Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
False-Positive Results with a Commercially Available West Nile Virus Immunoglobulin M Assay --- United States, 2008
In September 2008, CDC, the Food and Drug Administration (FDA), and state health departments began a nationwide investigation into an increase in false-positive test results obtained with a commercially available West Nile virus (WNV) immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay (ELISA). The investigation revealed that, in the United States, one lot of the commercially available test kits was the source of the false-positive results (1). That lot was recalled, and a second lot distributed outside the United States also was recalled (1). During July 1--September 30, 2008, the kit lot implicated in the United States resulted in positive tests on 568 specimens collected from 518 patients in 42 states and the District of Columbia (DC). A total of 166 (29%) specimens were retested at CDC, and 119 (72%) had false-positive results. A higher false-positive percentage were found among patients without evidence of neuroinvasive disease (77%) than patients with evidence of neuroinvasive disease (47%). Of the 518 patients, 249 (48%) had been reported to CDC as persons with WNV disease; however, only 45 (18%) had confirmatory testing that supported their inclusion in national surveillance data. Commercially available WNV test kits should be used to determine a presumptive diagnosis of WNV neuroinvasive disease. These kits should not be used to test specimens from persons without compatible illness, and any positive result should be confirmed by additional testing at a state health department or CDC.
WNV infection is a nationally notifiable disease. Cases of WNV disease are reported by state health departments to CDC through ArboNET, an Internet-based, passive surveillance system.* Cases reported to ArboNET must have clinical evidence of compatible illness and laboratory evidence of recent WNV infection (2). Based on patients' clinical signs and symptoms, WNV cases are classified as neuroinvasive disease (i.e., encephalitis, meningitis, or acute flaccid paralysis) or nonneuroinvasive disease (i.e., other febrile illness). Four FDA-cleared WNV serologic assays are commercially available for use in the United States. These assays are labeled for use on serum to aid in a presumptive diagnosis of WNV infection in patients who have clinical symptoms consistent with neuroinvasive disease. According to product inserts (3--6), all positive results obtained with these assays should be confirmed by plaque reduction neutralization test (PRNT) or by using current CDC guidelines for laboratory diagnosis of this disease (7).
Initial Investigation
In summer 2008, three state health departments independently contacted CDC regarding positive WNV IgM antibody test results in patients who lacked clinical or epidemiologic evidence of WNV infection. All of these tests results originated from one large commercial laboratory that was using the PanBio WNV IgM ELISA test kit manufactured by Inverness Medical (Princeton, New Jersey). On September 5, 2008, the New York State Department of Health's Wadsworth Center laboratory reported that 13 (86%) of 15 specimens testing positive for WNV IgM antibodies at the commercial laboratory in August were negative upon retesting at the state laboratory. On September 10, CDC notified all state health departments of the potential problem and initiated an investigation into the cause of the false-positive test results (1).
In late September, one of the affected commercial laboratories sent a convenience sample of 64 specimens that had yielded positive or negative WNV IgM antibodies results to CDC and the kit's manufacturer for retesting. This evaluation identified two lots of the kit with higher false-positive rates (20% and 56%) than the expected rate calculated from data in the package insert (2% [95% confidence interval = 0%--9%]). On October 8, these two lots were recalled voluntarily by the manufacturer. The lot with the 56% false-positive rate had been distributed to four laboratories in the United States and was used for testing specimens during July--September (Figure 1). The other lot was distributed outside the United States (1). On October 14, a CDC health advisory was distributed (1), and the investigation was expanded to determine the scope and impact of the problem in the United States.
Expanded Investigation
In September, CDC, along with state and local health departments, surveyed the four laboratories that had received the recalled kit lot to determine the number of positive specimens obtained using the lot and to collect corresponding demographic information regarding these patients. State health departments provided additional information regarding WNV confirmatory testing performed in state laboratories, patient clinical syndromes (e.g., neuroinvasive or nonneuroinvasive), and case status as reported to ArboNET.
The recalled WNV ELISA kit lot had produced positive results for 568 specimens obtained from 518 patients in 42 states and DC (Figure 2). Of the 488 patients for whom clinical information was known, 83 (17%) had symptoms consistent with WNV neuroinvasive disease, 242 (50%) had symptoms consistent with nonneuroinvasive disease, and 163 (33%) had no symptoms consistent with WNV disease.
During October--December, 166 (29%) available specimens of the 568 that tested positive with the implicated kit lot were identified and sent to CDC to be retested using WNV IgM microsphere immunofluorescence assay (MIA) and IgM capture ELISA.† Based on retesting, specimens were classified as false-positive, true-positive, or indeterminate. Of the 166 retested, 45 (27%) were classified as true-positive and 119 (72%) as false-positive results; two specimens had an indeterminate result. The retested specimens came from 160 patients; clinical syndrome was known for 157 of these patients. Of the 157, a higher percentage of false-positives was found among patients without evidence of neuroinvasive disease (77% [98 of 127]) than among patients with evidence of neuroinvasive disease (47% [14 of 30)]) (p<0.001 by chi-square test).
Of the 518 patients testing positive for WNV with the implicated kit lot, 249 (48%) had been reported to ArboNET as having WNV disease. However, only 45 (18%) of these 249 cases had confirmatory testing supporting their inclusion as WNV disease cases; 77 (31%) cases did not have evidence of WNV infection based on subsequent laboratory testing, and 127 (51%) cases had no further testing performed. For the remaining 269 (52%) of the 518 patients, case investigation by state health departments found no illness clinically compatible with WNV disease; therefore, these patients were not reported to ArboNET.
Reported by: DF Neitzel, MS, MM Kemperman, MPH, Minnesota Dept of Health. S Semple, MS, New Jersey Dept of Health and Senior Svcs. S Wong, PhD, Wadsworth Center; J Hallisey, MPH, New York State Dept of Health. MA Feist, TK Miller, MPH, North Dakota Dept of Health. WM Chung, MD, Dallas County Dept of Health and Human Svcs. S Hojvat, PhD, P Summers, MS, Food and Drug Admin. RS Lanciotti, PhD, AJ Panella, MPH, J Laven, O Kosoy, MS, JA Lehman, RS Nasci, PhD, M Fischer, MD, JE Staples, MD, Arboviral Diseases Br, E Zielinski-Gutierrez, DrPH, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; KB Janusz, DVM, EIS Officer, CDC.
Editorial Note:
After detection of WNV in the United States in 1999, diagnostic testing initially was performed only at CDC and later at state public health laboratories. In recent years, commercially available WNV diagnostic assays have been offered at an increasing number of commercial laboratories (8). Positive test results obtained using these assays help provide a presumptive diagnosis of WNV infection in patients with neuroinvasive disease; however, all positive assay results should be confirmed by further laboratory testing (3--6).
This investigation determined that use of one WNV IgM ELISA kit lot at four laboratories in the United States produced a substantial number of false-positive test results and inflated the number of WNV disease cases initially reported to ArboNET for 2008. The manufacturer voluntarily recalled the implicated lot and is working with FDA to improve the quality control and batch release procedures for its WNV IgM ELISA kits. In accordance with Clinical Laboratory Improvement Amendments (CLIA) regulations, commercial laboratories that perform diagnostic testing, including for WNV, also should monitor the ongoing performance of the kits they use (9,10). Before this investigation, confirmatory testing had been performed on <10% of the 568 specimens that had tested positive with the recalled kit lot. Health-care providers should consider that commercially available WNV IgM kits are only intended to help provide a presumptive diagnosis of WNV neuroinvasive disease when requesting testing and interpreting the results. In addition, commercial laboratories should work with public health laboratories to ensure that confirmatory testing is performed on all presumptive positive results.
The findings in this report are subject to at least two limitations. First, only 29% of the specimens that tested positive at CDC were available for retesting, limiting the precision with which the actual number and proportion of false-positive tests could be determined. Second, the impact of false-positive results on patient diagnosis and management was not assessed.
This multistate investigation required a considerable public health response to notify health-care providers, retest specimens, and reevaluate WNV cases reported to ArboNET. Applying the 72% false-positive proportion to all 568 specimens testing positive with the recalled kit lot, an estimated 400 specimens were incorrectly identified as positive for WNV IgM antibodies. Given that large proportion of false-positives, CDC recommended that state health departments not classify patients as having WNV disease if the only laboratory evidence was from the recalled kit lot. States have since reevaluated affected cases to arrive at the final WNV disease totals for 2008 (available at http://www.cdc.gov/westnile).
Acknowledgments
This report is based, in part, on contributions of members of the WNV False-Positive IgM ELISA Investigation Team, which includes state and local vector-borne disease coordinators and state public health laboratory workers.
References
- CDC. CDC health advisory: false-positive results with a commercially available West Nile virus IgM ELISA kit. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www2a.cdc.gov/han/archivesys/viewmsgv.asp?alertnum=00278.
- CDC. Neuroinvasive and non-neuroinvasive domestic arboviral diseases. 2004 case definition. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www.cdc.gov/ncphi/disss/nndss/casedef/arboviral_current.htm.
- Inverness Medical Innovations Australia Pty Ltd. PanBio WNV IgM capture ELISA test product overview. Available at http://www.panbio.com/download/PB0057.pdf.
- InBios International, Inc. West Nile detect IgM capture ELISA package insert. Available at http://www.inbios.com/cms/file/900015_2%20West%20Nile%20Detect%20IgM%20Capture%20ELISA%20Human%20Insert.pdf.
- Focus Diagnostics, Inc. West Nile virus IgM capture DxSelect package insert. Available at http://www.focusdx.com/focus/packageinsert/el0300M.pdf.
- Spectral Diagnostics, Inc. Spectral West Nile virus IgM test. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf5/k052519.pdf.
- CDC. Laboratory diagnosis. In: Epidemic/epizootic West Nile virus in the United States: guidelines for surveillance, prevention, and control. Fort Collins, CO: US Department of Health and Human Services, CDC; 2003. Available at http://www.cdc.gov/ncidod/dvbid/westnile/resources/wnvguidelines2003.pdf.
- Lehman J, Janusz K, Fischer M, Staples JE. West Nile virus testing performed at public health and commercial laboratories [Poster]. Presented at the Tenth National Conference on West Nile Virus in the United States. Savannah, GA: February 19--20, 2009.
- Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments overview. Baltimore, MD: US Department of Health and Human Services, Centers for Medicare & Medicaid Services; 2009. Available at http://www.cms.hhs.gov/clia.
- CDC. Current CLIA regulations §493.1256, §493.1281(b), §493.1282 (a and b), and §493.1289. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://wwwn.cdc.gov/clia/regs/toc.aspx.
* Available at http://www.cdc.gov/ncidod/dvbid/westnile/index.htm.
† An additional 58 (10%) of the 568 positive specimens were retested at state public health laboratories. Various assays were used; therefore, the results are not directly comparable to those from CDC. Nonetheless, of the 58 retested, a percentage similar to that found at CDC (64%) had false-positive results. In addition, false-positive percentages similar to those found at CDC were detected in persons without evidence of neuroinvasive disease (88%) and with evidence of neuroinvasive disease (33%) (p<0.001 by chi-square).
FIGURE 1. Number of specimens (N = 568) testing positive for West Nile virus immunoglobulin M antibodies, using one lot from a commercially available test kit that was later recalled, by week --- United States, July--September 2008
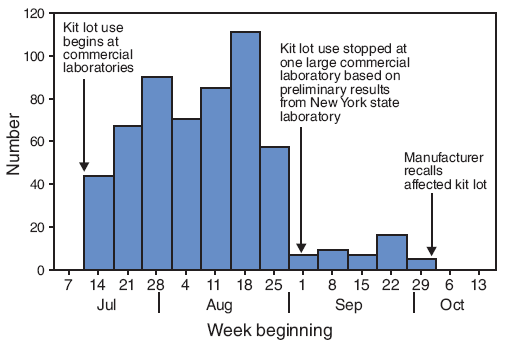
Alternative Text: The figure above shows the 568 specimens testing positive for West Nile virus immunoglobulin M antibodies, using one lot from a commercially available test kit that was later recalled, by week of test in the United States from July through September 2008. From the week beginning July 14 to the week beginning August 25, 40 to 120 specimens tested positive. After one large laboratory stopped using the kit lot on September 1, the number testing positive dereased sharply. The affected kit lot was recalled by the manufacturer the week beginning September 29.
FIGURE 2. Number of persons (N = 518) testing positive for West Nile virus immunoglobulin M antibodies using one lot from a commercially available test kit that was later recalled --- United States, July--September 2008
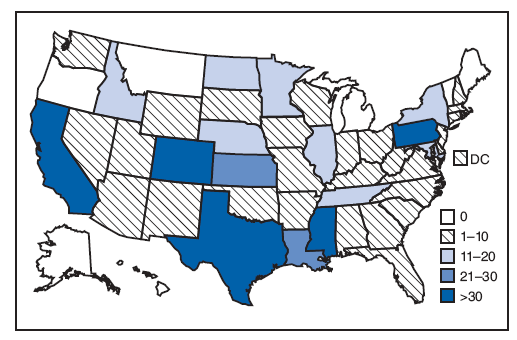
Alternative Text: The figure is a map of the United States showing the 518 persons testing positive for West Nile virus immunoglobulin M antibodies, using one lot from a commercially available test kit that was later recalled, by state, from July through September 2008. The number of persons per state ranged from zero to more than 30.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/7/2009


