 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Investigation of a Genotype Cluster of Tuberculosis Cases --- Detroit, Michigan, 2004--2007In August 2007, the Detroit Department of Health and Wellness Promotion, Michigan Department of Community Health (MDCH), and CDC investigated a genotype cluster of eight tuberculosis (TB) cases in U.S.- born patients in the Detroit metropolitan area. The cases had been reported during December 2004--April 2007. The first case was reported in a patient (the index patient) whose drug-susceptible TB subsequently developed multidrug resistance. Seven additional cases were reported in patients with Mycobacterium tuberculosis genotypes that matched the genotype of the index patient. These included one case of multidrug-resistant (MDR) TB in a young relative of the index patient and one case in the index patient's parent, who died from TB meningitis. This report describes the investigation and illustrates the importance of ensuring that each case of TB disease is promptly recognized and successfully treated and that all close contacts of TB patients are identified, evaluated, and treated for latent TB infection if indicated (1,2). TB genotyping is laboratory-based testing used to analyze the genetic material of TB bacteria. TB genotyping results, when combined with epidemiologic data, help identify persons with TB disease involved in the same chain of recent transmission. CDC's National Tuberculosis Genotyping Service was initiated in January 2004 to enable rapid genotyping of isolates from every patient in the United States with culture-positive TB (3). In 2007, genotyping information was available for 86% of culture-positive TB cases nationwide (3) and nearly 100% of cases in Michigan. The national service contracts with the MDCH laboratory, which provides M. tuberculosis genotyping results in 10--14 working days from two polymerase chain reaction (PCR)-based genotyping tests: spoligotyping and mycobacterial interspersed repetitive units (MIRU) typing (4). For this investigation, genotype-matched cases were defined as those whose isolates had matching spoligotype and MIRU patterns.* To further distinguish strains for isolates with identical PCR results, confirmatory restriction fragment length polymorphism (RFLP) testing (4) was conducted on isolates from both the early (December 2004) and later (July 2006) disease course for the index patient and from all seven suspected secondary cases in the TB cluster. All RFLP patterns matched, including both the index patient's early drug-susceptible TB isolate and later MDR TB isolate, implying that rather than being infected by a new MDR TB strain, the index patient remained infected with the initially drug-susceptible TB strain that developed resistance during the course of treatment. During December 2004--April 2007, when the eight genotype-matched cases were identified, approximately 350 additional cases of TB were diagnosed in the Detroit metropolitan area; however, none of those cases, nor any other Michigan cases, had isolates matching the genotype cluster described in this report. In December 2004, the index patient, an unemployed adult with a history of excessive alcohol and illicit drug use and unstable housing arrangements (i.e., living with various friends and family members), was first evaluated in a local emergency department for cough, hemoptysis, fever, fatigue, and night sweats of 1-month duration. Acid fast-bacilli (AFB) smear-positive, cavitary TB was diagnosed, and the patient began standard treatment with the four first-line TB drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) (5). Initial drug-susceptibility testing (DST) on an isolate from the patient indicated the patient's TB strain was susceptible to all first-line drugs. The patient initially agreed to receive directly observed therapy (DOT), a mainstay of TB treatment in which patients are observed to ingest each dose of medication to maximize the likelihood of completion of therapy (5). Approximately 5 weeks later, in February 2005, the patient began missing DOT appointments, and the local health department began exploring legal options, such as confinement via court order for treatment, to ensure patient adherence. However, during February--April 2005, the patient was lost to follow-up. A contact investigation conducted during December 2004--January 2005, after the patient's disease was first diagnosed, included five household contacts, all of whom had negative initial tuberculin skin test (TST) results. A second round of skin testing was planned for 8--10 weeks after the initial round (2). Despite numerous attempts by health department staff members, four household contacts, including the patient's parent, declined a second evaluation. The one contact who was retested (with the permission of an adult in the home), the patient's child, had a second negative TST result in April 2005. In April 2005, the index patient began picking up TB medications at the health department each month. The patient's AFB sputum smear test results were negative for the first time, but became positive again by June; DOT was not enforced during this period. From initial diagnosis through June 2005, the index patient's sputum specimens remained culture positive. During July--December 2005, the patient again was lost to follow-up and received no treatment for TB. In January 2006, the patient returned to the health department with cough and malaise. At that time, the patient's radiographs showed worsening cavitary disease; the AFB sputum smear result was positive, and DST still indicated drug-susceptible TB. The patient was restarted on isoniazid, rifampin, and pyrazinamide but did not comply with DOT. In September 2006, DST results on an M. tuberculosis isolate collected from the index patient in July 2006 indicated MDR TB (i.e., resistance to isoniazid and rifampin). The isolate was susceptible to pyrazinamide, ethambutol, streptomycin, ciprofloxacin, kanamycin, ethionamide, cycloserine, and capreomycin. The patient was prescribed an MDR TB treatment regimen of ethambutol, pyrazinamide, moxifloxacin, and streptomycin. However, in December 2006, the patient's sputum remained AFB smear positive and culture positive for MDR TB, despite consistently taking prescribed medication via DOT during September--December 2006, according to clinic records. In December 2006, a parent of the index patient died from unrecognized TB meningitis. The otherwise healthy parent had reported chronic headaches and lower back pain during the fall of 2006, progressing to weight loss, fatigue, and general debilitation; human immunodeficiency virus serologies were not tested. Mycobacteria culture results were not available until after the parent's death. Cerebrospinal fluid cultures revealed M. tuberculosis with a genotype that matched that of the index patient. DST results on the parent's isolate indicated drug-susceptible TB, suggesting that transmission from the index patient had occurred before July 2006, when the index patient was first known to have MDR TB. After the parent's culture and autopsy results became available, the health department decided to revisit and intensify the investigation of the index patient's contacts, focusing on family members because the index patient remained unwilling to name social contacts. In February 2007, a young relative of the index patient who spent considerable time in the same house (not the patient's child), had a positive TST result (25 mm induration). The child was asymptomatic, and a chest radiograph showed left hilar lymphadenopathy, which was not interpreted as TB. No medications were started, and the child was scheduled to return 2--3 weeks later for reevaluation. Six weeks later, this young patient was hospitalized for cough, fever, night sweats, and weight loss. The child's chest radiographs were consistent with TB pneumonia; sputum smear results were AFB negative. A sputum culture was positive for MDR TB, suggesting that the young relative had been infected by the index patient after July 2006, when the index patient was first known to have MDR TB. Because of the death and pediatric MDR TB diagnosis associated with the index patient's TB, in August 2007 the health department invited MDCH and CDC to assist in its investigation of the other cases in this genotype cluster. During December 2004--April 2007, in addition to the index patient and the two relatives, five other patients had matching genotypes (Table 1). Three of the five had drug-susceptible TB: a known social contact of the index patient and two persons with unconfirmed social contact who frequented the same neighborhood. The other two patients had M. tuberculosis isolates with a different drug-resistance pattern (pyrazinamide monoresistance) and lacked any clear epidemiologic links to the index patient or the other cases. All patients except the index patient's parent and young relative reported excessive alcohol use (Table 2). The patients ranged in age from 15 to 47 years (median: 37.5 years). Five of the seven patients in this cluster who were eligible for DOT did not receive it consistently. During the entire investigation, a total of 79 contacts of the eight patients in this cluster were identified. Fifty-one (65%) contacts were fully evaluated. Of these, two had a self-reported history of previous completion of TB treatment. Five (10%) of the 51 had a positive TST result and began therapy for latent TB infection. Of the 28 contacts who were not fully evaluated, 14 (50%) could not be located, 11 (39%) moved to another state, and three (11%) declined evaluation. No additional cases were identified. As of February 2009, the index patient was clinically stable with negative AFB sputum smear and culture results and improvement noted on chest radiographs. The patient continues to receive MDR TB treatment by DOT. The patient will receive treatment through at least May 2009 to complete 18--24 months of appropriate TB therapy (5). The index patient's young relative with MDR TB and the remaining five patients in the cluster have all successfully completed TB treatment. Reported by: M Dixon, MD, City of Detroit Dept of Health and Wellness Promotion; A Knecht, MPH, P Davidson, PhD, Michigan Dept of Community Health. V Green, MSPH, T Cropper, D Tuckey, MPH, L Lambert, MPH, M Haddad, MSN, Div of TB Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; S Bamrah, MD, J Finks, DVM, EIS officers, CDC. Editorial Note:Results of this cluster investigation revealed that at least four, and likely six, TB patients were involved in the same chain of transmission. These patients included the index patient, a young relative with MDR TB, the index patient's parent who died of TB meningitis, a known social contact, and two persons with unconfirmed social contact who frequented the same neighborhood. TB genotyping can help detect TB outbreaks earlier by highlighting unsuspected relationships among patients (6). In this cluster, several patients were unwilling to name social contacts, and TB genotyping proved useful in establishing otherwise undisclosed relationships. The result of this more rigorous investigation demonstrated ongoing transmission among a larger group of patients than originally identified. However, although TB genotyping is useful in establishing connections between patients, it cannot be used without also pursuing epidemiologic links. Two of the eight patients described in this cluster probably were not part of the same transmission chain, based on having a unique DST pattern (resistance to pyrazinamide only) and no clear epidemiologic link to the other six patients in this cluster. TB is a nationally notifiable infectious disease; successful treatment of TB benefits not only the individual but also the community (5). In this outbreak, the index patient probably was contagious for >1,000 days. Multiple interrelated factors contributed to treatment interruptions and inconsistent DOT, including the index patient's excessive alcohol and illicit drug use and unstable housing arrangements and a general misunderstanding and mistrust among patients and their contacts of the health department's responsibility for TB patient care. DOT is a key component and an important example of the many measures used in patient-centered case management. DOT ensures a patient's adherence to treatment, prevents development of drug resistance, and should be considered for all TB patients (5). The sufficiency of laws that authorize and support public health agencies' use of DOT and other roles in preventing the spread of TB can vary by jurisdiction (7). More recently, CDC and some of its public health partners have explored various approaches to strengthening public health agencies' legal preparedness for TB control and prevention. These approaches include 1) development of tools such as model legislative provisions that state policy makers and public health officials might use for examining existing laws regarding TB control, 2) table-top exercises for assessing understanding of jurisdiction-specific laws for TB control, and 3) informational guides, such as a handbook on TB control law, designed for public health practitioners and their legal counsel. This cluster also demonstrates the importance of TB contact investigations to prevent disease. A key challenge in the control of TB in the United States is conducting thorough investigations to protect the contacts of persons with infectious TB. Suboptimal contact investigations might occur when the persons with TB, such as those described in this report, are unable or unwilling to cooperate with the health department, or when public health resources for TB control measures are limited (2). Acknowledgments This report is based, in part, on contributions by D Kissner and the staff of the City of Detroit Dept of Health and Wellness Promotion, Communicable Disease/TB Control and Prevention Section; D Berry, M Wilkins, and C Miller, Michigan Dept of Community Health; A Buff, P Cruise, D Hardge, B Heath, J Keller, A Locke, P Moonan, M O'Rourke, and M Patterson, Div of TB Elimination, CDC; and R Goodman, Public Health Law Program, CDC. References
* Spoligotype 677737607760731 and MIRU patterns 224225163321 or 224225-63321. Table 1 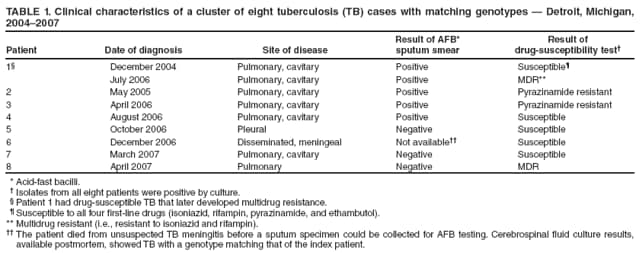 Return to top. Table 2 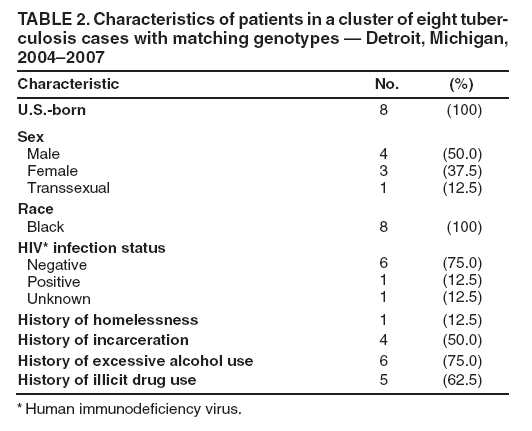 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/12/2009 |
|||||||||
|