences that HIV infection could impose on the epidemiology of TB in the United States. Basis for Recommendations of Control of TB Among Persons with HIV Infection HIV counseling and testing. Knowledge of the presence of HIV infection among patients with TB is useful for surveillance purposes to ensure that an optimal drug regimen is chosen for treatment (5), refer persons for HIV primary care if the case is newly detected, and guide decisions about contact investigations. TB is frequently the first illness that brings a person who has not previously received a diagnosis of HIV infection into the health-care system. Voluntary counseling and testing for HIV is recommended for all patients with TB (5), but this recommendation has not been fully implemented, and reporting of HIV among persons with TB is incomplete (14). In 2003, HIV testing was performed for <50% of patients reported with TB in the United States, and only 63% of persons in the age group at greatest risk (persons aged 25--44 years) were tested (14). HIV counseling and testing has also been recommended for contacts of persons with TB (302). However, recent data indicate that contacts of HIV-infected persons with TB have a high rate of HIV infection but that contacts of persons with TB without HIV infection do not (301). HIV testing for other persons with LTBI should be limited to those who have clinical or behavioral risk factors for HIV infection. Case detection. HIV coinfection affects the clinical and radiographic manifestations of TB. HIV-infected patients are more likely than persons without HIV infection to have extrapulmonary and miliary TB (379,380), and those who have pulmonary TB tend to have atypical findings (e.g., they are less likely to have apical cavities and are more likely to have lower lobe or instititial infiltrates and mediastinal or paratracheal lymphadenopathy). These atypical features are heavily dependent on the patient's CD4 cell count; those with CD4 cell counts >300 cells/µL usually have manifestations, such as upper lobe cavitary infiltrates (274). Persons with HIV infection might also have pulmonary TB despite a normal chest radiograph (274,379). HIV-infected patients are also vulnerable to other pulmonary and systemic infections such as Pneumocystis carinii and pneumococcal pneumonias and disseminated M. avium complex disease. Although the symptoms and signs of TB are usually different to the trained clinician from those caused by other prevalent invasive pathogens (273,381), HIV co-infection often results in delay in the diagnosis of TB as a result of altered clinical and radiographic manifestations (23). Undetected transmission of M. tuberculosis to HIV-infected persons can have serious sequelae (136). A substantial outbreak of TB in a prison in South Carolina in 1999 demonstrated the widespread consequences of an unrecognized TB case in a congregate setting with a substantial number of HIV-infected persons (37). In that outbreak, 32 TB cases and 96 tuberculin skin test conversions resulted from a single unrecognized case. Similar outbreaks have occurred in hospitals (53,244), HIV-living facilities and day-treatment programs (136), and homeless shelters (33). Such outbreaks underscore the importance of aggressive TB screening and treatment in settings in which HIV-infected persons congregate. Screening for TB in those settings has been successfully conducted by using symptom checklists, tuberculin skin testing, and chest radiographs (37,118,136). Case management. Management of TB among persons with HIV infection is complex. Drugs used to treat TB and those employed in combination antiretroviral therapy have overlapping toxicities and potentially dangerous drug interactions (382). Paradoxical responses to TB therapy are more common among HIV-infected persons (383). Use of multiple potentially toxic medications also provides further challenge to adherence with TB treatment. Therefore, integration of management of both HIV infection and TB is critical to the success of management of both. Comprehensive case management, including DOT, is particularly important (5). Among HIV-infected TB patients, more favorable outcomes and survival have been associated with DOT than with self-administered therapy (384). ATS/CDC/IDSA guidelines should be consulted for recommendations on length and mode of treatment and selection of drug regimens (5). Finally, patients with HIV and TB bear the brunt of two conditions that are associated with clinical and social complexities that can be personally overwhelming. Both HIV infection and TB are associated with stigmatization, and patients with these concomitant conditions often suffer from isolation and a lack of social support. Contact investigation. Despite controversy as to whether HIV-coinfected patients with TB are more or less infectious than HIV-seronegative patients (385,386), they are clearly capable of transmitting M. tuberculosis; contacts of the two populations of patients have comparable rates of LTBI (369,387). The higher risk for progressing rapidly from exposure to M. tuberculosis to TB disease means that all of the medical and public health interventions (case detection and reporting, initiation of an effective drug regimen, and identification and evaluation of contacts) are more urgent when working to control HIV-associated TB (388). Although offering HIV counseling and testing to all contacts of persons with infectious TB has been recommended (302), this undertaking would be resource-intensive. Whereas prevalence of HIV infection among contacts of HIV-infected persons is high, prevalence among contacts of persons with TB without HIV infection or with undetermined status is negligible (301). Targeted testing and treatment of LTBI. HIV coinfection is the most important known risk factor for persons with LTBI acquiring active TB (317,371,372). Treatment of LTBI is effective in reducing the risk for progression to TB disease among HIV coinfected persons (372,389). Thus, all possible efforts should be made to ensure that HIV-infected persons are tested for M. tuberculosis infection and that those found to have latent infection receive and complete a course of treatment. In addition, knowledge of the HIV status of persons being evaluated for LTBI is desirable 1) in interpreting the tuberculin skin test result (e.g., >5 mm of induration is considered a positive test among persons with HIV infection [4]) and 2) in counseling persons with positive skin test results about the risks and benefits of treatment for LTBI (the role of QFT-G for testing persons with HIV infection for LTBI has not been determined). According to current guidelines (302), persons being evaluated for LTBI should also be screened for HIV infection by using self-reported clinical and behavioral risk factors. Institutional infection control. Infection-control measures recommended to prevent transmission of M. tuberculosis have been effective in limiting exposure of HIV-infected persons, including patients, visitors, and staff members, to M. tuberculosis in hospitals, extended care facilities, and correctional facilities (9,244). Nevertheless, the risk for rapid progression from exposure to TB disease means that HIV-infected persons should continue to be advised of any potential sites of institutional exposure so an informed choice regarding employment or volunteering can be made. Control of TB Among Homeless PersonsThe persistence of TB among homeless persons in the United States is a major public health problem. The homeless population is not insubstantial; in 1995, an estimated 5 million persons (2.5% of adult U.S. residents) either were or had recently been homeless, living in streets or shelters, or marginally housed (e.g., living on public support in residential hotels) (390). TB incidence is high among homeless persons, and evidence exists of considerable transmission of M. tuberculosis. Among 2,774 homeless persons enrolled during 1990--1994 in San Francisco, California, 25 incident cases were identified for 1992--1996, for an annual rate of 270 cases/100,00 population (118). Among 20 M. tuberculosis isolates from incident cases that were subjected to genotyping study, 15 (75%) were clustered, indicating chains of transmission in the population. Other molecular epidemiology studies also have identified homelessness as an important risk factor for clustering of M. tuberculosis isolates (33,119,391,392). Shelters are key sites of TB transmission among homeless persons throughout the United States (27,33,118--120,166, 391--393). In Los Angeles, California during March 1994--May 1997, three homeless shelters were sites of TB transmission for 55 (70%) of 79 homeless patients (33). In Fort Worth, Texas during 1995--1996, clusters of cases among homeless persons occurred simultaneously in four homeless shelters (27). In Alabama, genotyping of isolates from TB cases reported in 1994-98 revealed an undetected statewide outbreak of TB that was traced to transmission in a correctional facility and in two homeless shelters (166). In an outbreak in a shelter in Syracuse, New York, during 1997--1998, a shelter resident was probably infectious for 10 months before receiving a diagnosis; ventilation in the shelter was poor, and the population included vulnerable persons with risk factors that included HIV infection, substance abuse, and malnutrition (120). Multiple barriers to the control of TB among homeless persons have been identified. Delays in detection of infectious cases have been reported (20); in a computer simulation study that modeled multiple strategies for TB control among homeless persons, a 10% improvement in access to treatment led to greater declines in disease and death after 10 years than comparable improvements in treatment programs (394). Traditional methods of conducting contact investigations often fail to identify contacts of homeless persons with TB (30,119,120). Difficulties also have been encountered in completing treatment for homeless patients with active TB (395) and LTBI (167,184). Basis for Recommendations for Control of TB Among Homeless Persons Surveillance and case detection. Delays in diagnosis and treatment of TB among homeless persons might occur as a result of delays in seeking medical care (181) and to the failure of medical providers to detect TB among those seeking care (20). Homeless persons with TB are disproportionately likely to receive care in hospital EDs and other urgent care clinics (232). For example, during 1994--1996, homeless persons in Atlanta, Georgia, were more likely than other patients to receive a diagnosis in a hospital ED (184). On the basis of sputum AFB smear results and radiologic findings, homeless persons had more advanced disease at the time of diagnosis, another indication that they received diagnoses later in the course of their disease (184). Shelters have proved to be effective sites for case detection by use of screening procedures among homeless persons. During May 1996--February 1997, among 127 homeless persons in Alabama for whom shelter-based screening was conducted by using symptom evaluation, sputum culture, and chest radiographs as the screening package, four (3.1%) persons had TB disease (281). Symptom evaluation alone was not proven to be useful. In a similar study from London, United Kingdom, that employed symptom evaluation, tuberculin skin testing, and chest radiography, 1.5% of homeless persons were determined to have TB (396). On the basis of findings of a high prevalence of TB in shelter-using homeless populations, certain communities have implemented compulsory screening of shelter residents based on symptom evaluation or tuberculin skin testing with radiographs for those with positive tests. One such program in Portland, Oregon, initiated in 1985, was associated with an 89% reduction in TB morbidity in the geographic area served by participating shelters during 1980--1995 (397). The implementation of a similar screening program in shelters in Denver, Colorado, in 1995 led to lower rates of active TB and reduced transmission of TB disease, as demonstrated by less genotype clustering by DNA fingerprinting (167). Both screening programs were based on symptom evaluation, tuberculin skin testing, and chest radiography. The decrease in TB morbidity in both these studies was attributed to shelter-based case detection through screening activities. Case management. Completion of treatment for active TB is more difficult for homeless persons, particularly those who report substance abuse, including alcohol abuse (395). Homeless persons with active TB are at high risk for poor adherence even with enhanced DOT and are more likely to default and move from the area of initial diagnosis. They are also more likely to have legal action taken in the form of court-ordered treatment or detention. Comprehensive case management that includes a variety of incentives and enablers, including food, temporary housing, transportation vouchers, and treatment for substance abuse and mental illness has improved rates of treatment completion in this population. Costs for homeless persons who are hospitalized for initial treatment of active TB have been $2,000 more than costs for persons who were not homeless (398). Excess hospital utilization could be attributable to social considerations, clinical indications (especially the need to render a patient noninfectious before discharge to a congregate living setting), or concerns about adherence to the plan of treatment. In San Diego, California, a novel housing program that used hostels facilitated the completion of treatment of TB in homeless persons (399). Completion rates of 84%--100% were achieved for persons housed at a designated hostel in 1995. Certain TB-control programs in cities with substantial homeless populations routinely provide temporary or longer-term housing in attempts to improve completion of treatment. The California Department of Health allots funds for temporary housing of persons with TB to each of its county and local jurisdictions. The U.S. Department of Housing and Urban Development also provides funding for housing patients with TB. The beneficial impact on treatment outcomes of an integrated approach to managing homeless patients with TB has been emphasized (394). For example, a social care and health follow-up program among homeless patients in Spain was associated with a decrease in TB rates from 32.4/100,000 in 1987 to 19.8 cases/100,000 in 1992, and better completion rates and reduced costs for hospitalizations were also documented (400). In Massachusetts, 58 (34.5%) of 214 persons hospitalized in a dedicated inpatient unit for difficult TB patients during 1990--1995 were homeless (401). Regardless of the case-management plan that is chosen, all such interventions should take into consideration the importance of addressing major gaps in knowledge, attitudes, and beliefs about TB among homeless persons (181). Contact investigation. Contact investigations for cases of TB among homeless persons are particularly challenging. Homeless patients with TB often fail to identify contacts during routine investigation (30). Completing a contact evaluation in identified contacts and completing treatment for LTBI among contacts that are homeless are often difficult (320,391,402). Interpretation of the results of tuberculin skin testing of contacts of homeless cases is problematic because the background prevalence of positive tuberculin skin tests in the population is usually higher than that of the general population. As with contact investigations among other populations at high risk, discerning when a contact investigation has become a targeted testing program is often difficult. A proposed alternative approach to conducting contact investigations of homeless persons is to focus on possible sites or locations of exposure, such as shelters (391,393). Targeted testing for and treatment of LTBI. When homeless persons are identified as a population at high risk on the basis of the local epidemiology of TB, targeted testing and treatment protocols tailored to local circumstances should be developed. However, low rates of completion of therapy for LTBI are commonly observed (167,184,402). For example, among 7,232 inner city residents (including homeless persons) screened for LTBI during 1994--1996 in Atlanta, Georgia, 4,701 (65%) completed tuberculin skin testing; of 809 (17%) who had a positive test, 409 (50%) were candidates for isoniazid therapy, and 84 (20%) completed treatment (184). In another study conducted in San Francisco, California, during 1991--1994 that was designed to improve adherence, two novel interventions (biweekly preventive DOT with either a $5 incentive or a peer health adviser) were compared with the usual method of self-supervised treatment (402). Even though completion of treatment was not high for any of the three groups, multivariate analysis indicated that independent predictors of completion were being offered the monetary incentive and residence in a hotel or other stable housing at entry into the study. That report confirmed an earlier finding that advocated offering monetary incentives (320). Institutional and environmental controls. Efforts have been made to reduce transmission of TB in shelters for homeless persons by enhancing institutional control measures. These efforts have included reducing shelter size (13), improving ventilation systems, and using germicidal ultraviolet light (280). Control of TB Among Detainees and PrisonersCorrectional facilities in the United States include jails and prisons, which serve different but complementary functions. Jails serve as pretrial detention centers and house persons (detainees) awaiting trial and those sentenced to <1 year of incarceration. Local and county governments operate the majority of jails. Jails are characterized by rapid turnover of detainees with short lengths of stay. Prisons serve as sites of detention for persons (prisoners) who have been sentenced and will be incarcerated for a known length of time, generally >1 year. State governments, the federal government, and the military all operate prison systems. On any given day, approximately 2 million persons in the United States are incarcerated; 1.4 million of those are imprisoned, and the remainder are detained in jails. Approximately 6 million persons are incarcerated in jails or prisons each year for variable lengths of time (124,125). Detainees and prisoners represent the poorest and most medically underserved segments of the U.S. population, the same population segments at risk for LTBI and TB disease (124,252,253). Persons entering prisons have usually spent time in jail, and detainees and prisoners eventually reenter the community. Consequently, TB outbreaks among detainees, prisoners, and the general population of a geographic area are interrelated (127,403), and close coordination of TB-control activities is needed between health programs in correctional facilities and jurisdictional public health agencies. Prisons have long been identified as sites of transmission of M. tuberculosis to other inmates and workers (38,139,404--408), including those with HIV infection (38,139,405,408). In addition, time spent in jail is a risk factor for subsequent acquisition of TB (127,250,256), an indication that jails often are also sites of transmission. Correctional facilities are among the most important sites of transmission of M. tuberculosis in the United States. Failure to detect TB in correctional facilities results in TB outbreaks, which have been well documented (37,139,404--408). Outbreaks of multidrug-resistant TB involving inmates and staff, including HIV-infected persons, were a prominent component of the 1985--1992 TB resurgence in the United States (404,405,409--411). However, outbreaks have continued to occur (37,139), even though TB control, including control of M. tuberculosis transmission, in the United States has improved. Basis for Recommendations on Control of TB Among Detainees and Prisoners Case detection and case management. Despite the importance of jails and prisons in sustaining and amplifying the reservoir of TB in the United States (127,405,407), little is known about the optimal means of case detection of TB among detainees and prisoners. The majority of prisons have adopted a case-detection strategy that is based on a survey of TB symptoms obtained on admission, in which all entrants are tested for M. tuberculosis infection <14 days of admission; universal chest radiographs of all entrants are rarely offered (410). No data have been published supporting the effectiveness of symptom surveys and testing for M. tuberculosis infection for detecting cases of TB and preventing transmission within jail systems, although screening by tuberculin skin testing was effective in controlling TB in one prison system (411). Certain substantial urban jails perform chest radiographs on all persons entering the institution in an attempt to minimize transmission of TB (283,412), and data indicate that this approach is cost effective (412). Because nearly all prison entrants have first been detained in a jail system, effective TB case-detection programs in jails will substantially decrease the probability that persons with undetected active TB will be admitted to prison. Once cases are detected, strategies similar to those used in the community have led to high rates of successful treatment completion (413). A particular problem for case management in a jail setting is the unanticipated release of detainees, which often precludes the development of an effective discharge plan. Strategies to better coordinate discharges with public health authorities should be promoted. Contact investigation. Continuing outbreaks of TB in correctional facilities (37,139) underscore the importance of prompt and thorough contact investigations in jails and prisons. Contact investigations in correctional facilities involve two steps: 1) identifying and evaluating persons exposed before the source-case was incarcerated, and 2) identifying and evaluating persons exposed during incarceration of the source-case. Effective case detection is important to limit the size of the latter group. Contact investigations often need to be conducted broadly, among more than one facility, because of the movement of detainees within the correctional system (414). Conducting contact investigations based on the concentric circle method is difficult in correctional institutions. Frequently, a single infected person can expose up to several hundred persons both before and after incarceration. Cases involving persons who were exposed before incarceration should be managed by the jurisdictional public health agency for the community in which the person lived before arrest. For the jurisdictional public health agency to carry out those contact investigations effectively, prompt notification and case reporting by the detention facility is necessary. Guidelines for conducting contact investigations in jails have been published (258). Targeted testing and treatment of LTBI. Targeted testing and treatment of latent TB among detainees and prisoners has been described in detail (415--417). Because of the high risk for transmission of M. tuberculosis in correctional facilities, inmates incarcerated for >14 days usually receive a test for M. tuberculosis infection as part of TB case detection. Detainees and prisoners with LTBI often are considered to be candidates for treatment of latent TB (124,252,253). Prisons often are an ideal setting for effective treatment of LTBI because of known location of the patient, length of stay, prohibition of illicit drugs and alcohol, and a predictable diet. Nevertheless, achieving high rates of completion of treatment for LTBI in prisons or jails has been difficult (257,416,417). The majority of jail detainees are released in <14 days of entry. If treatment for LTBI is started in the jail setting, community follow-up after release from jail is essential. Without specific interventions to assure such follow-up, the probability of completion of treatment might be <10% (256,257,418). Recent developments in short-course treatment of latent TB with a combination of rifampin and pyrazinamide for 2 months offered promise in improving treatment completion rates (419). However, the toxicity of this regimen precludes its routine use (324), and this combination should generally not be used for the treatment of LTBI in correctional settings because the rates of toxicity have been similar to those observed in the wider community. In addition, detainees and prisoners have high rates of hepatitis C infection, making them especially prone to serious hepatotoxicity. Institutional infection control. Correctional institutions have been sites of virulent outbreaks of TB, including multidrug-resistant TB, that have involved HIV-infected inmates and staff (37,139,405,408). Common findings in these outbreaks have included the failure to isolate persons with active TB quickly. Another common finding has been disease associated with rapid transmission of M. tuberculosis when immunosuppressed detainees and prisoners are housed together. An effective infection-control program can decrease the likelihood of TB transmission in correctional institutions (420). Guidelines to assist correctional institutions in developing effective infection-control programs have been published (258). Control of TB in Health-Care Facilities and Other High-Risk EnvironmentsDuring the 1985--1992 TB resurgence in the United States, TB cases resulted from transmission of M. tuberculosis in settings where patients with infectious TB congregated closely with susceptible persons (52--54,170,421). This epidemiologic disease pattern had not been recognized in the United States since the development of effective drugs against TB starting in the 1950s. Hospitals and other health-care facilities were the primary, but not the only, sites of transmission (405,406,408), and HIV-infected persons were prominent among those who contracted M. tuberculosis infection and rapidly acquired TB disease (52--54,170,406,408). Although incidence of TB in health-care facilities has been markedly reduced because of the development and deployment of effective infection-control measures (56,422--424) and decreasing incidence of TB in different communities, TB disease attributable to recent transmission of M. tuberculosis in other settings has not only persisted but has been recognized in a wide variety of sites and settings and become an established epidemiologic pattern. As a consequence of the changed epidemiology of TB in the United States, the primary strategies now required to control the disease include measures for its prevention in settings in which a risk for transmission of M. tuberculosis exists (Box 4). Recommendations for infection-control measures in high-risk settings are provided in this statement. The approach to control of TB and other airborne infections that was developed for health-care facilities (10) is the most successful model and is outlined in detail in this statement. Recommendations are also provided for control of transmission of M. tuberculosis in extended care facilities, correctional facilities, homeless shelters and other high-risk settings. Control of TB in Health-Care FacilitiesStrategies for control of TB in health-care facilities, which also are applicable for other settings in which high-risk persons congregate, are based on comprehensive guidelines issued by CDC in 1994 (10). New CDC guidelines for preventing transmission of M. tuberculosis in health-care facilities will be published in 2005. A draft† of these guidelines has been published in the Federal Register. In the assessment of institutional risk for TB, three levels of risk (low, medium, and potential ongoing transmission) are recommended, based on the recent experience with TB in the institution and in the community it serves. The recommended frequency of testing of employees for M. tuberculosis infection varies, depending on the institution's level of risk. The tuberculin skin test is recommended for testing HCWs and other employees with a risk for exposure to M. tuberculosis. QFT-G is also approved for detecting LTBI; guidelines for the use of QFT-G will be published in the MMWR. The risk for TB associated with health-care facilities is related to the incidence of TB in the community served by the facility and to the efficacy of infection-control measures (422). Implementation of infection-control guidelines (10) has markedly reduced risk for exposure to TB in health-care facilities during the past decade (56,422--424) and has also contributed to the decreasing numbers of TB cases. Implementation of effective infection-control measures in the medical workplace is thus an important element of broader national and international strategies to prevent transmission of TB (244). Epidemiologic investigations of the early outbreaks of TB in health-care facilities, including those involving multidrug-resistant cases, indicated that transmission usually occurred because of failure to identify and isolate patients with infectious forms of TB. In certain instances, diagnosis of TB was delayed as a result of the atypical presentation of TB among patients with HIV infection, especially those with low CD4 counts. Transmission was also facilitated by 1) the intermingling of patients with undiagnosed TB with patients who were highly susceptible; 2) inadequate laboratory facilities or delayed laboratory reporting; and 3) delayed institution of effective therapy. Other factors facilitating transmission included a lack of negative pressure respiratory isolation rooms, recirculation of air from respiratory isolation rooms to other parts of the hospital, failure to isolate patients until they were no longer infectious, allowing isolated patients to leave their rooms without wearing a mask, and leaving respiratory isolation room doors open (52--54,170,421,425,426). CDC guidelines recommend a hierarchy of TB infection-control measures (10). In order of importance, these measures are administrative controls, engineering controls, and personal respiratory protection (PRP) (Box 7). Administrative controls consist of measures to reduce the risk for exposure to persons with infectious TB, including screening of patients for symptoms and signs of TB at the time of admission, isolating those with suspected disease, establishing a diagnosis, and promptly initiating standard therapy (5). Engineering control measures are designed to reduce dissemination of droplet nuclei containing M. tuberculosis from infectious patients and include the use of airborne infection isolation rooms. The third level (and the lowest on the hierarchy of controls) is the use of PRP devices such as N-95 respirators. Respirator usage for the prevention of TB is regulated by the Occupational and Health Safety Administration under the general industry standard for respiratory protection§. In implementing a comprehensive infection control program for TB, institutions should first conduct a risk assessment to determine what measures are applicable. Risk for transmission of M. tuberculosis varies widely, and procedures that are appropriate for an institution in an area of high TB incidence (e.g., an inner-city hospital or homeless shelter in a metropolitan high-incidence area) differ from those applicable to an institution located in a low incidence area that is rarely used by patients with TB. The jurisdictional public health TB-control program should assist in the development of the assessment, which should include data on the epidemiology of TB in the community served by the institution and the number of TB patients receiving evaluation and care. The institutional risk for TB can be stratified according to the size of the institution and the number of patients with TB as low risk, medium risk, or potential ongoing transmission. Hospitals with >200 beds that provided care for fewer than six patients with TB during the previous year are categorized as low risk whereas those that cared for six or more patients are categorized as medium risk. For hospitals with <200 beds, those with fewer than three TB patients in the previous year are considered low risk, and those with three or more cases are considered medium risk. Outpatient clinics, outreach programs, or home health settings that provide care for fewer than three patients with TB per year are considered low risk, and those that care for three or more patients are considered medium risk. TB clinics, outreach programs, and other settings in which HCWs are responsible for the care of persons with TB are classified as medium risk. Any institution, clinic, or setting with evidence of recent patient-to-patient or patient-to-employee transmission of M. tuberculosis or of ongoing or unresolved transmission should be classified as having potential ongoing transmission until effective control measures have been implemented and transmission is interrupted. Potential ongoing transmission is a temporary classification. When transmission of M. tuberculosis is suspected at a facility, an immediate investigation should be undertaken that includes consultation with public health officials or experts in hospital epidemiology and infection control. Evidence of potential transmission of M. tuberculosis includes clusters of conversions of tests for M. tuberculosis infection among employees from negative to positive, increased rates of positive tests for M. tuberculosis infection among employees, an employee with potentially infectious TB, unrecognized TB among patients or employees, and recognition of identical strains on genotyping of M. tuberculosis isolates from patients or employees. How often employees at health-care facilities and other at-risk sites for M. tuberculosis infection are tested depends on the risk assessment. The positive predictive value of the tuberculin skin test is low when populations with a low prevalence of infection with M. tuberculosis are tested (424,427). Consequently, frequent testing by using that method in low-incidence, low-risk settings is discouraged. In addition, false-positive tests have been reported when institutions changed brands of Purified Protein Derivative (PPD) reagent, for example from Tubersol® to Aplisol® (427). At the time of employment, all HCWs should undergo baseline testing (with two-step testing if the tuberculin skin test is used and no testing was performed during the preceding year) (10). Those in medium-risk settings should be tested annually. Follow-up testing is recommended for workers in low-risk settings only if exposure to a patient with infectious TB (i.e., a patient not initially isolated but later found to have laryngeal or pulmonary TB) has occurred. Institutions in which ongoing transmission of M. tuberculosis is documented should carry out testing for M. tuberculosis infection of HCWs at risk every 3 months until transmission has been terminated. Employees testing positive for M. tuberculosis infection should receive a chest radiograph to exclude TB disease and should be evaluated for the treatment of LTBI based on current recommendations (4,324). Compliance with therapy for LTBI among HCWs, including clinicians, has historically been poor (428--430). Employee health clinics and infection-control departments should emphasize to HCWs the importance of completion of therapy for LTBI. In a comprehensive infection-control program that encourages HCWs to complete treatment for LTBI, higher completion rates have been reported (431,432). Control of Transmission of M. tuberculosis in Other High-Risk Settings Extended Care facilities. Elderly persons residing in a nursing home are almost twice as likely to acquire TB as those living in the community (252,433,434). Certain considerations for control of TB in hospitals apply also to extended care facilities, including 1) maintaining a high index of suspicion for the disease; 2) promptly detecting cases and diagnosing disease; 3) isolating infectious persons and initiating standard therapy; 4) identifying and evaluating contacts; and 5) conducting contact investigations when indicated. The value of treating LTBI in elderly residents of nursing homes so as to prevent future outbreaks has been documented (435). In 1990, CDC published recommendations for TB control in extended care facilities (433). Those long-term care facilities that do not have airborne-infection-isolation rooms should transfer patients suspected to have infectious TB to other facilities (including acute-care hospitals) until the disease is ruled in or out and treatment is started if indicated and continued until the patient is noninfectious (10). The risk assessment and frequency of testing for LTBI for employees at long-term care facilities are similar to those described previously. Residents should be tested on admission to the facility and should provide a history and undergo physical examination to identify symptoms and signs of TB. Residents with LTBI should be offered treatment according to current recommendations (4,324), with careful monitoring for drug toxicity. Correctional facilities. Common findings in outbreaks of TB in correctional facilities were the failure to recognize and isolate patients with TB and rapid progression of outbreaks when immunosuppressed detainees were housed together (405,406,408). Because of the substantial numbers of cases of TB infection and disease that might result from outbreaks at correctional facilities and the natural movement of inmates from incarceration to the general population, correctional facilities should be viewed as being among the most important sites of transmission of M. tuberculosis in the United States (128,436). Guidelines for control of TB transmission in correctional facilities (123) have emphasized that the infection-control principles developed for health-care facilities (10) are also applicable to correctional facilities. In prisons and jails, the most important activity in TB infection control is efficient detection of infectious TB cases, including those that are prevalent among persons entering the facility and those that arise during detention. A prompt diagnostic evaluation, respiratory isolation (including transfer out of the facility if airborne-infection-isolation rooms are not available), and institution of a standard treatment regimen are urgent priorities when suspected cases are encountered. If this process is delayed, a substantial number of persons might be exposed as a result of the congregate living arrangements that characterize correctional facilities. Because of crowded conditions that favor the spread of M. tuberculosis (420) and the high prevalence of HIV infection among prisoners (255), contact investigations should be undertaken immediately once a case of TB has occurred at a facility. In a study conducted in the Maryland state correctional system, prisons that conducted programs for targeted testing and treatment of LTBI among inmates experienced lower rates of tuberculin skin test conversions, an indication that this measure can contribute to successful infection control (420). A template is now available to assist jails in instituting an effective infection-control program (258). Shelters for homeless persons. As with correctional facilities, homeless shelters are important sites of transmission of M. tuberculosis and an important cause of the continuing high incidence of TB among the homeless population (33,118). Effective infection-control strategies in those venues are use of M. tuberculosis genotyping for rapid identification of clustered cases and sites of transmission (27,33), screening shelter users for TB disease, wide-ranging contact investigations, and engineering controls, including ultraviolet germicidal irradiation (437). A systematic shelter- based program for targeted testing and treatment of LTBI in Denver was also demonstrated to decrease incidence of TB in the homeless population (167). Because crowding and poor ventilation are often prevalent in shelters, infection-control efforts should also include engineering modifications to decrease exposure to M. tuberculosis. A guide to assist shelters in improving the safety of their environment through modifications in ventilation, air filtration, and the introduction of ultraviolet germicidal irradiation has been published (438). Other high-risk settings. As the incidence of TB has receded in recent years, new patterns of transmission have become evident. Epidemiologic investigations prompted by an increase in the incidence in TB in a community or state or by the identification of clusters of cases with identical M. tuberculosis genotype patterns have detected transmission in such venues as crack houses (137) and bars (27). In addition, transmission has been identified in association with certain social activities that are not typically considered in routine contact investigations; a church choir (140), a floating card game (172), exotic dancers and their contacts (38), a transgender social network (34), and persons who drink together in multiple drinking establishments (439). Although special techniques have been developed for exploring chains of transmission of M. tuberculosis in complex social networks (439), transmission of M. tuberculosis in such settings is not amenable to prevention by available infection-control strategies. These newly identified patterns of transmission of M. tuberculosis might be too complex to be detected and controlled by traditional approaches, and real-time M. tuberculosis genotyping capable of identifying unsuspected linkages among incident cases might be increasingly useful (131). This new TB threat, transmission in previously unknown settings, has emerged at a time when local TB-control programs often are not prepared to respond. As TB morbidity decreases in the United States and TB-control programs necessarily contract, new approaches will emerge, particularly in low-incidence areas. One model envisions that local public HCWs who do not work exclusively on TB are served by regional TB supervisors, who in turn are supported by statewide consultants and CDC specialists (172). Research Needs to Enhance TB ControlImplementation of the recommendations contained in this statement will likely improve TB control and allow progress to be made toward eliminating TB in the United States. However, achieving TB elimination as defined by ACET (i.e., one annual case of TB per one million population [11]) will require substantial advancements in the technology of diagnosis, treatment, and prevention of the disease. IOM has estimated that at the current rate of decline, approximately 6% annually, eliminating TB in the United States would take >70 years (2). New tools are needed for the diagnosis, treatment, and prevention of TB to accelerate the decline in TB incidence and reach the elimination threshold sooner (1,2,45). In addition, improved tests for the diagnosis of TB and LTBI and more effective drugs to treat them are needed to reduce the substantial worldwide burden of disease and death resulting from TB (44). AFB smear microscopy and the tuberculin skin test, the most commonly used tests for the diagnosis of TB and latent infection respectively, derive from technology developed in the 19th Century; the only available vaccine against TB, BCG, dates from the early 20th Century; and rifampin, the most recent novel compound for treatment of TB, was introduced in 1963. In the long term, the development of a new and effective vaccine would have the greatest impact on the global epidemic of TB, and the United States should lead the research and advocacy efforts to develop such a vaccine (180,440). However, other advances in TB diagnosis and treatment might substantially improve the control of TB in the United States. Better means to diagnose and treat LTBI are needed immediately. Breakthrough diagnostics and drugs that would facilitate the more effective usage of this therapeutic intervention to prevent TB would have an immediate and lasting effect on the incidence of the disease in the United States by affecting at least three of the major challenges to TB control in the United States: the substantial pool of persons with LTBI, TB among foreign-born persons, and TB among contacts of persons with infectious TB (Box 1). Public health interventions to control TB should be based on practices that have been demonstrated to be effective. Because an established scientific basis is lacking for certain fundamental principles of TB control, including certain recommendations contained in this statement, logic, experience, and expert opinion have been used to guide clinical and public health practice to control TB. In the preparation of these recommendations for TB control, deficiencies in evidence were frequently noted. Better understanding is needed of which persons among the millions of foreign-born persons that enter the United States each year (Table 8) are at sufficient additional risk for TB to warrant public health intervention. The approaches recommended for the development of programs for targeted testing of LTBI need additional verification. The new concepts of identifying contacts of infectious TB cases (439) require refinement. The optimal method of reducing the concentration of M. tuberculosis in ambient air in venues such as homeless shelters is not yet defined (438). Methods to monitor and evaluate TB control programs, and in particular, new activities such outbreak surveillance and response (441), should be delineated and standardized. The epidemiology of TB in the United States is constantly changing. Recent examples, as noted throughout this statement, are the increase in TB among foreign-born persons, the upsurge in reports of TB outbreaks, and the persistent high incidence of the disease among U.S.-born non-Hispanic blacks. Epidemiologic studies, including economic analyses, are needed to augment surveillance data and facilitate decisions about allocation of effort and resources to address newly identified facets of the epidemiology of TB. As new diagnostics are introduced to TB control, operational, economic, and behavioral studies will be needed to determine their most effective use. For example, QFT, a new diagnostic test for LTBI, was licensed in 2001, and early research indicated that this new test might have advantages over the tuberculin skin test in distinguishing between latent M. tuberculosis infection and infection with nontuberculous mycobacteria or vaccination with BCG (102). However, guidelines on testing for LTBI recommended that QFT should not be used in the evaluation of contacts of infectious cases of TB, for children aged <17 years, for pregnant women, or for patients with immunocompromising conditions, including HIV infection, because of a lack of data from studies in those populations (103). A newer version of the test, QFT-G, was licensed in 2004. The role of this new test in these populations has not been determined Thus, considerable research remains to be done to delineate the advantages this new test can bring to TB control. Despite the best efforts of national, state, and local TB programs, nonadherence to prescribed treatment for TB and latent infection remains a major barrier to TB elimination. As evidence of the importance of that intervention, completion of a course of treatment is the first national performance standard for TB (Table 4). For the outcome of TB treatment to be improved, both patient and health-care provider behaviors related to adherence to TB treatment must be understood, and that understanding should be used to design and implement methods for improving adherence. Although considerable research has been conducted in this field, no comprehensive effort has been undertaken to examine and compile the results and identify best practices. Gaps in knowledge remain, and the need exists to develop and implement a comprehensive behavioral and social science research agenda to address these deficiencies. Graded Recommendations for the Control and Prevention of Tuberculosis (TB)Recommendations for TB Laboratory Services
Case Detection and Primary Prevention Strategy
Case Management
Contact Investigation
Targeted Testing and Treatment of LTBI
Surveillance
Case Detection
Case Management
Contact Investigation
Targeted Testing and Treatment of LTBI
HIV Counseling and Testing
Case Detection
Case Management
Contact Investigation
Targeted Testing and Treatment of LTBI
Institutional Infection Control
Surveillance and Case Detection
Case Management
Contact Investigation
Targeted Testing and Treatment of LTBI
Institutional and Environmental Controls
Case Detection and Case Management
Contact Investigation
Targeted Testing and Treatment of LTBI
Institutional Infection Control
Acknowledgments The following persons provided constructive and helpful insights: WJ Burman, MD, Denver Public Health, Denver, Colorado. EP Desmond, MD, California Department of Health Services, Richmond; PC Hopewell, MD, San Francisco General Hospital, University of California, San Francisco; A Green Rush, Francis J. Curry National Tuberculosis Center, San Francisco, California. RJ O'Brien, MD, Foundation for Innovative New Diagnostics, Geneva, Switzerland. T Oemig, Wisconsin Division of Public Health, Madison, Wisconsin. PM Small, MD, The Bill and Melinda Gates Foundation, Seattle; G Wang, MD, Puget Sound Neighborhood Health Centers, Seattle, Washington. KG Castro, MD, MF Iademarco, MD, L Nelson, MD, TR Navin, MD, T Shinnick, MD, Div of TB Elimination, National Center for HIV, STD, & TB Prevention, Coordinating Center for Infectious Diseases, CDC. A Lipavsky and B Nodell also assisted in the preparation of this report. References
* Wisconsin Department of Health and Family Services. HFS 145. Control of Communicable Diseases. Available at http://www.legis.state.wi.us/rsb/code/hfs/hfs145.pdf. † Draft Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings. Federal Register 2004;69:70457--8. § Personal Protective Equipment, 29 C.F.R. Sect. 1910.134 (2003). Terms and Abbreviations Used in This Report
Joint Subcommittee of the American Thoracic Society (ATS), the Infectious Diseases Society
of America (ISDA), and CDC Chair: Charles M. Nolan, MD, Seattle-King County Department of Public Health, Seattle, Washington (American Thoracic Society). Co-chairs: Henry M. Blumberg, MD, Emory University School of Medicine, Atlanta, Georgia (Infectious Diseases Society of America), Zachary Taylor, MD, National Center for HIV, STD, and TB Prevention, CDC, Atlanta, Georgia. Members: John Bernardo, MD, Boston University School of Medicine, Boston, Massachusetts; Patrick J. Brennan, MD, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania; Nancy E. Dunlap, MD, PhD, University of Alabama at Birmingham, Birmingham, Alabama; Charles L. Daley, MD, National Jewish Medical and Research Center, Denver, Colorado; Wafaa M. El-Sadr, MD, Harlem Hospital and Columbia University, New York City, New York; Sue Etkind, MS, Massachusetts Department of Public Health, Jamaica Plain, Massachusetts; Mark FitzGerald, MD, University of British Columbia, Vancouver, British Columbia, Canada; James B. McAuley, MD, Rush Medical College, Chicago, Illinois; Marisa Moore, MD, Centers for Disease Control and Prevention, Atlanta, Georgia; Noreen L. Qualls, DrPH, Centers for Disease Control and Prevention, Atlanta, Georgia; Randall R. Reves, MD, Denver Public Health, Denver, Colorado; Sarah E. Royce, MD, California Department of Health Services, Berkeley, California; Max Salfinger, MD, Wadsworth Center, New York State Department of Health, Albany, New York; Jeffrey R. Starke, MD, Texas Children's Hospital and Baylor College of Medicine, Houston, Texas; Wanda Walton, PhD, Centers for Disease Control and Prevention, Atlanta, Georgia; Stephen E. Weis, DO, University of North Texas Health Science Center at Fort Worth, Fort Worth, Texas; and Jan Young, MS, California Department of Health Services, Berkeley, California. Table 1 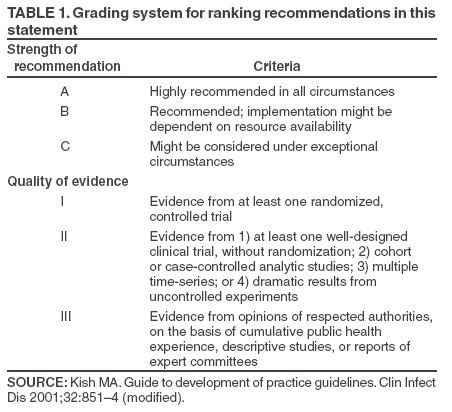 Return to top. Figure 1 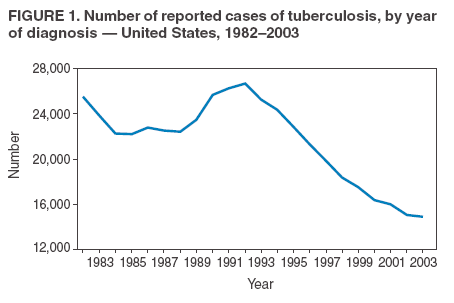 Return to top. Box 1 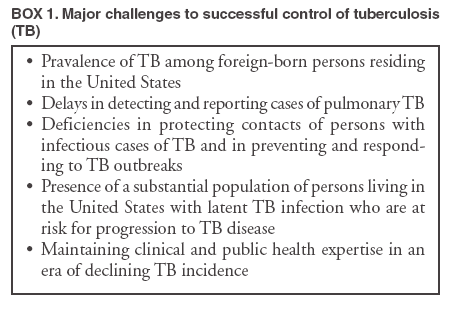 Return to top. Table 2 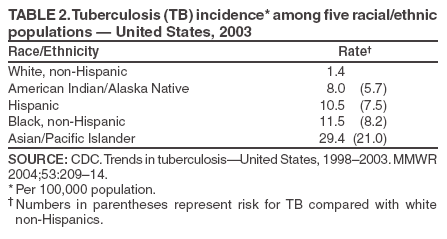 Return to top. Figure 2 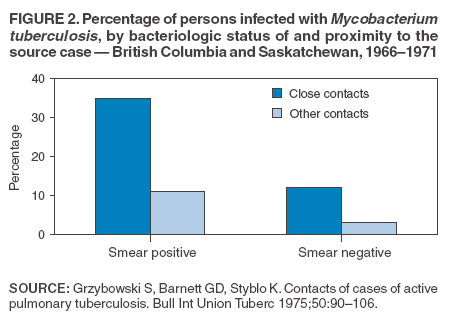 Return to top. Box 2 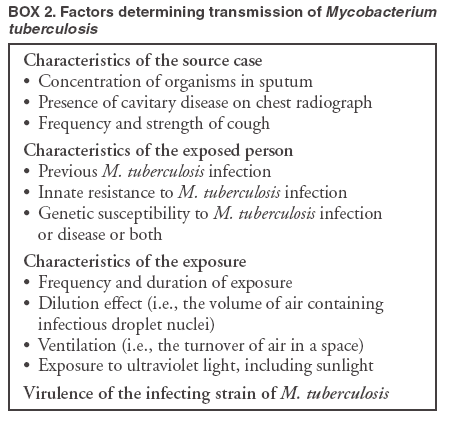 Return to top. Table 3  Return to top. Figure 3 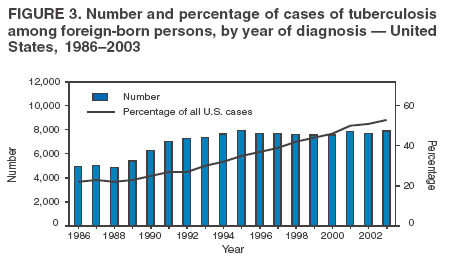 Return to top. Box 3 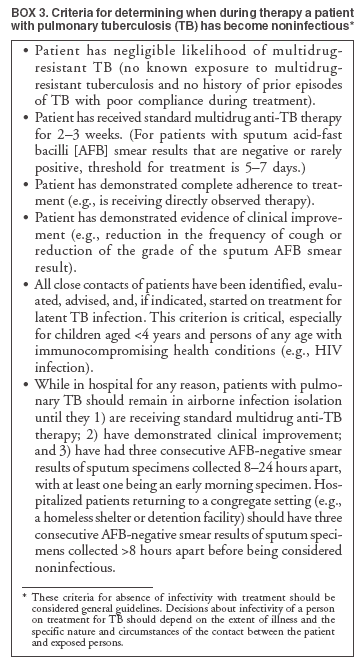 Return to top. Table 4 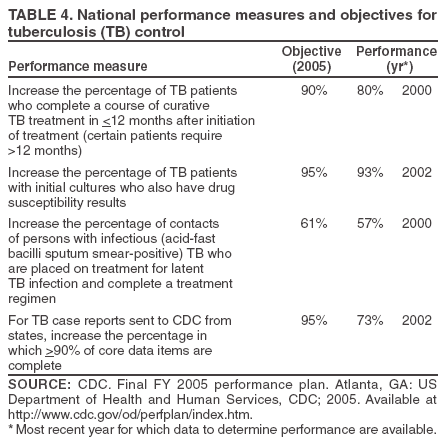 Return to top. Figure 4  Return to top. Box 4 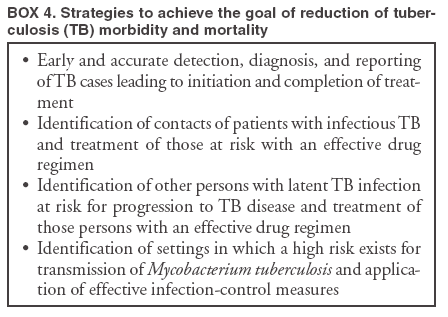 Return to top. Table 5 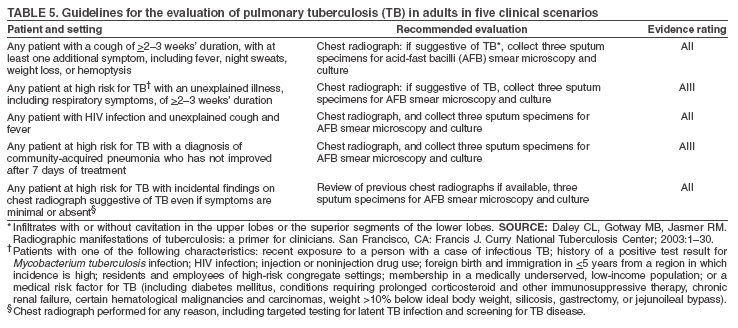 Return to top. Box 5 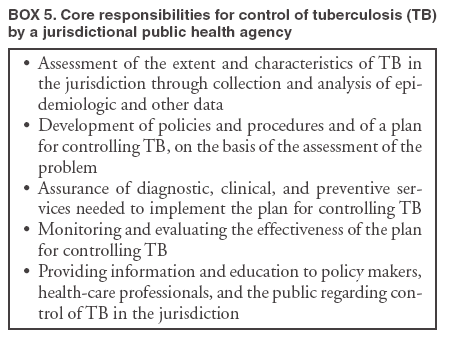 Return to top. Table 6 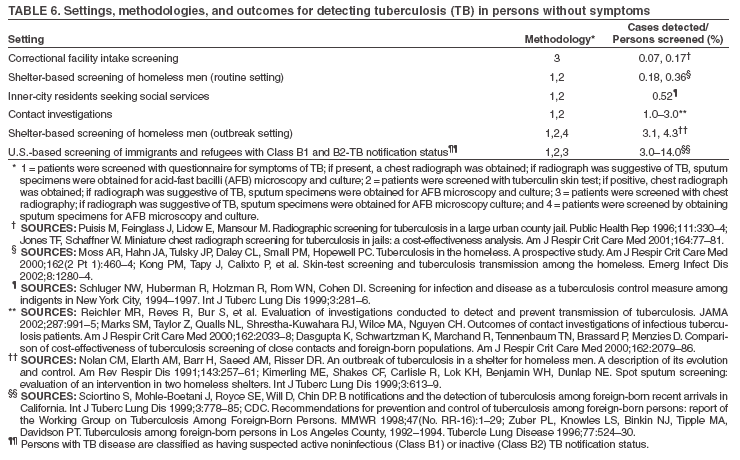 Return to top. Box 6 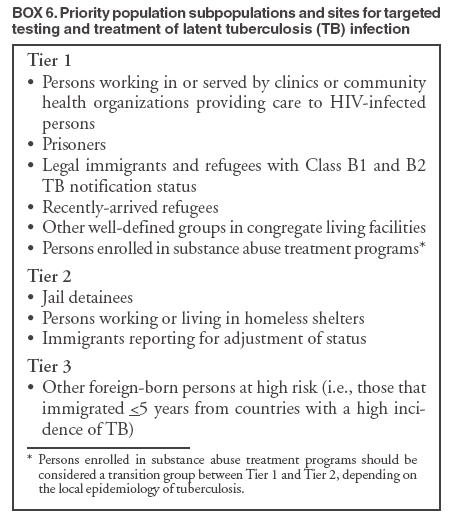 Return to top. Table 7 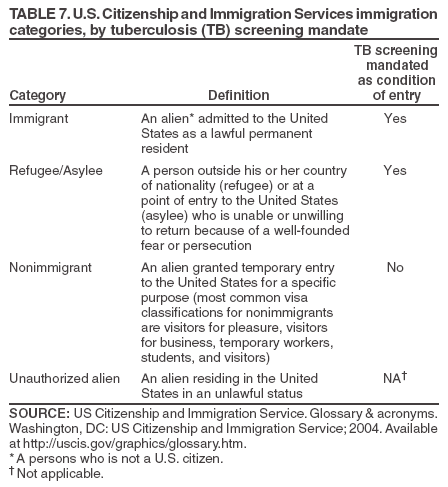 Return to top. Box 7 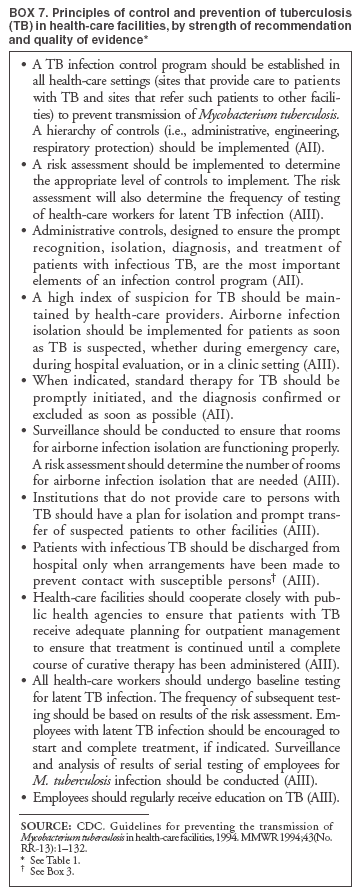 Return to top. Table 8  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/19/2005 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|