 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
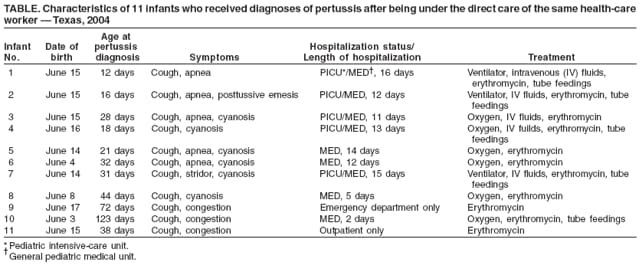
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hospital-Acquired Pertussis Among Newborns --- Texas, 2004On July 10, 2004, staff members at a children's hospital in Texas noted that six infants with pertussis diagnosed by clinical symptoms and confirmed by polymerase chain reaction (PCR) testing had all been born during June 4--16 at the same area general hospital. The infants had symptoms consistent with pertussis, including cough, congestion, cyanosis, emesis, or apnea. Infection-control personnel at the general hospital (general hospital A), children's hospital (children's hospital A), and the county health department investigated and determined that an outbreak of pertussis among 11 newborns at general hospital A had occurred after direct exposure to a health-care worker (HCW) with pertussis. This report describes the outbreak investigation and highlights the importance of following recommendations to administer tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine to HCWs to prevent transmission of pertussis to patients. Immediately after identification of the six infants with pertussis at children's hospital A, hospital staff members reviewed newborn nursery charts at general hospital A. One staff member (HCW A) was identified as having directly cared for all six infants during their stay in the newborn nursery. Review of work logs for all shifts identified four additional hospital workers who had been present while the six infants were in the newborn nursery. From early to mid-June until July 17, while working in the newborn nursery at general hospital A, HCW A had exhibited symptoms of pertussis, including cough, posttussive emesis, and dyspnea. Her spouse reportedly had similar symptoms after he returned from a trip to California, 2--3 weeks before HCW A began exhibiting her symptoms. HCW A, aged 24 years, had been fully vaccinated for pertussis during early childhood. HCW A and a nursery coworker with cough symptoms were tested for pertussis by PCR; only HCW A tested positive. On July 17, HCW A was furloughed from general hospital A for 5 days and treated with erythromycin. Her husband also was prescribed erythromycin. After obtaining Institutional Review Board approval from the institutions involved, staff members at children's hospital A reviewed the charts and laboratory records of all patients aged <4 months who had received a diagnosis of pertussis during June--August 2004. During that period, no additional cases of pertussis were reported to the county health department from facilities other than children's hospital A. A case of pertussis was defined in accordance with the Council of State and Territorial Epidemiologists (CSTE) case definition for pertussis, with one variation. The CSTE case definition for pertussis is a cough illness lasting at least 2 weeks with one of the following symptoms and no other apparent cause (as reported by a health professional): paroxysms of coughing, inspiratory "whoop," or posttussive vomiting. Confirmatory criteria consist of either isolation of B. pertussis from a clinical specimen or positive PCR assay for B. pertussis. For this investigation, that definition was modified to include infants with cough illness of any duration so that the definition might cover cases in newborns in the first 2 weeks of life. PCR amplification and detection of a 114 nucleotide segment of the B. pertussis IS481 sequence (1) was conducted using nucleic acid extracted from nasopharyngeal swabs. The review of laboratory records and charts at children's hospital A revealed that 29 infants aged <4 months met the case definition for pertussis during June--August. Of these 29 infants, 11 (including the six previously known patients) had been born at general hospital A and directly exposed to HCW A in the newborn nursery. All 11 had been treated at children's hospital A with erythromycin and recovered; none developed hypertrophic pyloric stenosis, which has been reported as a complication of treatment of infants with erythromycin (2). Five of the infants required admission to the pediatric intensive-care unit (PICU), and four were treated in the general pediatric medical unit; one infant was treated in the emergency department, and one was treated as an outpatient (Table). Median age of the 11 infants born at general hospital A was 31 days at the time of pertussis diagnosis, compared with a median age of 61 days for the other 18 infants with diagnosed pertussis, who were born at 12 other general hospitals during June--August. On July 21, 2004, the county health department directed general hospital A to contact the families of all infants who had been in its newborn nursery during May 31--July 17 so that the infants could be screened for respiratory symptoms and administered antibiotics as needed. Families of 158 infants who had been in the newborn nursery during May 31--July 17 were contacted, and a total of 110 infants returned to general hospital A. Eighteen of the 110 had cough but were PCR negative; they received erythromycin prophylaxis. Two infants had cough and also were PCR positive; they were treated for pertussis, and one was admitted to children's hospital A. In addition, three family members reported cough or runny nose but were PCR negative; they were treated with erythromycin. During the period that HCW A exhibited symptoms, she directly cared for 113 infants, 11 of whom subsequently had a diagnosis of pertussis, resulting in an attack rate of 9.7%. One other possible case was identified in a sibling aged 3 years. Interviews with families when they brought their infants back to general hospital A for screening, revealed no other exposures to pertussis. No secondary cases of pertussis among HCWs at either general hospital A or children's hospital A were discovered. After HCW A was furloughed and treated, no new cases of pertussis were identified during September--October 2004 in infants born at general hospital A. Reported by: JL Hood, MPH, DK Murphey, MD, JJ Dunn, PhD, children's hospital A, Texas. Editorial Note:Pertussis is a highly contagious, vaccine-preventable illness caused by Bordetella pertussis infection. Complications of pertussis (e.g., seizures, pneumonia, encephalopathy, and cardiovascular compromise) can occur, especially in infants aged <1 year. Deaths from pertussis occur most frequently among infants; the case-fatality rate is 1.8% for newborns and infants aged <2 months (3). From 1980--1989 to 1990--1999, the number of infant deaths from pertussis increased from 61 (1.67 deaths per million) to 93 (2.40 deaths per million) (4). Newborns most commonly acquire pertussis from adults with undiagnosed disease (5). Reports on outbreaks of pertussis in health-care facilities and neonatal nurseries have been published previously (6,7). In 2004, the reported incidence of pertussis in the United States nearly tripled compared with 2001, and the number of reported cases exceeded any year since 1959 (8). This increase might have resulted, in part, from increased use of more sensitive PCR testing (8). CDC recommendations call for culture confirmation of infection in one or more cases in an outbreak. However, in the outbreak described in this report, no culture confirmation was performed. The medical staff at children's hospital A requested PCR testing, as did the local health department. Current molecular detection methods for detection of B. pertussis have high sensitivity compared with culture, but occasionally can be prone to false positives, depending on the target sequences, interpretation of results, and subjects tested (9). In a recent report describing outbreaks of respiratory illness mistakenly attributed to pertussis, PCR was used inappropriately as a mass screening tool on a large number of persons who did not meet the CSTE case definition for pertussis (9). For the infants described in this report, a high index of suspicion for pertussis was based on clinical symptoms, and PCR testing was used to confirm diagnoses of pertussis. HCW A also met the CTSE case definition for pertussis. In 2005, Tdap vaccine was licensed by the Food and Drug Administration for use in adolescents and adults. In December 2006, the Advisory Committee on Immunization Practices (ACIP) recommended use of Tdap vaccine for HCWs with direct patient contact and for adults who have or might have close contact with infants aged <12 months (3). This recommendation was based on the documented risk for transmission of pertussis in health-care facilities. Despite the costs involved for health-care facilities, one study suggests the return on investment from vaccinating HCWs with Tdap vaccine is twice the cost of the vaccine (10). Widespread implementation of Tdap vaccination of adolescents and adults as recommended by ACIP can reduce the risk for pertussis in the community and the incidence of pertussis transmission in health-care facilities. This outbreak also highlights the importance of rapid recognition of pertussis transmission in health-care settings and rapid response from hospital and public health practitioners to identify the source and prevent more extensive spread of disease, particularly among vulnerable newborns and infants. Acknowledgment The findings in this report are based, in part, on observations first made by S Roderick. References
 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/4/2008 |
|||||||||
|