Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
National Laboratory Inventories for Wild Poliovirus Containment --- Western Pacific Region, 2008
In the future, when wild poliovirus (WPV) transmission is interrupted worldwide, facilities holding WPV materials will represent the only remaining repository of the virus. Maintaining the number of such facilities at a minimum and at an appropriate biosafety standard (laboratory containment) reduces the risk for a facility-associated reintroduction of WPV. In May 1999, the World Health Assembly (WHA) urged all member states to begin the process leading to laboratory containment of WPV (1). The World Health Organization (WHO) global action plan for laboratory containment of WPV issued in 1999 indicated a staged approach that begins with a national survey of all biomedical facilities (Phase I); the purpose of the survey is to alert institutions and facilities to the need for containment, encourage reduction of WPV materials, and develop a national inventory of facilities holding such materials. The survey and inventory provide a facility database for use in all subsequent steps toward global poliovirus containment. In May 2008, WHA urged all WHO member states to complete Phase I activities outlined in the WHO Global Action Plan for Laboratory Containment of Wild Polioviruses (2,3). In the WHO Western Pacific Region (WPR), Phase I surveys of 77,260 laboratories in the 37 countries and areas of WPR were conducted during 1999--2008. A total of 45 laboratories were identified as holding WPV materials in 2008. This report describes completion of Phase I containment activities by WPR countries, and updates a previous report on Phase I completion in the European Region and global progress (4).
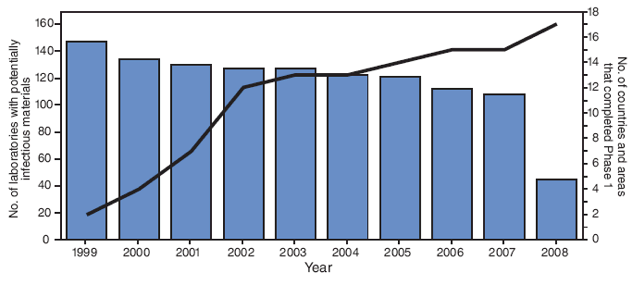
Specific guidelines for Phase I activities in the WPR were issued in 1999 that advised member states and areas* to conduct national surveys of biomedical facilities,† communicate the need for WPV containment, develop a database of facilities, and compile a national inventory of laboratories identified to possess WPV infectious or potentially infectious materials (WPV materials).§ Facilities were to be advised to dispose of unneeded WPV materials and to handle retained WPV materials under appropriate biosafety conditions. In 1999, the Regional Commission for the Certification of Poliomyelitis Eradication in the Western Pacific (WPR RCC) established progress toward completion of Phase I containment as a requirement for certification of the region as polio-free (5). Preliminary information on Phase I activities was reported by each member state to the WHO regional office annually. When WPR was certified as polio free in October 2000, all member states/areas had initiated Phase I, but only four member states/areas had completed it (Figure).
Strategies for identifying and surveying biomedical facilities differed among WPR countries according to population size, administrative and health infrastructure, economic development, and political structure. In small member states (e.g., Brunei Darussalam and Macao [China]), laboratory surveys were easier to complete by the small number of facilities, the majority of which were under government jurisdiction. In member states with less developed laboratory infrastructures (e.g., Cambodia and the 21 Pacific island countries and areas), health staff at district levels identified the facilities in the country and assessed freezer capacity and power supply to exclude facilities not capable of preserving polioviruses. Containment officials at the national level then focused survey efforts on the facilities assumed to be at higher risk for retaining WPV materials (i.e., those with research or teaching functions). In member states with more developed laboratory infrastructures (e.g., Australia and Republic of Korea), the database of facilities was compiled from preexisting lists of licensed laboratories supplemented with member lists of professional institution associations (e.g., biosafety associations and microbiological societies), and lists for laboratory accreditation or quality control. In the majority of countries, the surveys were completed by calling or visiting nonresponding laboratories. Completeness of the surveys was assessed by a systematic quality-assurance procedure provided by WHO.
By 2006, Phase I was complete in all countries except China and Japan, which have vast biomedical laboratory infrastructures. During 2000--2003, Phase I surveys in China were conducted in facilities operating under the jurisdiction of five Chinese ministries/agencies.¶ During 2005--2008, a comprehensive approach was initiated which included 1) surveying nearly 50,000 biomedical laboratories under the jurisdiction of the Ministry of Health identified by compiling information at the county, prefecture, and provincial levels; 2) performing a pilot survey in selected provinces of biomedical facilities among 46 ministries and agencies other than the Ministry of Health; and 3) expanding surveys to all provinces and ministries based on the lessons learned from the pilot. Survey completion was facilitated by regulations on safe handling of pathogenic agents issued after confirmed laboratory-acquired SARS infections during the 2003 epidemic (6,7).
In Japan, a nationwide survey was implemented during 2000--2002 by the Ministry of Health, Labor, and Welfare (MHLW) covering 7,865 facilities with an overall return rate of 53.8%. An expanded survey conducted during 2004--2005 achieved a response rate of >99% from the 12,142 facilities under the jurisdiction of the MHLW and 1,367 facilities under the Ministry of Education, Culture, Sport, Science, and Technology, the two ministries overseeing facilities considered most likely to have WPV materials. A further total of 560 facilities were surveyed under the jurisdiction of the remaining ministries. The number of biomedical laboratories surveyed within each facility was not reported. An additional targeted survey of 80 high-risk facilities identified through a search of published poliovirus research was conducted in 2008 to further validate the earlier surveys. Among the 88 laboratories in these facilities, 82 (93%) had been surveyed previously; one laboratory of the six that were not surveyed previously reported holding WPV materials.**
In the WPR, a total of 77,260 biomedical facilities responded to the Phase I surveys for all countries, including 55,688 facilities in China and 14,069 facilities in Japan (Table). Of all biomedical facilities responding to the surveys, 89% (68,831) were clinical diagnostic laboratories, primarily under the jurisdiction of ministries of health; only 32 (0.05%) of these were found to hold WPV materials, 27 of which are laboratories within the Global Polio Laboratory Network (one in Australia, 25 in China, and one in Japan). Of the regional total, 3,838 facilities were listed as being at high risk for retaining WPV materials, 94% of which were located in Australia, China, Japan, and Republic of Korea (Table); 11 (0.3%) of these reported having WPV materials.†† Any facility that had reported retention of WPV materials in a given survey was resurveyed annually. The number of facilities/laboratories retaining WPV materials decreased during the course of Phase I implementation, resulting in a decline from 147 facilities in 1999 to 107 in 2006 to 45 in 2008 (two in Australia, 27 in China, 15 in Japan, and one in the Republic of Korea) (Figure).
All member states documented laboratory containment activities through standardized reports reviewed by the WHO regional office, a panel of experts external to the process, and the RCC. For the two member states with the most complex laboratory infrastructure (China and Japan), the external review of the process included site visits to critical academic and research institutions. In December 2008, the WPR RCC accepted the final reports from China and Japan and declared Phase I WPV laboratory containment complete for the region.
Reported by: National containment coordinators, Western Pacific Region member states; B Bayutas, S Roesel, World Health Organization Western Pacific Regional Office, Manila, Philippines. C Wolff, Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Task Force for Global Health, Decatur, Georgia. Global Immunization Div, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note:
WPR has joined the WHO European Region in completing Phase I WPV laboratory containment activities (4). The WHO Region of the Americas (AMR), the remaining polio-free region, did not initiate containment activities at the time of certification in 1994 because the policies were not yet developed. The AMR RCC has accepted the final Phase 1 reports from 24 (69%) of 35 member states, including the United States. The other 11 AMR countries have reported completion of Phase 1 and are anticipated to submit final reports by the end of 2009. In the three polio-endemic WHO regions (8),§§ 43 (62%) of 69 polio-free countries and areas have completed Phase I activities.
Multiple challenges were faced in the 10 years required to complete Phase I in the WPR. Prioritization for WPV containment activities weakened in the ministries of health of many countries after regional certification. China and Japan had to access laboratories under the jurisdiction of a wide range of government agencies in addition to the ministries of health. During 2001--2006, identification of vaccine-derived polioviruses (9), which are considered equivalent as WPV for containment purposes, required several WPR countries to resurvey some facilities.
Despite the challenges, successful completion of Phase I activities is achievable even in countries with highly complex laboratory infrastructures. The only polio-free countries with comparable laboratory infrastructures remaining to complete Phase I activities are Egypt and South Africa. India, which remains polio-endemic in 2009, also has a complex laboratory infrastructure and will require similar efforts. Tangential benefits of Phase I were noted in the WPR and other regions. Authorities of many member states found that the national survey and inventory process led to a better understanding of the laboratory infrastructure of the country, a strengthened process for laboratory registration, and an increased awareness of the importance of maintaining biosafety standards.
A major accomplishment in the WPR was a progressive voluntary reduction in number of facilities retaining WPV from 147 provisionally reported in 1999 to 45 in 2008, certified by the ministries of health. Authorities in the four relevant WPR countries have indicated the intention to reduce further the number of facilities holding WPV materials.
Subsequent phases of WPV containment are outlined in the newly developed WHO Global Action Plan to Minimize Poliovirus Facility-Associated Risk in the Post-Eradication/Post-OPV Era (10). A revised draft edition will be available for public review and comment before the end of 2009.¶¶ This action plan includes containment of oral poliovirus vaccine (OPV) Sabin strains as well, and establishes the goal of reducing the number of facilities holding WPV worldwide to <20 in the post-eradication/post-OPV era, including vaccine manufacturers. Nonviable poliovirus reagents can replace live polioviruses in national surveillance and diagnostic facilities. Phase II of the action plan begins after evident interruption of WPV transmission in one of the four remaining endemic countries, during which member states are requested to establish long-term national policies and regulations for destruction and/or containment of WPV materials. Completion of Phase I in all countries of two WHO regions and the majority of countries in the other four regions, as of the end of 2008, provides a solid base for subsequent polio containment phases.
References
- World Health Assembly. 52nd session resolution WHA52.8. Eradication of poliomyelitis. Geneva, Switzerland: World Health Organization; 1999. Available at http://apps.who.int/gb/archive/pdf_files/wha52/ew8.pdf.
- World Health Assembly. 61st session resolution WHA61.1. Poliomyelitis: mechanism for management of potential risks to eradication. Geneva, Switzerland: World Health Organization; 2008. Available at http://apps.who.int/gb/ebwha/pdf_files/wha61-rec1/a61_rec1-part2-en.pdf.
- World Health Organization. WHO global action plan for laboratory containment of wild polioviruses. 2nd edition. Geneva, Switzerland: World Health Organization; 2004. Available at http://www.polioeradication.org/content/publications/who-vb-03-729.pdf.
- CDC. National laboratory inventory for global poliovirus containment---European Region, June 2006. MMWR 2006;55:916--8.
- World Health Organization Regional Committee for the Western Pacific. 50th session resolution WPR/RC50.R2. Eradication of poliomyelitis in the region. Manila, Philippines: World Health Organization Regional Committee for the Western Pacific; 1999. Available at http://www.wpro.who.int/rcm/en/archives/rc50/rc_resolutions/wpr_rc50_r02.htm.
- Wang M, Du L, Zhou DH, et al. [Study on the epidemiology and measures for control on severe acute respiratory syndrome in Guangzhou city][Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2003;24:353--7.
- Wilder-Smith A, Low JG. Risk of respiratory infections in health care workers: lessons on infection control emerge from the SARS outbreak. Southeast Asian J Trop Med Public Health. 2005;36:481--8.
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, 2008. MMWR 2009;58:308--12.
- CDC. Update on vaccine-derived polioviruses. MMWR 2006;55:1093--7.
- World Health Organization. WHO global action plan to minimize poliovirus facility-associated risk in the post-eradication/post-OPV era [draft]. Geneva, Switzerland: World Health Organization; 2009. Available at http://www.polioeradication.org/content/publications/gapiiiworkingdraft_07.pdf.
* American Samoa, Australia, Brunei Darussalam, Cambodia, China, Commonwealth of the Cook Islands, Fiji, French Polynesia, Guam, Hong Kong (China), Japan, Kiribati, Lao People's Democratic Republic, Macao (China), Malaysia, Marshall Islands, Micronesia, Mongolia, Nauru, New Caledonia, New Zealand, Niue, Northern Mariana Islands, Palau, Papua New Guinea, Philippines, Pitcairn Islands, Republic of Korea, Samoa, Singapore, Solomon Islands, Republic of Tokelau, Tonga, Tuvalu, Vanuatu, Vietnam, and Federated States of Wallis and Futuna.
† Facilities holding WPV infectious or potentially infectious materials (WPV materials) include diagnostic laboratories with frozen stool specimens, institutions with current or past research on polioviruses, teaching and industrial facilities that use poliovirus as a test virus, and vaccine manufacturers.
§ Infectious materials include clinical materials from persons with confirmed WPV infections; environmental sewage, or water samples in which WPV is present; and replication products containing WPV. Potentially infectious materials include feces, respiratory secretions, environmental sewage, and untreated water samples of unknown origin or collected for any purpose at a time and in a geographic area where presence of WPVs was suspected, and the replication products of such materials. Replication products include cell culture isolates, reference stocks, and laboratory derivatives in poliovirus-permissive cells or animals. For the purposes of containment, vaccine-derived poliovirus materials are treated as equivalent to WPV materials (3).
¶ Ministry of Health, Ministry of Education, State Environmental Protection Administration, State Drug Administration, and Chinese Academy of Science.
** Another facility was added to the national inventory after the survey, when newly requested reference strains were transferred from Japan's National Institute of Infectious Diseases.
†† The other two facilities holding WPV materials were regulatory and production facilities.
§§ African Region, Eastern Mediterranean Region, and South-East Asia Region.
¶¶ Additional information available at http://www.polioeradication.org.
FIGURE. Number of biomedical facilities reporting wild poliovirus (WPV) materials* and number of World Health Organization (WHO) member states and areas† having completed Phase I of the WPV containment process, by year --- WHO Western Pacific Region, 1999--2008
* WPV infectious and potentially infectious materials. Additional information available at http://www.polioeradication.org/content/publications/who-vb-03-729.pdf.
† Of the 37 member states/areas, the 21 Pacific island countries and areas are presented as a block and include American Samoa, Commonwealth of the Cook Islands, Fiji, French Polynesia, Guam, Kiribati, Marshall Islands, Micronesia, Nauru, New Caledonia, Niue, Northern Mariana Islands, Palau, Pitcairn Islands, Samoa, Solomon Islands, Republic of Tokelau, Tonga, Tuvalu, Vanuatu, and Federated States of Wallis and Futuna. The 16 other member states and areas are Australia, Brunei Darussalam, Cambodia, China, Hong Kong (China), Japan, Lao People's Democratic Republic, Macao (China), Malaysia, Mongolia, New Zealand, Papua New Guinea, Philippines, Republic of Korea, Singapore, and Vietnam.
Alternative Text: The figure above shows the number of biomedical facilities reporting wild poliovirus (WPV) materials and number of World Health Organization member states and areas having completed Phase I of the WPV containment process, by year from 1999-2008. According to the figure, when the Western Pacific Region was certified as polio-free in October 2000, all member states/areas had initiated Phase I, but only four member states/areas had completed it.
|
TABLE. Number of biomedical facilities surveyed for the presence of wild poliovirus (WPV) materials,* by country/area and type of facility, 1999--2008, and number retaining WPV materials in 2008 --- World Health Organization Western Pacific Region |
||||||
|---|---|---|---|---|---|---|
|
Country/Area |
Diagnostic |
Teaching/ Research |
Industrial |
Other/Unknown |
Total |
Retaining WPV materials |
|
Australia |
2,026 |
197 |
0 |
85 |
2,308 |
2† |
|
Brunei Darussalam |
12 |
2 |
0 |
0 |
14 |
|
|
Cambodia |
0 |
10 |
0 |
233 |
243 |
|
|
China |
52,502 |
1,704 |
379 |
1,103 |
55,688 |
27† |
|
Hong Kong (China) |
190 |
19 |
47 |
0 |
256 |
|
|
Japan |
10,865 |
1,280 |
1,285 |
639 |
14,069 |
15† |
|
Lao People's Democratic Republic |
23 |
2 |
0 |
1 |
26 |
|
|
Macao (China) |
9 |
3 |
1 |
1 |
14 |
|
|
Malaysia |
431 |
43 |
0 |
81 |
555 |
|
|
Mongolia |
53 |
6 |
0 |
12 |
71 |
|
|
New Zealand |
60 |
44 |
3 |
2 |
109 |
|
|
Pacific island countries and areas§ |
17 |
4 |
0 |
6 |
27 |
|
|
Papua New Guinea |
19 |
2 |
0 |
5 |
26 |
|
|
Philippines |
2,114 |
40 |
0 |
609 |
2,763 |
|
|
Republic of Korea |
299 |
427 |
37 |
4 |
767 |
1 |
|
Singapore |
83 |
43 |
19 |
38 |
183 |
|
|
Vietnam |
128 |
12 |
1 |
0 |
141 |
|
|
Total |
68,831 |
3,838 |
1,772 |
2,819 |
77,260 |
45† |
|
* WPV infectious and potentially infectious materials. Additional information available at http://www.polioeradication.org/content/publications/who-vb-03-729.pdf. † Includes 27 laboratories in the Global Polio Laboratory Network: Australia (one), China (25), and Japan (one). § The 21 Pacific island countries and areas are presented as a block and include American Samoa, Commonwealth of the Cook Islands, Fiji, French Polynesia, Guam, Kiribati, Marshall Islands, Micronesia, Nauru, New Caledonia, Niue, Northern Mariana Islands, Palau, Pitcairn Islands, Samoa, Solomon Islands, Republic of Tokelau, Tonga, Tuvalu, Vanuatu, and Federated States of Wallis and Futuna. |
||||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/9/2009


