 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention and Control of InfluenzaRecommendations of the Advisory Committee on Immunization Practices (ACIP)An erratum has been published for this article. To view the erratum, please click here. Prepared by The material in this report was prepared for publication by the National Center for Infectious Diseases, James M. Hughes, M.D., Director; Division of Viral and Rickettsial Diseases, James W. LeDuc, Ph.D., Acting Director; the National Immunization Program, Walter A. Orenstein, M.D., Director; and the Epidemiology and Surveillance Division, Melinda Wharton, M.D., Director. SummaryThis report updates the 2001 recommendations by the Advisory Committee on Immunization Practices (ACIP) regarding the use of influenza vaccine and antiviral agents (MMWR 2001;50[No. RR-4]:1--44). The 2002 recommendations include new or updated information regarding 1) the timing of influenza vaccination by risk group; 2) influenza vaccine for children aged 6--23 months; 3) the 2002--2003 trivalent vaccine virus strains: A/Moscow/10/99 (H3N2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Hong Kong/330/2001-like strains; and 4) availability of certain influenza vaccine doses with reduced thimerosal content. A link to this report and other information related to influenza can be accessed at the website for the Influenza Branch, Division of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC, at http://www.cdc.gov/ncidod/diseases/flu/fluvirus.htm. IntroductionEpidemics of influenza typically occur during the winter months and are responsible for an average of approximately 20,000 deaths/year in the United States (1,2). Influenza viruses also can cause pandemics, during which rates of illness and death from influenza-related complications can increase dramatically worldwide. Influenza viruses cause disease among all age groups (3--5). Rates of infection are highest among children, but rates of serious illness and death are highest among persons aged >65 years and persons of any age who have medical conditions that place them at increased risk for complications from influenza (3,6--8). Influenza vaccination is the primary method for preventing influenza and its severe complications. In this report from the Advisory Committee on Immunization Practices (ACIP), the primary target groups recommended for annual vaccination are 1) groups who are at increased risk for influenza-related complications (e.g., persons aged >65 years and persons of any age with certain chronic medical conditions); 2) persons aged 50--64 years, because this group has an elevated prevalence of certain chronic medical conditions; and 3) persons who live with or care for persons at high risk (e.g., health-care workers and household members who have frequent contact with persons at high risk and can transmit influenza to persons at high risk). Vaccination is associated with reductions in influenza-related respiratory illness and physician visits among all age groups, hospitalization and death among persons at high risk, otitis media among children, and work absenteeism among adults (9--18). Although influenza vaccination levels increased substantially during the 1990s, further improvements in vaccine coverage levels are needed, chiefly among persons aged <65 years at high risk. The ACIP recommends using strategies to improve vaccination levels, including using reminder/recall systems and standing orders programs (19,20). Although influenza vaccination remains the cornerstone for the control and treatment of influenza, information is also presented regarding antiviral medications, because these agents are an adjunct to vaccine. Primary Changes and Updates in the RecommendationsThe 2002 recommendations include five principal changes or updates, as follows:
Influenza and Its BurdenBiology of Influenza Influenza A and B are the two types of influenza viruses that cause epidemic human disease (21). Influenza A viruses are further categorized into subtypes on the basis of two surface antigens: hemagglutinin (H) and neuraminidase (N). Influenza B viruses are not categorized into subtypes. Since 1977, influenza A (H1N1) viruses, influenza A (H3N2) viruses, and influenza B viruses have been in global circulation. Influenza A (H1N2) viruses that probably emerged after genetic reassortment between human A (H3N2) and A (H1N1) viruses have been detected recently in many countries. Both influenza A and B viruses are further separated into groups on the basis of antigenic characteristics. New influenza virus variants result from frequent antigenic change (i.e., antigenic drift) resulting from point mutations that occur during viral replication. Influenza B viruses undergo antigenic drift less rapidly than influenza A viruses. A person's immunity to the surface antigens, especially hemagglutinin, reduces the likelihood of infection and severity of disease if infection occurs (22). Antibody against one influenza virus type or subtype confers limited or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype (23). Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the incorporation of >1 new strains in each year's influenza vaccine. Clinical Signs and Symptoms of Influenza Influenza viruses are spread from person-to-person primarily through the coughing and sneezing of infected persons (21). The incubation period for influenza is 1--4 days, with an average of 2 days (24). Adults and children typically are infectious from the day before symptoms begin until approximately 5 days after illness onset. Children can be infectious for a longer period, and very young children can shed virus for <6 days before their illness onset. Severely immunocompromised persons can shed virus for weeks (25--27). Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms (e.g., fever, myalgia, headache, severe malaise, nonproductive cough, sore throat, and rhinitis) (28). Respiratory illness caused by influenza is difficult to distinguish from illness caused by other respiratory pathogens on the basis of symptoms alone (see Role of Laboratory Diagnosis). Reported sensitivities and specificities of clinical definitions for influenza-like illness that include fever and cough have ranged from 63% to 78% and 55% to 71%, respectively, compared with viral culture (29,30). Sensitivity and predictive value of clinical definitions can vary, depending on the degree of co-circulation of other respiratory pathogens and the level of influenza activity (31). Influenza illness typically resolves after a limited number of days for the majority of persons, although cough and malaise can persist for >2 weeks. Among certain persons, influenza can exacerbate underlying medical conditions (e.g., pulmonary or cardiac disease), lead to secondary bacterial pneumonia or primary influenza viral pneumonia, or occur as part of a coinfection with other viral or bacterial pathogens (32). Influenza infection has also been associated with encephalopathy, transverse myelitis, Reye syndrome, myositis, myocarditis, and pericarditis (32). Hospitalizations and Deaths from Influenza The risks for complications, hospitalizations, and deaths from influenza are higher among persons aged >65 years, very young children, and persons of any age with certain under-lying health conditions than among healthy older children and younger adults (1,2,7,9,33--35). Estimated rates of influenza-associated hospitalizations have varied substantially by age group in studies conducted during different influenza epidemics (Table 1). Among children aged 0--4 years, hospitalization rates have ranged from approximately 500/100,000 population for those with high-risk conditions to 100/100,000 population for those without high-risk conditions (36--39). Within the 0--4 age group, hospitalization rates are highest among children aged 0--1 years and are comparable to rates found among persons aged >65 years (38,39) (Table 1). During influenza epidemics from 1969--1970 through 1994--1995, the estimated overall number of influenza-associated hospitalizations in the United States ranged from approximately 16,000 to 220,000/epidemic. An average of approximately 114,000 influenza-related excess hospitalizations occurred per year, with 57% of all hospitalizations occurring among persons aged <65 years. Since the 1968 influenza A (H3N2) virus pandemic, the greatest numbers of influenza-associated hospitalizations have occurred during epidemics caused by type A (H3N2) viruses, with an estimated average of 142,000 influenza-associated hospitalizations per year (40). Influenza-related deaths can result from pneumonia as well as from exacerbations of cardiopulmonary conditions and other chronic diseases. In studies of influenza epidemics occurring from 1972--1973 through 1994--1995, excess deaths (i.e., the number of influenza-related deaths above a projected baseline of expected deaths) occurred during 19 of 23 influenza epidemics (41) (unpublished data, Influenza Branch, Division of Viral and Rickettsial Diseases [DVRD], National Center for Infectious Diseases [NCID], CDC, 1998). During those 19 influenza seasons, estimated rates of influenza-associated deaths ranged from approximately 30 to >150 deaths/100,000 persons aged >65 years (unpublished data, Influenza Branch, DVRD, NCID, CDC, 1998). Older adults account for >90% of deaths attributed to pneumonia and influenza (42). From 1972--1973 through 1994--1995, >20,000 influenza-associated deaths were estimated to occur during each of 11 different U.S. epidemics, and >40,000 influenza-associated deaths were estimated for each of 6 of these 11 epidemics (41) (unpublished data, Influenza Branch, DVRD, NCID, CDC, 1998). In the United States, pneumonia and influenza deaths might be increasing in part because the number of older persons is increasing (43). Options for Controlling InfluenzaIn the United States, the main option for reducing the impact of influenza is immunoprophylaxis with inactivated (i.e., killed virus) vaccine (see Recommendations for Using Influenza Vaccine). Vaccinating persons at high risk for complications each year before seasonal increases in influenza virus circulation is the most effective means of reducing the impact of influenza. Vaccination coverage can be increased by administering vaccine to persons during hospitalizations or routine health-care visits before the influenza season, rendering special visits to physicians' offices or clinics unnecessary. When vaccine and epidemic strains are well-matched, achieving increased vaccination rates among persons living in closed settings (e.g., nursing homes and other chronic-care facilities) and among staff can reduce the risk for outbreaks by inducing herd immunity (14). Vaccination of health-care workers and other persons in close contact with persons in groups at high risk can also reduce transmission of influenza and subsequent influenza-related complications. Using influenza-specific antiviral drugs for chemoprophylaxis or treatment of influenza is a key adjunct to vaccine (see Recommendations for Using Antiviral Agents for Influenza). However, antiviral medications are not a substitute for vaccination. Influenza vaccines are standardized to contain the hemagglutinins of strains (i.e., typically two type A and one type B), representing the influenza viruses likely to circulate in the United States in the upcoming winter. The vaccine is made from highly purified, egg-grown viruses that have been made noninfectious (i.e., inactivated) (44). Subvirion and purified surface-antigen preparations are available. Because the vaccine viruses are initially grown in embryonated hens' eggs, the vaccine might contain limited amounts of residual egg protein. Manufacturing processes differ by manufacturer. Manufacturers might use different compounds to inactivate influenza viruses and add antibiotics to prevent bacterial contamination. Package inserts should be consulted for additional information. Influenza vaccine distributed in the United States might also contain thimerosal, a mercury-containing compound, as the preservative (45). Thimerosal has been used as a preservative in vaccines since the 1930s. Although no evidence of harm caused by low levels of thimerosal in vaccines has been reported, in 1999, the U.S. Public Health Service and other organizations recommended that efforts be made to reduce the thimerosal content in vaccines to decrease total mercury exposure, chiefly among infants and pregnant woman (45,46). Since mid-2001, routinely administered, noninfluenza childhood vaccines for the U.S. market have been manufactured either without or with only trace amounts of thimerosal to provide a substantial reduction in the total mercury exposure from vaccines for children (47). For the 2002--2003 influenza season, a limited number of individually packaged doses (i.e., single-dose syringes) of reduced thimerosal-content influenza vaccine (<1 mcg thimerosal/0.5 mL-dose) will be available. Thus far, reduced thimerosal content vaccine is available from one manufacturer, Evans Vaccines. This manufacturer's vaccine is approved for use in persons aged >4 years (see Vaccine Use for Young Children, By Manufacturer). Multidose vials and single-dose syringes of influenza vaccine containing approximately 25 mcg thimerosal/0.5 mL-dose are also available as they have been in past years. Because of the known risks for severe illness from influenza infection and the benefits of vaccination, and because a substantial safety margin has been incorporated into the health guidance values for organic mercury exposure, the benefit of influenza vaccine with reduced or standard thimerosal content outweighs the theoretical risk, if any, from thimerosal (45,48). The removal of thimerosal from other vaccines further reduces the theoretical risk from thimerosal in influenza vaccines. The trivalent influenza vaccine recommended for the 2002--2003 season includes A/Moscow/10/99 (H3N2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Hong Kong/330/2001-like antigens. For the A/Moscow/10/99 (H3N2)-like antigen, manufacturers will use the antigenically equivalent A/Panama/2007/99 (H3N2) virus. For the B/Hong Kong/330/2001-like antigen, the actual B strains that will be included in the vaccine will be announced later. These viruses will be used because of their growth properties and because they are representative of influenza viruses likely to circulate in the United States during the 2002--2003 influenza season. Because circulating influenza A (H1N2) viruses are a reasortant of influenza A (H1N1) and (H3N2) viruses, antibody directed against influenza A (H1N1) and influenza (H3N2) vaccine strains will provide protection against circulating influenza A (H1N2) viruses. Effectiveness of Inactivated Influenza Vaccine The effectiveness of influenza vaccine depends primarily on the age and immunocompetence of the vaccine recipient and the degree of similarity between the viruses in the vaccine and those in circulation. The majority of vaccinated children and young adults develop high postvaccination hemagglutination-inhibition antibody titers (49--51). These antibody titers are protective against illness caused by strains similar to those in the vaccine (50--53). When the vaccine and circulating viruses are antigenically similar, influenza vaccine prevents influenza illness among approximately 70%--90% of healthy adults aged <65 years (10,13,54,55). Vaccination of healthy adults also has resulted in decreased work absenteeism and decreased use of health-care resources, including use of antibiotics, when the vaccine and circulating viruses are well-matched (10--13,55,56). Children as young as age 6 months can develop protective levels of antibody after influenza vaccination (49,50,57--60), although the antibody response among children at high risk might be lower than among healthy children (61,62). In a randomized study among children aged 1--15 years, inactivated influenza vaccine was 77%--91% effective against influenza respiratory illness and was 44%--49%, 74%--76%, and 70%--81% effective against influenza seroconversion among children aged 1--5, 6--10, and 11--15 years, respectively (51). One study (63) reported a vaccine efficacy of 56% against influenza illness among healthy children aged 3--9 years, and another study (64) found vaccine efficacy of 22%--54% and 60%--78% among children with asthma aged 2--6 years and 7--14 years, respectively. A 2-year randomized study of children aged 6--24 months determined that >89% of children seroconverted to all three vaccine strains during both years; vaccine efficacy was 66% (95% confidence intervals [CI] = 34% and 82%) against culture-confirmed influenza during year 1 among 411 children and was --7% (95% CI = --247% and 67%) during year 2 among 375 children. However, no overall reduction in otitis media was reported (65). Other studies report that using trivalent inactivated influenza vaccine decreases the incidence of influenza-associated otitis media among young children by approximately 30% (17,18). Older persons and persons with certain chronic diseases might develop lower postvaccination antibody titers than healthy young adults and thus can remain susceptible to influenza-related upper respiratory tract infection (66--68). A randomized trial among noninstitutionalized persons aged >60 years reported a vaccine efficacy of 58% against influenza respiratory illness, but indicated that efficacy might be lower among those aged >70 years (69). The vaccine can also be effective in preventing secondary complications and reducing the risk for influenza-related hospitalization and death (14--16,70). Among elderly persons living outside nursing homes or similar chronic-care facilities, influenza vaccine is 30%--70% effective in preventing hospitalization for pneumonia and influenza (16,71). Among elderly persons residing in nursing homes, influenza vaccine is most effective in preventing severe illness, secondary complications, and deaths. Among this population, the vaccine can be 50%--60% effective in preventing hospitalization or pneumonia and 80% effective in preventing death, although the effectiveness in preventing influenza illness often ranges from 30% to 40% (72,73). Cost-Effectiveness of Influenza Vaccine Influenza vaccination can reduce both health-care costs and productivity losses associated with influenza illness. Economic studies of influenza vaccination of persons aged >65 years conducted in the United States have reported overall societal cost-savings and substantial reductions in hospitalization and death (16,71,74). Studies of adults aged <65 years have reported that vaccination can reduce both direct medical costs and indirect costs from work absenteeism (9,11--13,55). Reductions of 34%--44% in physician visits, 32%--45% in lost workdays (11,13), and 25% in antibiotic use for influenza-associated illnesses have been reported (13). One cost-effectiveness analysis estimated a cost of approximately $60--$4,000/illness averted among healthy persons aged 18--64 years, depending on the cost of vaccination, the influenza attack rate, and vaccine effectiveness against influenza-like illness (55). Another cost-benefit economic model estimated an average annual savings of $13.66/person vaccinated (75). In the second study, 78% of all costs prevented were costs from lost work productivity, whereas the first study did not include productivity losses from influenza illness. Economic studies specifically evaluating the cost-effectiveness of vaccinating persons aged 50--64 years are not available, and the number of studies that examine the economics of routinely vaccinating children are limited (9,76--78). However, in a study that included all age groups, cost-utility improved with increasing age and among those with chronic medical conditions (9). Among persons aged >65 years, vaccination resulted in a net savings per quality-adjusted-life-year (QALY) gained and resulted in costs of $23--$256/QALY among younger age groups. Additional studies of the relative cost-effectiveness and cost-utility of influenza vaccination among children and among adults aged <65 years are needed and should be designed to account for year-to-year variations in influenza attack rates, illness severity, and vaccine efficacy when evaluating the long-term costs and benefits of annual vaccination. Vaccination Coverage Levels Among persons aged >65 years, influenza vaccination levels increased from 33% in 1989 (79) to 66% in 1999 (80), surpassing the Healthy People 2000 goal of 60% (81). Although 1999 influenza vaccination coverage reached the highest levels recorded among black, Hispanic, and white populations, vaccination levels among blacks and Hispanics continue to lag behind those among whites (80,82). In 1999, the influenza vaccination rates among persons aged >65 years were 68% among non-Hispanic whites, 50% among non-Hispanic blacks, and 55% among Hispanics (80). Possible reasons for the increase in influenza vaccination levels among persons aged >65 years through 1999 include greater acceptance of preventive medical services by practitioners, increased delivery and administration of vaccine by health-care providers and sources other than physicians, new information regarding influenza vaccine effectiveness, cost-effectiveness, and safety, and the initiation of Medicare reimbursement for influenza vaccination in 1993 (9,15,16,72,73,83,84). Influenza vaccination levels among persons interviewed during 2000 were not substantially different from 1999 levels among persons aged >65 years (64% in 2000 versus 66% in 1999) and persons aged 50--64 years (35% in 2000 versus 34% in 1999) (80). The percentage of adults interviewed during the first quarter of 2001 who reported influenza vaccination during the past 12 months was lower than the percentage reported by adults interviewed during the first quarter of 2000 (63% versus 68% among those aged >65 years; 32% versus 37% among those aged 50--64 years). Delays in influenza vaccine supply during fall 2000 probably contributed to these declines in vaccination levels (see Vaccine Supply). Continued annual monitoring is needed to determine the effects of vaccine supply delays and other factors on vaccination coverage among persons aged >50 years. The Healthy People 2010 objective is to achieve vaccination coverage for 90% of persons aged >65 years (85). Additional strategies are needed to achieve this Healthy People 2010 objective in all segments of the population and to reduce racial and ethnic disparities in vaccine coverage. In 1997 and 1998, vaccination rate estimates among nursing home residents were 64%--82% and 83%, respectively (86,87). The Healthy People 2010 goal is to achieve influenza vaccination of 90% of nursing home residents, an increase from the Healthy People 2000 goal of 80% (81,85). In 2000, the overall vaccination rate for adults aged 18--64 years with high-risk conditions was 32%, far short of the Healthy People 2000 goal of 60% (unpublished data, National Immunization Program [NIP], CDC, 2000) (81). Among persons aged 50--64 years, 44% of those with chronic medical conditions and 31% of those without chronic medical conditions received influenza vaccine. Only 25% of adults aged <50 years with high-risk conditions were vaccinated. Reported vaccination rates of children at high risk are low. One study conducted among patients in health maintenance organizations reported influenza vaccination rates ranging from 9% to 10% among children with asthma (88), and a rate of 25% was reported among children with severe-to-moderate asthma who attended an allergy and immunology clinic (89). However, a study conducted in a pediatric clinic demonstrated an increase in the vaccination rate of children with asthma or reactive airways disease of 5%--32% after implementing a reminder/recall system (90). Increasing vaccination coverage among persons who have high-risk conditions and are aged <65 years, including children at high risk, is the highest priority for expanding influenza vaccine use. Annual vaccination is recommended for health-care workers. Nonetheless, the National Health Interview Survey (NHIS) indicated vaccination rates of only 34% and 38% among health-care workers in the 1997 and 2000 surveys, respectively (91) (unpublished NHIS data, NIP, CDC, 2002). Vaccination of health-care workers has been associated with reduced work absenteeism (10) and fewer deaths among nursing home patients (92,93). Limited information is available regarding the use of influenza vaccine among pregnant women. Among women aged 18--44 years without diabetes responding to the 1999 Behavioral Risk Factor Surveillance Survey, those reporting they were pregnant were less likely to report influenza vaccination during the past 12 months (9.6%) than those not pregnant (15.7%). Vaccination coverage among pregnant women did not substantially change during 1997--1999, whereas coverage among nonpregnant women increased from 14.4% in 1997. Similar results were determined by using the 1997--2000 NHIS data, excluding pregnant women who reported diabetes, heart disease, lung disease, and other selected high-risk conditions (unpublished NHIS data, NIP, CDC, 2002). Although not directly measuring influenza vaccination among women who were past the second trimester of pregnancy during influenza season, these data indicate low compliance with the ACIP recommendations for pregnant women (94). In a study of influenza vaccine acceptance by pregnant women, 71% who were offered the vaccine chose to be vaccinated (95). However, a 1999 survey of obstetricians and gynecologists determined that only 39% gave influenza vaccine to obstetric patients, although 86% agree that pregnant women's risk for influenza-related morbidity and mortality increases during the last two trimesters (96). Recommendations for Using Influenza VaccineInfluenza vaccine is strongly recommended for any person aged >6 months who is at increased risk for complications from influenza. In addition, health-care workers and other persons (including household members) in close contact with persons at high risk should be vaccinated to decrease the risk for transmitting influenza to persons at high risk. Influenza vaccine also can be administered to any person aged >6 months to reduce the probability of becoming infected with influenza. Target Groups for VaccinationPersons at Increased Risk for Complications Vaccination is recommended for the following groups of persons who are at increased risk for complications from influenza:
Approximately 35 million persons in the United States are aged >65 years; an additional 10--14 million adults aged 50--64 years, 15--18 million adults aged 18--49 years, and 8 million children aged 6 months--17 years have >1 medical conditions that are associated with an increased risk for influenza-related complications (unpublished data, NIP, CDC, 2002). Persons Aged 50--64 Years Vaccination is recommended for persons aged 50--64 years because this group has an increased prevalence of persons with high-risk conditions. Approximately 43 million persons in the United States are aged 50--64 years, and 10--14 million (24%--32%) have >1 high-risk medical conditions (unpublished data, NIP, CDC, 2002). Influenza vaccine has been recommended for this entire age group to increase the low vaccination rates among persons in this age group with high-risk conditions. Age-based strategies are more successful in increasing vaccine coverage than patient-selection strategies based on medical conditions. Persons aged 50--64 years without high-risk conditions also receive benefit from vaccination in the form of decreased rates of influenza illness, decreased work absenteeism, and decreased need for medical visits and medication, including antibiotics (10--13). Further, 50 years is an age when other preventive services begin and when routine assessment of vaccination and other preventive services has been recommended (97,98). Persons Who Can Transmit Influenza to Those at High Risk Persons who are clinically or subclinically infected can transmit influenza virus to persons at high risk for complications from influenza. Decreasing transmission of influenza from caregivers to persons at high risk might reduce influenza-related deaths among persons at high risk. Evidence from two studies indicates that vaccination of health-care workers is associated with decreased deaths among nursing home patients (92,93). Vaccination of health-care workers and others in close contact with persons at high risk, including household members, is recommended. The following groups should be vaccinated:
In addition, because children aged 0--23 months are at increased risk for influenza-related hospitalization (37--39), vaccination is encouraged for their household contacts and out-of-home caretakers, particularly for contacts of children aged 0--5 months because influenza vaccines have not been approved by the U.S. Food and Drug Administration (FDA) for use among children aged <6 months (see Healthy Young Children). Additional Information Regarding Vaccination of Specific PopulationsPregnant Women Influenza-associated excess deaths among pregnant women were documented during the pandemics of 1918--1919 and 1957--1958 (99--102). Case reports and limited studies also indicate that pregnancy can increase the risk for serious medical complications of influenza as a result of increases in heart rate, stroke volume, and oxygen consumption; decreases in lung capacity; and changes in immunologic function (103--106). A study of the impact of influenza during 17 interpandemic influenza seasons demonstrated that the relative risk for hospitalization for selected cardiorespiratory conditions among pregnant women enrolled in Medicaid increased from 1.4 during weeks 14--20 of gestation to 4.7 during weeks 37--42 in comparison with women who were 1--6 months postpartum (107). Women in their third trimester of pregnancy were hospitalized at a rate (i.e., 250/100,000 pregnant women) comparable with that of nonpregnant women who had high-risk medical conditions. By using data from this study, researchers estimated that an average of 1--2 hospitalizations could be prevented for every 1,000 pregnant women vaccinated. Because of the increased risk for influenza-related complications, women who will be beyond the first trimester of pregnancy (>14 weeks of gestation) during the influenza season should be vaccinated. Certain providers prefer to administer influenza vaccine during the second trimester to avoid a coincidental association with spontaneous abortion, which is common in the first trimester, and because exposures to vaccines traditionally have been avoided during the first trimester (108). Pregnant women who have medical conditions that increase their risk for complications from influenza should be vaccinated before the influenza season, regardless of the stage of pregnancy. A study of influenza vaccination of >2,000 pregnant women demonstrated no adverse fetal effects associated with influenza vaccine (109). However, additional data are needed to confirm the safety of vaccination during pregnancy. The majority of influenza vaccine distributed in the United States contains thimerosal, a mercury-containing compound, as a preservative, but influenza vaccine with reduced thimerosal content might be available in limited quantities (see Influenza Vaccine Composition). Thimerosal has been used in U.S. vaccines since the 1930s. No data or evidence exists of any harm caused by the level of mercury exposure that might occur from influenza vaccination. Because pregnant women are at increased risk for influenza-related complications and because a substantial safety margin has been incorporated into the health guidance values for organic mercury exposure, the benefit of influenza vaccine with reduced or standard thimerosal content outweighs the potential risk, if any, for thimerosal (45,48). Persons Infected with HIV Limited information is available regarding the frequency and severity of influenza illness or the benefits of influenza vaccination among persons with HIV infection (110,111). However, a retrospective study of young and middle-aged women enrolled in Tennessee's Medicaid program found that the attributable-risk for cardiopulmonary hospitalizations among women with HIV infection was higher during influenza seasons than during the peri-influenza periods. The risk for hospitalization was higher for HIV-infected women than for women with other well-recognized high-risk conditions, including chronic heart and lung diseases (112). Another study estimated that the risk for influenza-related death was 9.4--14.6/10,000 persons with AIDS, compared with rates of 0.09--0.10/10,000 among all persons aged 25--54 years and 6.4--7.0/10,000 among persons aged >65 years (113). Other reports demonstrate that influenza symptoms might be prolonged and the risk for complications from influenza increased for certain HIV-infected persons (114--116). Influenza vaccination has been demonstrated to produce substantial antibody titers against influenza among vaccinated HIV-infected persons who have minimal acquired immunodeficiency syndrome-related symptoms and high CD4+ T-lymphocyte cell counts (117--120). A limited, randomized, placebo-controlled trial determined that influenza vaccine was highly effective in preventing symptomatic, laboratory-confirmed influenza infection among HIV-infected persons with a mean of 400 CD4+ T-lymphocyte cells/mm3; a limited number of persons with CD4+ T-lymphocyte cell counts of <200 were included in that study (111). A nonrandomized study among HIV-infected persons determined that influenza vaccination was most effective among persons with >100 CD4+ cells and among those with <30,000 viral copies of HIV type 1/mL (116). Among patients who have advanced HIV disease and low CD4+ T-lymphocyte cell counts, influenza vaccine might not induce protective antibody titers (119,120); a second dose of vaccine does not improve the immune response in these persons (120,121). One study reported that HIV RNA levels increased transiently in one HIV-infected patient after influenza infection (122). Studies have demonstrated a transient (i.e., 2--4-week) increase in replication of HIV-1 in the plasma or peripheral blood mononuclear cells of HIV-infected persons after vaccine administration (119,123). Other studies using similar laboratory techniques have not documented a substantial increase in the replication of HIV (124--126). Deterioration of CD4+ T-lymphocyte cell counts or progression of HIV disease have not been demonstrated among HIV-infected persons after influenza vaccination compared with unvaccinated persons (120,127). Limited information is available concerning the effect of antiretroviral therapy on increases in HIV RNA levels after either natural influenza infection or influenza vaccination (110,128). Because influenza can result in serious illness and because influenza vaccination can result in the production of protective antibody titers, vaccination will benefit HIV-infected patients, including HIV-infected pregnant women. Breast-Feeding Mothers Influenza vaccine does not affect the safety of mothers who are breast-feeding or their infants. Breast-feeding does not adversely affect the immune response and is not a contraindication for vaccination. The risk for exposure to influenza during travel depends on the time of year and destination. In the tropics, influenza can occur throughout the year. In the temperate regions of the Southern Hemisphere, the majority of influenza activity occurs during April--September. In temperate climate zones of the Northern and Southern Hemispheres, travelers also can be exposed to influenza during the summer, especially when traveling as part of organized tourist groups that include persons from areas of the world where influenza viruses are circulating. Persons at high risk for complications of influenza who were not vaccinated with influenza vaccine during the preceding fall or winter should consider receiving influenza vaccine before travel if they plan to
No information is available regarding the benefits of revaccinating persons before summer travel who were already vaccinated in the preceding fall. Persons at high risk who received the previous season's vaccine before travel should be revaccinated with the current vaccine in the following fall or winter. Persons aged >50 years and others at high risk might wish to consult with their physicians before embarking on travel during the summer to discuss the symptoms and risks for influenza and the advisability of carrying antiviral medications for either prophylaxis or treatment of influenza. General Population In addition to the groups for which annual influenza vaccination is recommended, physicians should administer influenza vaccine to any person who wishes to reduce the likelihood of becoming ill with influenza (the vaccine can be administered to children aged >6 months), depending on vaccine availability (see Vaccine Supply). Persons who provide essential community services should be considered for vaccination to minimize disruption of essential activities during influenza outbreaks. Students or other persons in institutional settings (e.g., those who reside in dormitories) should be encouraged to receive vaccine to minimize the disruption of routine activities during epidemics. Studies indicate that rates of hospitalization are higher among young children than older children when influenza viruses are in circulation (36--38,129,130). The increased rates of hospitalization are comparable with rates for other groups considered at high risk for influenza-related complications. However, the interpretation of these findings has been confounded by co-circulation of respiratory syncytial viruses, which are a cause of serious respiratory viral illness among children and which frequently circulate during the same time as influenza viruses (131--133). Two recent studies have attempted to separate the effects of respiratory syncytial viruses and influenza viruses on rates of hospitalization among children who do not have high-risk conditions (37,38). Both studies reported that otherwise healthy children aged <2 years, and possibly children aged 2--4 years, are at increased risk for influenza-related hospitalization compared with older healthy children (Table 1). Among the Tennessee Medicaid population during 1973--1993, healthy children aged 6 months--<3 years had rates of influenza-associated hospitalization comparable with or higher than rates among children aged 3--14 years with high-risk conditions (Table 1) (37,39). Because children aged 6--23 months are at substantially increased risk for influenza-related hospitalizations, influenza vaccination of all children in this age group is encouraged when feasible. However, before a full recommendation to annually vaccinate all children aged 6--23 months can be made, ACIP, the American Academy of Pediatrics, and the American Academy of Family Physicians recognize that certain key concerns must be addressed. These concerns include increasing efforts to educate parents and providers regarding the impact of influenza and the potential benefits and risks of vaccination among young children, clarification of practical strategies for annual vaccination of children, certain ones of whom will require two doses within the same season, and reimbursement for vaccination. ACIP will provide updated information as these concerns are addressed. A full recommendation could be made by 2003--2005. In the interim, ACIP continues to strongly recommend influenza vaccination of adults and children aged >6 months who have high-risk medical conditions. The current inactivated influenza vaccine is not approved by FDA for use among children aged <6 months, the pediatric group at greatest risk for influenza-related complications (37). Vaccinating their household contacts and out-of-home caretakers might decrease the probability of influenza among these children. Persons Who Should Not Be VaccinatedInactivated influenza vaccine should not be administered to persons known to have anaphylactic hypersensitivity to eggs or to other components of the influenza vaccine without first consulting a physician (see Side Effects and Adverse Reactions). Prophylactic use of antiviral agents is an option for preventing influenza among such persons. However, persons who have a history of anaphylactic hypersensitivity to vaccine components but who are also at high risk for complications from influenza can benefit from vaccine after appropriate allergy evaluation and desensitization. Information regarding vaccine components can be found in package inserts from each manufacturer. Persons with acute febrile illness usually should not be vaccinated until their symptoms have abated. However, minor illnesses with or without fever do not contraindicate the use of influenza vaccine, particularly among children with mild upper respiratory tract infection or allergic rhinitis. Timing of Annual VaccinationVaccination in October and November The optimal time to vaccinate is usually during October--November. However, because of substantial vaccine distribution delays during the 2000--2001 and 2001--2002 influenza seasons and the possibility of similar situations in future years, ACIP recommends that vaccine providers focus their vaccination efforts in October and earlier on persons at high risk and health-care workers. Vaccination of children aged <9 years who are receiving vaccine for the first time should also begin in October because they need a booster dose 1 month after the initial dose. Vaccination of all other groups should begin in November, including household members of persons at high risk, healthy persons aged 50--64 years, and other persons who wish to decrease their risk for influenza infection. Materials to assist providers in prioritizing early vaccination are available at http://www.cdc.gov/nip/flu/Provider.htm (for information regarding vaccination of travelers, see the Travelers section in this report). Vaccination in December and Later After November, certain persons who should or want to receive influenza vaccine remain unvaccinated. In addition, substantial amounts of vaccine have remained unused during the past two influenza seasons. To improve vaccine coverage and use, chiefly among persons at high risk and health-care workers, influenza vaccine should continue to be offered in December and throughout the influenza season as long as vaccine supplies are available, even after influenza activity has been documented in the community. In the United States, seasonal influenza activity can begin to increase as early as November or December, but influenza activity has not reached peak levels in the majority of recent seasons until late December through early March (Table 2). Therefore, although the timing of influenza activity can vary by region, vaccine administered after November is likely to be beneficial in the majority of influenza seasons. Adults develop peak antibody protection against influenza infection 2 weeks after vaccination (134,135). Vaccination Before October To avoid missed opportunities for vaccination of persons at high risk for serious complications, such persons should be offered vaccine beginning in September during routine health-care visits or during hospitalizations, if vaccine is available. In facilities housing older persons (e.g., nursing homes), vaccination before October typically should be avoided because antibody levels in such persons can begin to decline within a limited time after vaccination (136). Timing of Organized Vaccination Campaigns Persons planning substantial organized vaccination campaigns should consider scheduling these events after mid-October because the availability of vaccine in any location cannot be ensured consistently in the early fall. Scheduling campaigns after mid-October will minimize the need for cancellations because vaccine is unavailable. Campaigns conducted before November should focus efforts on vaccination of persons at high risk, health-care workers, and household contacts of persons at high-risk to the extent feasible. DosageDosage recommendations vary according to age group (Table 3). Among previously unvaccinated children aged <9 years, two doses administered >1 months apart are recommended for satisfactory antibody responses. If possible, the second dose should be administered before December. Among adults, studies have indicated limited or no improvement in antibody response when a second dose is administered during the same season (137--139). Even when the current influenza vaccine contains >1 antigens administered in previous years, annual vaccination with the current vaccine is necessary because immunity declines during the year after vaccination (140,141). Vaccine prepared for a previous influenza season should not be administered to provide protection for the current season. Vaccine Use Among Young Children, By ManufacturerProviders should use influenza vaccine that has been approved by FDA for vaccinating children aged 6 months--3 years. Influenza vaccines from Wyeth Laboratories, Inc. (Flushield®) and Aventis Pasteur, Inc. (Fluzone® split-virus) are approved for use among persons aged >6 months. Influenza vaccine from Evans Vaccines Ltd. (Fluvirin®) is labeled in the United States for use only among persons aged >4 years because data to demonstrate efficacy among younger persons have not been provided to FDA. RouteThe intramuscular route is recommended for influenza vaccine. Adults and older children should be vaccinated in the deltoid muscle. A needle length of >1 inches can be considered for these age groups because needles <1 inch might be of insufficient length to penetrate muscle tissue in certain adults and older children (142). Infants and young children should be vaccinated in the anterolateral aspect of the thigh (47). Side Effects and Adverse ReactionsWhen educating patients regarding potential side effects, clinicians should emphasize that 1) inactivated influenza vaccine contains noninfectious killed viruses and cannot cause influenza; and 2) coincidental respiratory disease unrelated to influenza vaccination can occur after vaccination. Local Reactions In placebo-controlled studies among adults, the most frequent side effect of vaccination is soreness at the vaccination site (affecting 10%--64% of patients) that lasts <2 days (13,143--145). These local reactions typically are mild and rarely interfere with the person's ability to conduct usual daily activities. One study (62) reported 20%--28% of asthmatic children aged 9 months--18 years had local pain and swelling and another study (60) reported that 23% of children aged 6 months--4 years with chronic heart or lung disease had local reactions. A different study (59) reported no difference in local reactions among 53 children aged 6 months--6 years with high-risk medical conditions or among 305 healthy children aged 3--12 years in a placebo-controlled trial of inactivated influenza vaccine. In a study of 12 children aged 5--32 months, no substantial local or systemic reactions were noted (146). Systemic Reactions Fever, malaise, myalgia, and other systemic symptoms can occur after vaccination and most often affect persons who have had no prior exposure to the influenza virus antigens in the vaccine (e.g., young children) (147,148). These reactions begin 6--12 hours after vaccination and can persist for 1--2 days. Recent placebo-controlled trials demonstrate that among older persons and healthy young adults, administration of split-virus influenza vaccine is not associated with higher rates of systemic symptoms (e.g., fever, malaise, myalgia, and headache) when compared with placebo injections (13,143--145). Less information from published studies is available for children compared with adults. In a study of 791 healthy children (51), postvaccination fever was noted among 11.5% of children aged 1--5 years, 4.6% among children aged 6--10 years, and 5.1% among children aged 11--15 years. Among children at high risk, one study of 52 children aged 6 months--4 years reported fever among 27% and irritability and insomnia among 25% (60); a study among 33 children aged 6--18 months reported that one child had irritability and one had a fever and seizure after vaccination (149). No placebo comparison was made in these studies. However, in pediatric trials of A/New Jersey/76 swine influenza vaccine, no difference occurred between placebo and split-virus vaccine groups in febrile reactions after injection, although the vaccine was associated with mild local tenderness or erythema (59). Limited data regarding potential adverse events after influenza vaccination are available from the Vaccine Adverse Event Reporting System (VAERS). During January 1, 1991--July 16, 2001, VAERS received 789 reports of adverse events among children aged <18 years, including 89 reporting adverse events among children aged 6--23 months. The number of influenza vaccine doses received by children during this time period is unknown. The most frequently reported events were fever, injection-site reactions, and rash (unpublished data, CDC, 2001). Because of the limitations of spontaneous reporting systems, determining causality for specific types of adverse events, with the exception of injection-site reactions, is usually not possible by using VAERS data alone. Immediate --- presumably allergic --- reactions (e.g., hives, angioedema, allergic asthma, and systemic anaphylaxis) rarely occur after influenza vaccination (150). These reactions probably result from hypersensitivity to certain vaccine components; the majority of reactions probably are caused by residual egg protein. Although influenza vaccines contain only a limited quantity of egg protein, this protein can induce immediate hypersensitivity reactions among persons who have severe egg allergy. Persons who have experienced hives, have had swelling of the lips or tongue, or have experienced acute respiratory distress or collapse after eating eggs should consult a physician for appropriate evaluation to help determine if vaccine should be administered. Persons who have documented immunoglobulin E (IgE)-mediated hypersensitivity to eggs --- including those who have had occupational asthma or other allergic responses to egg protein --- might also be at increased risk for allergic reactions to influenza vaccine, and consultation with a physician should be considered. Protocols have been published for safely administering influenza vaccine to persons with egg allergies (151,152). Hypersensitivity reactions to any vaccine component can occur. Although exposure to vaccines containing thimerosal can lead to induction of hypersensitivity, the majority of patients do not experience reactions to thimerosal when it is administered as a component of vaccines, even when patch or intradermal tests for thimerosal indicate hypersensitivity (153,154). When reported, hypersensitivity to thimerosal usually has consisted of local, delayed-type hypersensitivity reactions (153). Guillain-Barré Syndrome The 1976 swine influenza vaccine was associated with an increased frequency of Guillain-Barré syndrome (GBS) (155,156). Among persons who received the swine influenza vaccine in 1976, the rate of GBS that exceeded the background rate was <10 cases/1,000,000 persons vaccinated with the risk of influenza vaccine-associated GBS higher among persons aged >25 years than persons <25 years (155). Evidence for a causal relationship of GBS with subsequent vaccines prepared from other influenza viruses is unclear. Obtaining strong epidemiologic evidence for a possible limited increase in risk is difficult for such a rare condition as GBS, which has an annual incidence of 10--20 cases/1,000,000 adults (157), and stretches the limits of epidemiologic investigation. More definitive data probably will require the use of other methodologies (e.g., laboratory studies of the pathophysiology of GBS). During three of four influenza seasons studied during 1977--1991, the overall relative risk estimates for GBS after influenza vaccination were slightly elevated but were not statistically significant in any of these studies (158--160). However, in a study of the 1992--1993 and 1993--1994 seasons, the overall relative risk for GBS was 1.7 (95% confidence interval = 1.0--2.8; p = 0.04) during the 6 weeks after vaccination, representing approximately 1 additional case of GBS/1,000,000 persons vaccinated. The combined number of GBS cases peaked 2 weeks after vaccination (161). Thus, investigations to date indicate no substantial increase in GBS associated with influenza vaccines (other than the swine influenza vaccine in 1976) and that, if influenza vaccine does pose a risk, it is probably slightly more than 1 additional case/1,000,000 persons vaccinated. Cases of GBS after influenza infection have been reported, but no epidemiologic studies have documented such an association (162,163). Substantial evidence exists that several infectious illnesses, most notably Campylobacter jejuni, as well as upper-respiratory tract infections typically are associated with GBS (157,164--166). Even if GBS were a true side effect of vaccination in the years after 1976, the estimated risk for GBS of approximately 1 additional case/1,000,000 persons vaccinated is substantially less than the risk for severe influenza, which could be prevented by vaccination among all age groups, and chiefly persons aged >65 years and those who have medical indications for influenza vaccination (Table 1) (see Hospitalizations and Deaths from Influenza). The potential benefits of influenza vaccination in preventing serious illness, hospitalization, and death greatly outweigh the possible risks for developing vaccine-associated GBS. The average case-fatality ratio for GBS is 6% and increases with age (157,167). No evidence indicates that the case-fatality ratio for GBS differs among vaccinated persons and those not vaccinated. The incidence of GBS among the general population is low, but persons with a history of GBS have a substantially greater likelihood of subsequently developing GBS than persons without such a history (158,168). Thus, the likelihood of coincidentally developing GBS after influenza vaccination is expected to be greater among persons with a history of GBS than among persons with no history of this syndrome. Whether influenza vaccination specifically might increase the risk for recurrence of GBS is unknown; therefore, avoiding vaccinating persons who are not at high risk for severe influenza complications and who are known to have developed GBS within 6 weeks after a previous influenza vaccination is prudent. As an alternative, physicians might consider the use of influenza antiviral chemoprophylaxis for these persons. Although data are limited, for the majority of persons who have a history of GBS and who are at high risk for severe complications from influenza, the established benefits of influenza vaccination justify yearly vaccination. Simultaneous Administration of Other Vaccines, Including Childhood VaccinesAdult target groups for influenza and pneumococcal polysaccharide vaccination overlap considerably (169). For persons at high risk who have not previously been vaccinated with pneumococcal vaccine, health-care providers should strongly consider administering pneumococcal polysaccharide and influenza vaccines concurrently. Both vaccines can be administered at the same time at different sites without increasing side effects (170,171). However, influenza vaccine is administered each year, whereas pneumococcal vaccine is not. A patient's verbal history is acceptable for determining prior pneumococcal vaccination status. When indicated, pneumococcal vaccine should be administered to patients who are uncertain regarding their vaccination history (169). No studies regarding the simultaneous administration of inactivated influenza vaccine and other childhood vaccines have been conducted. However, typically, inactivated vaccines do not interfere with the immune response to other inactivated or live vaccines (47), and children at high risk for influenza-related complications, including those aged 6--23 months, can receive influenza vaccine at the same time they receive other routine vaccinations. Strategies for Implementing These Recommendations in Health-Care SettingsSuccessful vaccination programs combine publicity and education for health-care workers and other potential vaccine recipients, a plan for identifying persons at high risk, use of reminder/recall systems, and efforts to remove administrative and financial barriers that prevent persons from receiving vaccine (19). Using standing orders programs is recommended for long-term care facilities (e.g., nursing homes and skilled nursing facilities) under the supervision of a medical director to ensure the administration of recommended vaccinations for adults. Other settings (e.g., inpatient and outpatient facilities, managed care organizations, assisted living facilities, correctional facilities, pharmacies, adult workplaces, and home health-care agencies) are encouraged to introduce standing orders programs as well (20). Persons for whom influenza vaccine is recommended can be identified and vaccinated in the settings described in the following sections. Outpatient Facilities Providing Ongoing Care Staff in facilities providing ongoing medical care (e.g., physicians' offices, public health clinics, employee health clinics, hemodialysis centers, hospital specialty-care clinics, and outpatient rehabilitation programs) should identify and label the medical records of patients who should receive vaccination. Vaccine should be offered during visits beginning in September and throughout the influenza season. The offer of vaccination and its receipt or refusal should be documented in the medical record. Patients for whom vaccination is recommended who do not have regularly scheduled visits during the fall should be reminded by mail, telephone, or other means of the need for vaccination. Outpatient Facilities Providing Episodic or Acute Care Beginning in each September, acute health-care facilities (e.g., emergency rooms and walk-in clinics) should offer vaccinations to persons for whom vaccination is recommended or provide written information regarding why, where, and how to obtain the vaccine. This written information should be available in languages appropriate for the populations served by the facility. Nursing Homes and Other Residential Long-Term Care Facilities During October and November each year, vaccination should be routinely provided to all residents of chronic-care facilities with the concurrence of attending physicians. Consent for vaccination should be obtained from the resident or a family member at the time of admission to the facility or anytime afterwards. All residents should be vaccinated at one time, preceding the influenza season. Residents admitted through March after completion of the facility's vaccination program should be vaccinated at the time of admission. Acute-Care Hospitals Persons of all ages (including children) with high-risk conditions and persons aged >50 years who are hospitalized at any time during September--March should be offered and strongly encouraged to receive influenza vaccine before they are discharged. In one study, 39%--46% of patients hospitalized during the winter with influenza-related diagnoses had been hospitalized during the preceding autumn (172). Thus, the hospital is a setting in which persons at increased risk for subsequent hospitalization can be identified and vaccinated. Using standing orders in hospitals increases vaccination rates among hospitalized persons (173). Visiting Nurses and Others Providing Home Care to Persons at High Risk Beginning in September, nursing-care plans should identify patients for whom vaccination is recommended, and vaccine should be administered in the home, if necessary. Caregivers and other persons in the household (including children) should be referred for vaccination. Other Facilities Providing Services to Persons Aged >50 Years Beginning in October, such facilities as assisted-living facilities, retirement communities, and recreation centers should offer unvaccinated residents and attendees vaccination on site before the influenza season. Staff education should emphasize the need for influenza vaccination. Health-Care Workers Beginning in October each year, health-care facilities should offer influenza vaccinations to all personnel, including night and weekend staff. Particular emphasis should be placed on providing vaccinations for persons who care for members of groups at high risk. Efforts should be made to educate health-care workers regarding the benefits of vaccination and the potential health consequences of influenza illness for themselves and their patients. Measures should be taken to provide all health-care workers convenient access to influenza vaccination at the work site, free of charge, as part of employee health programs. Influenza Vaccine SupplyIn 2000, difficulties with growing and processing the influenza A (H3N2) vaccine strain and other manufacturing problems resulted in substantial delays in the distribution of 2000--2001 influenza vaccine and fewer vaccine doses than were distributed in 1999 (174). In 2001, a less severe delay occurred. By December 2001, approximately 87.7 million doses of vaccine were produced, more than in any year except the 1976--1977 swine influenza vaccine campaign (175). In July 2001, ACIP issued supplemental recommendations in anticipation of the delay in 2001--2002 vaccine distribution (176). The possibility of future influenza vaccine delivery delays or vaccine shortages remains. Steps to address such situations include identification and implementation of ways to strengthen the influenza vaccine supply, to improve targeted delivery of vaccine to groups at high risk when delays or shortages are expected, and to encourage the administration of vaccine throughout the influenza season every year. Potential New VaccineIntranasally administered, cold-adapted, live, attenuated, influenza virus vaccines (LAIVs) are being used in Russia and have been under development in the United States since the 1960s (177--181). LAIVs have been studied as monovalent, bivalent, and trivalent formulations (180,181). LAIVs consist of live viruses that replicate in the upper respiratory tract, that induce minimal symptoms (i.e., are attenuated) and that replicate poorly at temperatures found in the lower respiratory tract (i.e., are temperature-sensitive). Possible advantages of LAIVs are their potential to induce a broad mucosal and systemic immune response, ease of administration, and the acceptability of an intranasal rather than intramuscular route of administration. In a 5-year study that compared trivalent inactivated vaccine and bivalent LAIVs (administered by nose drops) and that used related but different vaccine strains, the two vaccines were found to be approximately equivalent in terms of effectiveness (51,182). In a 1996--1997 study of children aged 15--71 months, an intranasally administered trivalent LAIV was 93% effective in preventing culture-positive influenza A (H3N2) and B infections, reduced febrile otitis media among vaccinated children by 30%, and reduced otitis media with concomitant antibiotic use by 35% compared with unvaccinated children (183). In a follow-up study during the 1997--1998 season, the trivalent LAIV was 86% effective in preventing culture-positive influenza among children, despite a suboptimal match between the vaccine's influenza A (H3N2) component and the predominant circulating influenza A (H3N2) virus (184). A study conducted among healthy adults during the same season found a 9%--24% reduction in febrile respiratory illnesses and a 13%--28% reduction in lost work days (185). No study has directly compared the efficacy or effectiveness of trivalent inactivated vaccine and trivalent LAIV. An application for licensure of a LAIV is under review by FDA. Recommendations for Using Antiviral Agents for InfluenzaAntiviral drugs for influenza are an adjunct to influenza vaccine for controlling and preventing influenza. However, these agents are not a substitute for vaccination. Four licensed influenza antiviral agents are available in the United States: amantadine, rimantadine, zanamivir, and oseltamivir. Amantadine and rimantadine are chemically related antiviral drugs known as adamantanes with activity against influenza A viruses but not influenza B viruses. Amantadine was approved in 1966 for chemoprophylaxis of influenza A (H2N2) infection and was later approved in 1976 for treatment and chemoprophylaxis of influenza type A virus infections among adults and children aged >1 years. Rimantadine was approved in 1993 for treatment and chemoprophylaxis of infection among adults and prophylaxis among children. Although rimantadine is approved only for chemoprophylaxis of infection among children, certain experts in the management of influenza consider it appropriate for treatment among children (186). Zanamivir and oseltamivir are chemically related antiviral drugs known as neuraminidase inhibitors, which inhibit neuraminidase and have activity against both influenza A and B viruses. Both zanamivir and oseltamivir were approved in 1999 for treating uncomplicated influenza infections. Zanamivir is approved for treating persons aged >7 years, and oseltamivir is approved for treatment for persons aged >1 years. In 2000, oseltamivir was approved for chemoprophylaxis of influenza among persons aged >13 years. The four drugs differ in terms of their pharmacokinetics, side effects, routes of administration, approved age groups, dosages, and costs. An overview of the indications, use, administration, and known primary side effects of these medications is presented in the following sections. Information contained in this report might not represent FDA approval or approved labeling for the antiviral agents described. Package inserts should be consulted for additional information. Role of Laboratory DiagnosisAppropriate treatment of patients with respiratory illness depends on accurate and timely diagnosis. The early diagnosis of influenza can reduce the inappropriate use of antibiotics and provide the option of using antiviral therapy. However, because certain bacterial infections can produce symptoms similar to influenza, bacterial infections should be considered and appropriately treated if suspected. In addition, bacterial infections can occur as a complication of influenza. Influenza surveillance information as well as diagnostic testing can aid clinical judgment and guide treatment decisions. The accuracy of clinical diagnosis of influenza based on symptoms alone is limited because symptoms from illness caused by other pathogens can overlap considerably with influenza (28--30). Influenza surveillance by state and local health departments and CDC can provide information regarding the presence of influenza viruses in the community. Surveillance can also identify the predominant circulating types, subtypes, and strains of influenza. Diagnostic tests available for influenza include viral culture, serology, rapid antigen testing, polymerase chain reaction (PCR) and immunofluorescence (24). Sensitivity and specificity of any test for influenza might vary by the laboratory that performs the test, the type of test used, and the type of specimen tested. Among respiratory specimens for viral isolation or rapid detection, nasopharyngeal specimens are typically more effective than throat swab specimens (187). As with any diagnostic test, results should be evaluated in the context of other clinical information available to the physician. Commercial rapid diagnostic tests are available that can be used by laboratories in outpatient settings to detect influenza viruses within 30 minutes (24,188). These rapid tests differ in the types of influenza viruses they can detect and whether they can distinguish between influenza types. Different tests can detect 1) only influenza A viruses; 2) both influenza A and B viruses but not distinguish between the two types; or 3) both influenza A and B and distinguish between the two. The types of specimens acceptable for use (i.e., throat swab, nasal wash, or nasal swab) also vary by test. The specificity and, in particular, the sensitivity of rapid tests are lower than for viral culture and vary by test. Because of the lower sensitivity of the rapid tests, physicians should consider confirming negative tests with viral culture or other means. Package inserts and the laboratory performing the test should be consulted for more details. Additional information regarding diagnostic testing is available at http://www.cdc.gov/ncidod/diseases/flu/flu_dx_table.htm. Despite the availability of rapid diagnostic tests, the collection of clinical specimens for viral culture is critical, because only culture isolates can provide specific information regarding circulating influenza subtypes and strains. This information is needed to compare current circulating influenza strains with vaccine strains, to guide decisions regarding influenza treatment and chemoprophylaxis, and to formulate vaccine for the coming year. Virus isolates also are needed to monitor the emergence of antiviral resistance and the emergence of novel influenza A subtypes that might pose a pandemic threat. Indications for UseTreatment When administered within 2 days of illness onset to otherwise healthy adults, amantadine and rimantadine can reduce the duration of uncomplicated influenza A illness, and zanamivir and oseltamivir can reduce the duration of uncomplicated influenza A and B illness by approximately 1 day compared with placebo (55,189--202). More clinical data are available concerning the efficacy of zanamivir and oseltamivir for treatment of influenza A infection than for treatment of influenza B infection (191--206). However, in vitro data and studies of treatment among mice and ferrets (207--214), in addition to clinical studies, have documented that zanamivir and oseltamivir have activity against influenza B viruses (195,199--201,205,206). None of the four antiviral agents has been demonstrated to be effective in preventing serious influenza-related complications (e.g., bacterial or viral pneumonia or exacerbation of chronic diseases). Evidence for the effectiveness of these four antiviral drugs is based principally on studies of patients with uncomplicated influenza (215). Data are limited and inconclusive concerning the effectiveness of amantadine, rimantadine, zanamivir, and oseltamivir for treatment of influenza among persons at high risk for serious complications of influenza (189,191,192,194,195,202,216--220). Fewer studies of the efficacy of influenza antivirals have been conducted among pediatric populations compared with adults (189,192,198,199,218,221,222). One study of oseltamivir treatment documented a decreased incidence of otitis media among children (199). To reduce the emergence of antiviral drug-resistant viruses, amantadine or rimantadine therapy for persons with influenza A illness should be discontinued as soon as clinically warranted, typically after 3--5 days of treatment or within 24--48 hours after the disappearance of signs and symptoms. The recommended duration of treatment with either zanamivir or oseltamivir is 5 days. Chemoprophylaxis Chemoprophylactic drugs are not a substitute for vaccination, although they are critical adjuncts in the prevention and control of influenza. Both amantadine and rimantadine are indicated for the chemoprophylaxis of influenza A infection, but not influenza B. Both drugs are approximately 70%--90% effective in preventing illness from influenza A infection (55,189,218). When used as prophylaxis, these antiviral agents can prevent illness while permitting subclinical infection and the development of protective antibody against circulating influenza viruses. Therefore, certain persons who take these drugs will develop protective immune responses to circulating influenza viruses. Amantadine and rimantadine do not interfere with the antibody response to the vaccine (189). Both drugs have been studied extensively among nursing home populations as a component of influenza outbreak control programs, which can limit the spread of influenza within chronic care institutions (189,217,223--225). Among the neuraminidase inhibitor antivirals, zanamivir and oseltamivir, only oseltamivir has been approved for prophylaxis, but community studies of healthy adults indicate that both drugs are similarly effective in preventing febrile, laboratory-confirmed influenza illness (efficacy: zanamivir, 84%; oseltamivir, 82%) (226,227). Both antiviral agents have also been reported to prevent influenza illness among persons given chemoprophylaxis after a household member was diagnosed with influenza (205,228). Experience with prophylactic use of these agents in institutional settings or among patients with chronic medical conditions is limited in comparison with the adamantanes (201,220,229--232). One 6-week study of oseltamivir prophylaxis among nursing home residents reported a 92% reduction in influenza illness (201,233). Use of zanamivir has not been reported to impair the immunologic response to influenza vaccine (200,234). Data are not available on the efficacy of any of the four antiviral agents in preventing influenza among severely immune compromised persons. When determining the timing and duration for administering influenza antiviral medications for prophylaxis, factors related to cost, compliance, and potential side effects should be considered. To be maximally effective as prophylaxis, the drug must be taken each day for the duration of influenza activity in the community. However, to be most cost-effective, one study of amantadine or rimantadine prophylaxis reported that the drugs should be taken only during the period of peak influenza activity in a community (235). Persons at High Risk Who Are Vaccinated After Influenza Activity Has Begun. Persons at high risk for complications of influenza still can be vaccinated after an outbreak of influenza has begun in a community. However, the development of antibodies in adults after vaccination can take approximately 2 weeks (134,135). When influenza vaccine is administered while influenza viruses are circulating, chemoprophylaxis should be considered for persons at high risk during the time from vaccination until immunity has developed. Children aged <9 years who receive influenza vaccine for the first time can require 6 weeks of prophylaxis (i.e., prophylaxis for 4 weeks after the first dose of vaccine and an additional 2 weeks of prophylaxis after the second dose). Persons Who Provide Care to Those at High Risk. To reduce the spread of virus to persons at high risk during community or institutional outbreaks, chemoprophylaxis during peak influenza activity can be considered for unvaccinated persons who have frequent contact with persons at high risk. Persons with frequent contact include employees of hospitals, clinics, and chronic-care facilities, household members, visiting nurses, and volunteer workers. If an outbreak is caused by a variant strain of influenza that might not be controlled by the vaccine, chemoprophylaxis should be considered for all such persons, regardless of their vaccination status. Persons Who Have Immune Deficiency. Chemoprophylaxis can be considered for persons at high risk who are expected to have an inadequate antibody response to influenza vaccine. This category includes persons infected with HIV, chiefly those with advanced HIV disease. No published data are available concerning possible efficacy of chemoprophylaxis among persons with HIV infection or interactions with other drugs used to manage HIV infection. Such patients should be monitored closely if chemoprophylaxis is administered. Other Persons. Chemoprophylaxis throughout the influenza season or during peak influenza activity might be appropriate for persons at high risk who should not be vaccinated. Chemoprophylaxis can also be offered to persons who wish to avoid influenza illness. Health-care providers and patients should make this decision on an individual basis. Control of Influenza Outbreaks in Institutions Using antiviral drugs for treatment and prophylaxis of influenza is a key component of institutional outbreak control. In addition to using antiviral medications, other outbreak control measures include instituting droplet precautions and establishing cohorts of patients with confirmed or suspected influenza, re-offering influenza vaccinations to unvaccinated staff and patients, restricting staff movement between wards or buildings, and restricting contact between ill staff or visitors and patients (236--238) (for additional information regarding outbreak control in specific settings, see Additional Information Regarding Influenza Infection Control Among Specific Populations). The majority of published reports concerning the use of antiviral agents to control institutional influenza outbreaks are based on studies of influenza A outbreaks among nursing home populations where amantadine or rimantadine were used (189,217,223--225,235). Less information is available concerning the use of neuraminidase inhibitors in influenza A or B institutional outbreaks (220,231,233). When confirmed or suspected outbreaks of influenza occur in institutions that house persons at high risk, chemoprophylaxis should be started as early as possible to reduce the spread of the virus. In these situations, having preapproved orders from physicians or plans to obtain orders for antiviral medications on short notice is useful. When institutional outbreaks occur, chemoprophylaxis should be administered to all residents --- regardless of whether they received influenza vaccinations during the previous fall --- and should continue for >2 weeks. If surveillance indicates that new cases continue to occur, chemoprophylaxis should be continued until approximately 1 week after the end of the outbreak. The dosage for each resident should be determined individually. Chemoprophylaxis also can be offered to unvaccinated staff who provide care to persons at high risk. Prophylaxis should be considered for all employees, regardless of their vaccination status, if the outbreak is caused by a variant strain of influenza that is not well-matched by the vaccine. In addition to nursing homes, chemoprophylaxis also can be considered for controlling influenza outbreaks in other closed or semiclosed settings (e.g., dormitories or other settings where persons live in close proximity). For example, chemoprophylaxis with rimantadine has been used successfully to control an influenza A outbreak aboard a cruise ship (239). To limit the potential transmission of drug-resistant virus during institutional outbreaks, whether in chronic or acute-care settings or other closed settings, measures should be taken to reduce contact as much as possible between persons taking antiviral drugs for treatment and other persons, including those taking chemoprophylaxis (see Antiviral Drug-Resistant Strains of Influenza). DosageDosage recommendations vary by age group and medical conditions (Table 4). Children Amantadine. Use of amantadine among children aged <1 year has not been adequately evaluated. The FDA-approved dosage for children aged 1--9 years for treatment and prophylaxis is 4.4--8.8 mg/kg/day, not to exceed 150 mg/day. Although further studies are needed to determine the optimal dosage for children aged 1--9 years, physicians should consider prescribing only 5 mg/kg/day (not to exceed 150 mg/day) to reduce the risk for toxicity. The approved dosage for children aged >10 years is 200 mg/day (100 mg twice a day); however, for children weighing <40 kg, prescribing 5 mg/kg/day, regardless of age, is advisable (219,240). Rimantadine. Rimantadine is approved for prophylaxis among children aged >1 years and for treatment and prophylaxis among adults. Although rimantadine is approved only for prophylaxis of infection among children, certain specialists in the management of influenza consider rimantadine appropriate for treatment among children (186). Use of rimantadine among children aged <1 year has not been adequately evaluated. Rimantadine should be administered in one or two divided doses at a dosage of 5 mg/kg/day, not to exceed 150 mg/day for children aged 1--9 years. The approved dosage for children aged >10 years is 200 mg/day (100 mg twice a day); however, for children weighing <40 kg, prescribing 5 mg/kg/day, regardless of age, is recommended (241). Zanamivir. Zanamivir is approved for treatment among children aged >7 years. The recommended dosage of zanamivir for treatment of influenza is two inhalations (one 5-mg blister per inhalation for a total dose of 10 mg) twice daily (approximately 12 hours apart) (200). Oseltamivir. Oseltamivir is approved for treatment among persons aged >1 year and for chemoprophylaxis among persons age >13 years. Recommended treatment dosages for children vary by the weight of the child: the dosage recommendation for children who weigh <15 kg is 30 mg twice a day; for children weighing >15--23 kg, the dosage is 45 mg twice a day; for those weighing >23--40 kg, the dosage is 60 mg twice a day; and for children weighing >40 kg, the dosage is 75 mg twice a day. The treatment dosage for persons >13 years is 75 mg twice daily. For children >13 years, the recommended dosage for prophylaxis is 75 mg once a day (201). Persons Aged >65 Years Amantadine. The daily dosage of amantadine for persons aged >65 years should not exceed 100 mg for prophylaxis or treatment, because renal function declines with increasing age. For certain older persons, the dosage should be further reduced. Rimantadine. Among older persons, the incidence and severity of central nervous system (CNS) side effects are substantially lower among those taking rimantadine at a dosage of 100 mg/day than among those taking amantadine at dosages adjusted for estimated renal clearance (242). However, chronically ill older persons have had a higher incidence of CNS and gastrointestinal symptoms and serum concentrations two to four times higher than among healthy, younger persons when rimantadine has been administered at a dosage of 200 mg/day (189). For prophylaxis among persons aged >65 years, the recommended dosage is 100 mg/day. For treatment of older persons in the community, a reduction in dosage to 100 mg/day should be considered if they experience side effects when taking a dosage of 200 mg/day. For treatment of older nursing home residents, the dosage of rimantadine should be reduced to 100 mg/day (241). Zanamivir and Oseltamivir. No reduction in dosage is recommended on the basis of age alone. Persons with Impaired Renal Function Amantadine. A reduction in dosage is recommended for patients with creatinine clearance <50 mL/min/1.73m2. Guidelines for amantadine dosage on the basis of creatinine clearance are found in the package insert. Because recommended dosages on the basis of creatinine clearance might provide only an approximation of the optimal dose for a given patient, such persons should be observed carefully for adverse reactions. If necessary, further reduction in the dose or discontinuation of the drug might be indicated because of side effects. Hemodialysis contributes minimally to amantadine clearance (240,243). Rimantadine. A reduction in dosage to 100 mg/day is recommended for persons with creatinine clearance <10 mL/min. Because of the potential for accumulation of rimantadine and its metabolites, patients with any degree of renal insufficiency, including older persons, should be monitored for adverse effects, and either the dosage should be reduced or the drug should be discontinued, if necessary. Hemodialysis contributes minimally to drug clearance (244). Zanamivir. Limited data are available regarding the safety and efficacy of zanamivir for patients with impaired renal function. Among patients with renal failure who were administered a single intravenous dose of zanamivir, decreases in renal clearance, increases in half-life, and increased systemic exposure to zanamivir were observed (200,245). However, a limited number of healthy volunteers who were administered high doses of intravenous zanamivir tolerated systemic levels of zanamivir that were substantially higher than those resulting from administration of zanamivir by oral inhalation at the recommended dose (246,247). On the basis of these considerations, the manufacturer recommends no dose adjustment for inhaled zanamivir for a 5-day course of treatment for patients with either mild-to-moderate or severe impairment in renal function (200). Oseltamivir. Serum concentrations of oseltamivir carboxylate (GS4071), the active metabolite of oseltamivir, increase with declining renal function (201,204). For patients with creatinine clearance of 10--30 mL/min (201), a reduction of the treatment dosage of oseltamivir to 75 mg once daily and in the prophylaxis dosage to 75 mg every other day is recommended. No treatment or prophylaxis dosing recommendations are available for patients undergoing routine renal dialysis treatment. Persons with Liver Disease Amantadine. No increase in adverse reactions to amantadine has been observed among persons with liver disease. Rare instances of reversible elevation of liver enzymes among patients receiving amantadine have been reported, although a specific relationship between the drug and such changes has not been established (248). Rimantadine. A reduction in dosage to 100 mg/day is recommended for persons with severe hepatic dysfunction. Zanamivir and Oseltamivir. Neither of these medications has been studied among persons with hepatic dysfunction. Persons with Seizure Disorders Amantadine. An increased incidence of seizures has been reported among patients with a history of seizure disorders who have received amantadine (249). Patients with seizure disorders should be observed closely for possible increased seizure activity when taking amantadine. Rimantadine. Seizures (or seizure-like activity) have been reported among persons with a history of seizures who were not receiving anticonvulsant medication while taking rimantadine (250). The extent to which rimantadine might increase the incidence of seizures among persons with seizure disorders has not been adequately evaluated. Zanamivir and Oseltamivir. Seizure events have been reported during postmarketing use of zanamivir and oseltamivir, although no epidemiologic studies have reported any increased risk for seizures with either zanamivir or oseltamivir use. RouteAmantadine, rimantadine, and oseltamivir are administered orally. Amantadine and rimantadine are available in tablet or syrup form, and oseltamivir is available in capsule or oral suspension form (178,179). Zanamivir is available as a dry powder that is self-administered via oral inhalation by using a plastic device included in the package with the medication. Patients will benefit from instruction and demonstration of correct use of this device (200). PharmacokineticsAmantadine Approximately 90% of amantadine is excreted unchanged in the urine by glomerular filtration and tubular secretion (223,251--254). Thus, renal clearance of amantadine is reduced substantially among persons with renal insufficiency, and dosages might need to be decreased (see Dosage) (Table 4). Rimantadine Approximately 75% of rimantadine is metabolized by the liver (218). The safety and pharmacokinetics of rimantadine among persons with liver disease have been evaluated only after single-dose administration (218,255). In a study of persons with chronic liver disease (the majority with stabilized cirrhosis), no alterations in liver function were observed after a single dose. However, for persons with severe liver dysfunction, the apparent clearance of rimantadine was 50% lower than that reported for persons without liver disease (241). Rimantadine and its metabolites are excreted by the kidneys. The safety and pharmacokinetics of rimantadine among patients with renal insufficiency have been evaluated only after single-dose administration (218,244). Further studies are needed to determine multiple-dose pharmacokinetics and the most appropriate dosages for patients with renal insufficiency. In a single-dose study of patients with anuric renal failure, the apparent clearance of rimantadine was approximately 40% lower, and the elimination half-life was approximately 1.6-fold greater than that among healthy persons of the same age (244). Hemodialysis did not contribute to drug clearance. In studies of persons with less severe renal disease, drug clearance was also reduced, and plasma concentrations were higher than those among control patients without renal disease who were the same weight, age, and sex (241,256). Zanamivir In studies of healthy volunteers, approximately 7%--21% of the orally inhaled zanamivir dose reached the lungs, and 70%--87% was deposited in the oropharynx (200,257). Approximately 4%--17% of the total amount of orally inhaled zanamivir is systemically absorbed. Systemically absorbed zanamivir has a half-life of 2.5--5.1 hours and is excreted unchanged in the urine. Unabsorbed drug is excreted in the feces (200,247). Oseltamivir Approximately 80% of orally administered oseltamivir is absorbed systemically (204). Absorbed oseltamivir is metabolized to oseltamivir carboxylate, the active neuraminidase inhibitor, primarily by hepatic esterases. Oseltamivir carboxylate has a half-life of 6--10 hours and is excreted in the urine by glomerular filtration and tubular secretion via the anionic pathway (201,258). Unmetabolized oseltamivir also is excreted in the urine by glomerular filtration and tubular secretion (258). Side Effects and Adverse ReactionsWhen considering the use of influenza antiviral medications (i.e., choice of antiviral drug, dosage, and duration of therapy), clinicians must consider the patient's age, weight, and renal function (Table 4); presence of other medical conditions; indications for use (i.e., prophylaxis or therapy); and the potential for interaction with other medications. Amantadine and Rimantadine Both amantadine and rimantadine can cause CNS and gastrointestinal side effects when administered to young, healthy adults at equivalent dosages of 200 mg/day. However, incidence of CNS side effects (e.g., nervousness, anxiety, insomnia, difficulty concentrating, and lightheadedness) is higher among persons taking amantadine than among those taking rimantadine (259). In a 6-week study of prophylaxis among healthy adults, approximately 6% of participants taking rimantadine at a dosage of 200 mg/day experienced >1 CNS symptoms, compared with approximately 13% of those taking the same dosage of amantadine and 4% of those taking placebo (259). A study of older persons also demonstrated fewer CNS side effects associated with rimantadine compared with amantadine (242). Gastrointestinal side effects (e.g., nausea and anorexia) occur in approximately 1%--3% of persons taking either drug, compared with 1% of persons receiving the placebo (259). Side effects associated with amantadine and rimantadine are usually mild and cease soon after discontinuing the drug. Side effects can diminish or disappear after the first week, despite continued drug ingestion. However, serious side effects have been observed (e.g., marked behavioral changes, delirium, hallucinations, agitation, and seizures) (240,249). These more severe side effects have been associated with high plasma drug concentrations and have been observed most often among persons who have renal insufficiency, seizure disorders, or certain psychiatric disorders and among older persons who have been taking amantadine as prophylaxis at a dosage of 200 mg/day (223). Clinical observations and studies have indicated that lowering the dosage of amantadine among these persons reduces the incidence and severity of such side effects (Table 4). In acute overdosage of amantadine, CNS, renal, respiratory, and cardiac toxicity, including arrhythmias, have been reported (240). Because rimantadine has been marketed for a shorter period than amantadine, its safety among certain patient populations (e.g., chronically ill and elderly persons) has been evaluated less frequently. Because amantadine has anticholinergic effects and might cause mydriasis, it should not be used for patients with untreated angle closure glaucoma (240). Zanamivir In a study of zanamivir treatment of influenza-like illness among persons with asthma or chronic obstructive pulmonary disease where study medication was administered after using a B2-agonist, 13% of patients receiving zanamivir and 14% of patients who received placebo (inhaled powdered lactose vehicle) experienced a >20% decline in forced expiratory volume in 1 second (FEV1) after treatment (200,202). However, in a study of persons with mild or moderate asthma who did not have influenza-like illness, 1 of 13 patients experienced bronchospasm after administration of zanamivir (200). In addition, during postmarketing surveillance, cases of respiratory function deterioration after inhalation of zanamivir have been reported. Certain patients had underlying airways disease (e.g., asthma or chronic obstructive pulmonary disease). Because of the risk for serious adverse events and because the efficacy has not been demonstrated among this population, zanamivir is generally not recommended for treatment for patients with underlying airway disease (200). If physicians decide to prescribe zanamivir to patients with underlying chronic respiratory disease after carefully considering potential risks and benefits, the drug should be used with caution under conditions of proper monitoring and supportive care, including the availability of short-acting bronchodilators (215). Patients with asthma or chronic obstructive pulmonary disease who use zanamivir are advised to 1) have a fast-acting inhaled bronchodilator available when inhaling zanamivir and 2) stop using zanamivir and contact their physician if they develop difficulty breathing (200). No clear evidence is available regarding the safety or efficacy of zanamivir for persons with underlying respiratory or cardiac disease or for persons with complications of acute influenza (215). Allergic reactions, including oropharyngeal or facial edema, have also been reported during postmarketing surveillance (200,220). In clinical treatment studies of persons with uncomplicated influenza, the frequencies of adverse events were similar for persons receiving inhaled zanamivir and those receiving placebo (i.e., inhaled lactose vehicle alone) (190--195,220). The most common adverse events reported by both groups were diarrhea; nausea; sinusitis; nasal signs and symptoms; bronchitis; cough; headache; dizziness; and ear, nose, and throat infections. Each of these symptoms was reported by <5% of persons in the clinical treatment studies combined (200). Oseltamivir Nausea and vomiting were reported more frequently among adults receiving oseltamivir for treatment (nausea without vomiting, approximately 10%; vomiting, approximately 9%) than among persons receiving placebo (nausea without vomiting, approximately 6%; vomiting, approximately 3%) (196,197,201,260). Among children treated with oseltamivir, 14.3% had vomiting, compared with 8.5% of placebo recipients. Overall, 1% discontinued the drug secondary to this side effect (199), whereas a limited number of adults enrolled in clinical treatment trials of oseltamivir discontinued treatment because of these symptoms (201). Similar types and rates of adverse events were found in studies of oseltamivir prophylaxis (201). Nausea and vomiting might be less severe if oseltamivir is taken with food (201,260). Use During PregnancyNo clinical studies have been conducted regarding the safety or efficacy of amantadine, rimantadine, zanamivir, or oseltamivir for pregnant women; only two cases of amantadine use for severe influenza illness during the third trimester have been reported (105,106). However, both amantadine and rimantadine have been demonstrated in animal studies to be teratogenic and embryotoxic when administered at very high doses (240,241). Because of the unknown effects of influenza antiviral drugs on pregnant women and their fetuses, these four drugs should be used during pregnancy only if the potential benefit justifies the potential risk to the embryo or fetus (see package inserts for additional information [200,201,240,241]). Drug InteractionsCareful observation is advised when amantadine is administered concurrently with drugs that affect CNS, especially CNS stimulants. Concomitant administration of antihistamines or anticholinergic drugs can increase the incidence of adverse CNS reactions (189). No clinically significant interactions between rimantadine and other drugs have been identified. Clinical data are limited regarding drug interactions with zanamivir. However, no known drug interactions have been reported, and no clinically important drug interactions have been predicted on the basis of in vitro data and data from studies involving rats (200,261). Limited clinical data are available regarding drug inter-actions with oseltamivir. Because oseltamivir and oseltamivir carboxylate are excreted in the urine by glomerular filtration and tubular secretion via the anionic pathway, a potential exists for interaction with other agents excreted by this pathway. For example, coadministration of oseltamivir and probenecid resulted in reduced clearance of oseltamivir carboxylate by approximately 50% and a corresponding approximate twofold increase in the plasma levels of oseltamivir carboxylate (201,258). No published data are available concerning the safety or efficacy of using combinations of any of these four influenza antiviral drugs. For more detailed information concerning potential drug interactions for any of these influenza antiviral drugs, package inserts should be consulted. Antiviral Drug-Resistant Strains of InfluenzaAmantadine-resistant viruses are cross-resistant to rimantadine and vice versa (262). Drug-resistant viruses can appear in approximately one third of patients when either amantadine or rimantadine is used for therapy (222,263,264). During the course of amantadine or rimantadine therapy, resistant influenza strains can replace sensitive strains within 2--3 days of starting therapy (263,265). Resistant viruses have been isolated from persons who live at home or in an institution where other residents are taking or have recently taken amantadine or rimantadine as therapy (266,267); however, the frequency with which resistant viruses are transmitted and their impact on efforts to control influenza are unknown. Amantadine- and rimantadine-resistant viruses are not more virulent or transmissible than sensitive viruses (268). The screening of epidemic strains of influenza A has rarely detected amantadine- and rimantadine-resistant viruses (263,269,270). Persons who have influenza A infection and who are treated with either amantadine or rimantadine can shed sensitive viruses early in the course of treatment and later shed drug-resistant viruses, especially after 5--7 days of therapy (222). Such persons can benefit from therapy even when resistant viruses emerge. Resistance to zanamivir and oseltamivir can be induced in influenza A and B viruses in vitro (271--278), but induction of resistance requires multiple passages in cell culture. By contrast, resistance to amantadine and rimantadine in vitro can be induced with fewer passages in cell culture (279,280). Development of viral resistance to zanamivir and oseltamivir during treatment has been identified but does not appear to be frequent (201,281--284). In clinical treatment studies using oseltamivir, 1.3% of posttreatment isolates from patients aged >13 years and 8.6% among patients aged 1--12 years had decreased susceptibility to oseltamivir (201). No isolates with reduced susceptibility to zanamivir have been reported from clinical trials, although the number of posttreatment isolates tested is limited (285), and the risk for emergence of zanamivir-resistant isolates cannot be quantified (200). Only one clinical isolate with reduced susceptibility to zanamivir, obtained from an immunocompromised child on prolonged therapy, has been reported (282). Available diagnostic tests are not optimal for detecting clinical resistance to the neuraminidase inhibitor antiviral drugs, and additional tests are being developed. (285,286). Postmarketing surveillance for neuraminidase inhibitor-resistant influenza viruses is being conducted (287). Sources of Information Regarding Influenza and Its SurveillanceInformation regarding influenza surveillance, prevention, detection, and control is available on CDC/NCID's website at http://www.cdc.gov/ncidod/diseases/flu/fluvirus.htm. Surveillance information is available through the CDC Voice Information System (influenza update) at 888-232-3228 or CDC Fax Information Service at 888-232-3299. During October--May, surveillance information is updated at least every other week. In addition, periodic updates regarding influenza are published in the MMWR (weekly). Additional information regarding influenza vaccine can be obtained at CDC/NIP's website at http://www.cdc.gov/nip/flu or by calling the NIP hotline at 800-232-2522 (English) or 800-232-0233 (Spanish). State and local health departments should be consulted concerning availability of influenza vaccine, access to vaccination programs, information related to state or local influenza activity, and for reporting influenza outbreaks and receiving advice concerning outbreak control. Additional Information Regarding Influenza Infection Control Among Specific PopulationsEach year, ACIP provides general, annually updated information regarding the control and prevention of influenza. Other reports on the control and prevention of influenza among specific populations (e.g., immunocompromised persons, health-care workers, hospitals, and travelers) are also available in the following publications:
References
Table 1 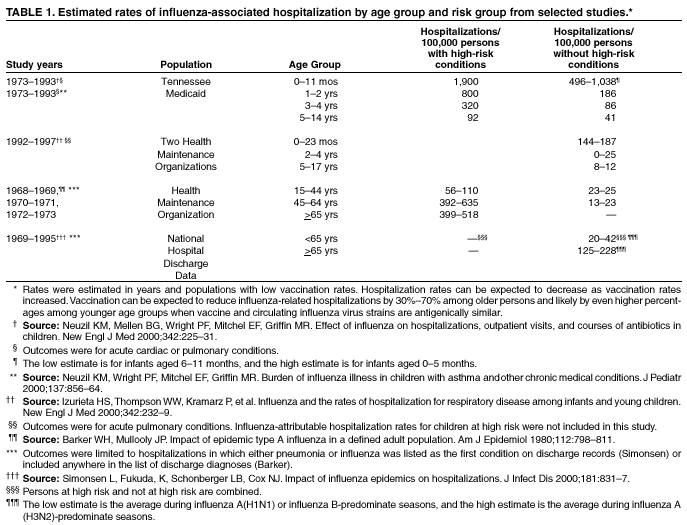 Return to top. Table 2  Return to top. Table 3 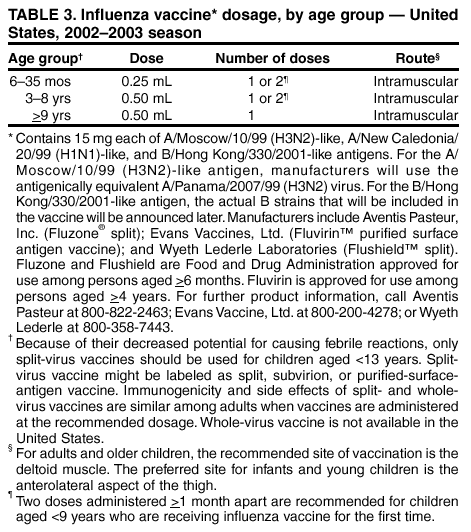 Return to top. Table 4 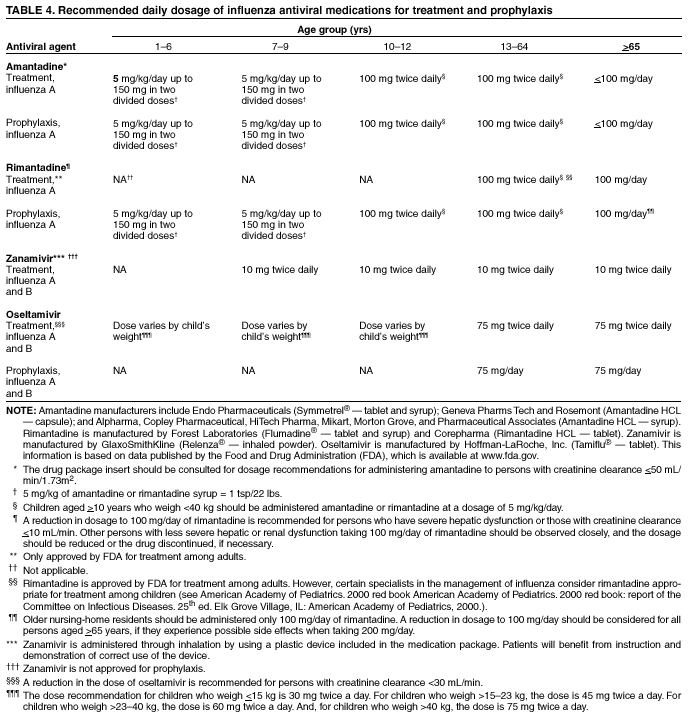 Return to top. Advisory Committee on Immunization PracticesMembership List, February 2002 Chairman: John F. Modlin, M.D., Professor of Pediatrics and Medicine, Dartmouth Medical School, Lebanon, New Hampshire. Executive Secretary: Dixie E. Snider, Jr., M.D., Associate Director for Science, Centers for Disease Control and Prevention, Atlanta, Georgia. Members: Robert B. Belshe, M.D., Saint Louis University Health Sciences Center, St. Louis, Missouri; Guthrie S. Birkhead, M.D., New York State Department of Health, Albany, New York; Dennis A. Brooks, M.D., Johnson Medical Center, Baltimore, Maryland; Jaime Deseda-Tous, M.D., San Jorge Children’s Hospital, San Juan, Puerto Rico; Celine I. Hanson, M.D., Texas Department of Health, Austin, Texas; Myron J. Levin, M.D., University of Colorado School of Medicine, Denver, Colorado; Margaret B. Rennels, M.D., University of Maryland School of Medicine, Baltimore, Maryland; John E. Salamone, Washington, D.C.; Natalie J. Smith, M.D., California Department of Health Services, Berkeley, California; Lucy S. Tompkins, M.D., Ph.D., Stanford University Medical Center, Stanford, California; Bonnie M. Word, M.D., Monmouth Junction, New Jersey; and Richard Zimmerman, M.D., University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania. Ex-Officio Members: James Cheek, M.D., Indian Health Service, Albuquerque, New Mexico; Col. Benedict M. Diniega, M.D., Department of Defense, Falls Church, Virginia; Geoffrey S. Evans, M.D., Health Resources and Services Administration, Rockville, Maryland; T. Randolph Graydon, Centers for Medicare and Medicaid Services, Baltimore, Maryland; Carole Heilman, Ph.D., National Institutes of Health, Bethesda, Maryland; Karen Midthun, M.D., Food and Drug Administration, Bethesda, Maryland; Martin G. Myers, M.D., National Vaccine Program Office, Atlanta, Georgia; and Kristin Lee Nichol, M.D., Veterans Administration Medical Center, Minneapolis, Minnesota. Liaison Representatives: American Academy of Family Physicians, Richard D. Clover, M.D., Louisville, Kentucky, and Martin Mahoney, M.D., Ph.D., Clarence, New York; American Academy of Pediatrics, Jon Abramson, M.D., Winston-Salem, North Carolina, and Gary Overturf, M.D., Albuquerque, New Mexico; American Association of Health Plans, Eric K. France, M.D., Denver, Colorado; American College of Obstetricians and Gynecologists, Stanley A. Gall, M.D., Louisville, Kentucky; American College of Physicians, Kathleen M. Neuzil, M.D., Seattle, Washington; American Medical Association, H. David Wilson, M.D., Grand Forks, North Dakota; American Pharmaceutical Association, Stephan L. Foster, Pharm.D., Memphis, Tennessee; Association of Teachers of Preventive Medicine, W. Paul McKinney, M.D., Louisville, Kentucky; Canadian National Advisory Committee on Immunization, Victor Marchessault, M.D., Cumberland, Ontario, Canada; Healthcare Infection Control Practices Advisory Committee, Jane D. Siegel, M.D., Dallas, Texas; Infectious Diseases Society of America, Samuel L. Katz, M.D., Durham, North Carolina, and William Schaffner, M.D., Nashville, Tennessee; London Department of Health, David M. Salisbury, M.D., London, United Kingdom; National Coalition for Adult Immunization, David A. Neumann, Ph.D., Bethesda, Maryland; National Immunization Council and Child Health Program, Mexico, Jose Ignacio Santos, M.D., Mexico City, Mexico; National Medical Association, Rudolph E. Jackson, M.D., Atlanta, Georgia; National Vaccine Advisory Committee, Georges Peter, M.D., Providence, Rhode Island; and Pharmaceutical Research and Manufacturers of America, Kevin Reilly, Radnor, Pennsylvania. Members of the Influenza Working GroupAdvisory Committee on Immunization Practices (ACIP), Bonnie M. Word, M.D., Chairman, T. Randolph Graydon, Martin G. Myers, M.D., Kristin Lee Nichol, M.D., Margaret B. Rennels, M.D., Natalie J. Smith, M.D., and Richard Zimmerman, M.D.; American Academy of Pediatrics, Jon Abramson, M.D.; American Association of Health Plans, Eric K. France, M.D.; American College of Obstetricians and Gynecologists, Stanley A. Gall, M.D.; Food and Drug Administration, Roland A. Levandowski, M.D.; American College of Physicians, Kathleen M. Neuzil, M.D.; Pharmaceutical Research and Manufacturers of America, Fred Ruben, M.D.; Infectious Diseases Society of America, William Schaffner, M.D.; Massachusetts Department of Health, Susan Lett, M.D.; American Academy of Family Physicians, Richard D. Clover, M.D.; and CDC, Nancy J. Cox, Ph.D., Keiji Fukuda, M.D., James A. Singleton, M.S., Marika Iwane, Ph.D., John K. Iskander, M.D., and Gina T. Mootrey, D.O.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/5/2002 |
|||||||||
This page last reviewed 4/5/2002
|