 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Mumps Epidemic --- Iowa, 2006In the United States, since 2001, an average of 265 mumps cases (range: 231--293 cases) have been reported each year,* and in Iowa, an average of five cases have been reported annually since 1996.† However, in 2006, by March 28, a total of 219 mumps cases§ had been reported in Iowa, and an additional 14 persons with clinically compatible symptoms were being investigated in three neighboring states (11 in Illinois, two in Nebraska, and one in Minnesota) in what has become the largest epidemic of mumps in the United States since 1988 (1). This report summarizes and characterizes the ongoing mumps epidemic in Iowa, the public health response, and recommendations for preventing further transmission. Mumps is an acute viral infection characterized by fever and nonsuppurative swelling of the salivary glands; an estimated 20%--30% of cases are asymptomatic. Complications can include inflammation of the testicles or ovaries, meningitis/encephalitis, spontaneous abortion, and deafness. During the prevaccine era, nearly everyone in the United States experienced mumps, and 90% of cases occurred among children aged <15 years. In 1977, Iowa law mandated 1 dose of measles, mumps, and rubella (MMR) vaccine for entry to public schools; in 1991, the mandate became 2 doses. For the 2004--05 school year, 97% of children entering school in Iowa had received 2 doses of MMR vaccine (2). The first reports to the Iowa Department of Public Health (IDPH) of mumps-like illness occurred in December 2005 at a university in eastern Iowa, where several students with glandular swelling were tested; two tested positive for mumps-specific IgM antibodies. In mid-January 2006, an isolate from an unrelated patient was cultured and identified as mumps virus at the University Hygienic Laboratory (Iowa's state public health laboratory). Viral isolates were sent to CDC, and the mumps strain was identified as genotype G. By mid-February, active surveillance had been initiated in seven geographic areas, including the campuses of the three largest universities in Iowa. Of the 219 cases reported in Iowa, the median patient age was 21 years (range: 3--85 years), with 48% of patients aged 17--25 years; 30% (34 of 114) were known to be college students. Of the 133 patients with investigated vaccine history, 87 (65%) had documentation of receiving 2 doses, 19 (14%) 1 dose, and eight (6%) no doses; vaccine status could not be documented in 19 (14%) patients. Among the 114 patients for whom symptomatic information was available, the most common symptoms were parotitis in 94 (83%) patients, submaxillary/sublingual gland swelling in 46 (40%), fever in 41 (36%), and sore throat in 36 (32%); average duration of illness was 5.1 days. Six (5%) patients reported complications (e.g., orchitis); one suspected case of encephalitis is being investigated. As of March 28, 2006, investigators had determined that only 36 (16%) of the 219 cases were linked epidemiologically (i.e., a source of infection was identified), suggesting frequent unapparent transmission. The source of the Iowa epidemic is unknown; however, the United Kingdom (UK) experienced a recent mumps epidemic that peaked during 2005 with approximately 56,000 cases and a high attack rate among young adults (3). The mumps strain in the UK epidemic also was identified as genotype G (4), and the UK epidemic has been linked to a 2005 mumps outbreak in the United States (5). To educate health-care professionals in Iowa regarding the epidemic and mumps, information has been distributed via Iowa's Health Alert Network (HAN), in weekly electronic newsletters, and via frequent conference calls. The IDPH website¶ has provided biweekly updates, county case counts, fact sheets, and guidance to local health departments and health-care facilities on case investigations. IDPH recommendations include 1) requesting at least 5 days of isolation for all patients (quarantine is not being used), 2) ensuring that students and staff members on all Iowa college campuses have had 2 doses of MMR or are immune from mumps (6), 3) assessing vaccination status of all health-care professionals in Iowa and offering vaccination where appropriate (7), and 4) sending all specimens collected from possible cases to University Hygienic Laboratory for testing. Despite control efforts and a highly vaccinated population, this epidemic has spread across Iowa and potentially to neighboring states. Ongoing investigations will focus on identifying actual vaccine coverage on college campuses, potential modes of mumps transmission, and the effectiveness of 1 or 2 doses of MMR. Reported by: Local Iowa public health departments; University Hygienic Laboratory, Iowa City; P Quinlisk, MD, M Harris, MPH, T Thornton, Iowa Dept of Public Health. L Flamigni, MD, EIS Officer, CDC. References
* Data available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5511md.htm#tab1. † Data available at http://www.idph.state.ia.us/adper/common/pdf/cade/decades.pdf. § Includes 150 confirmed, nine probable, and 60 suspect cases. Case definitions were modified from Council of State and Territorial Epidemiologists/CDC mumps case definitions for use in this outbreak. Confirmed: case that meets the clinical case definition (i.e., unilateral or bilateral tender, self-limited, swelling of the parotid or other salivary gland, lasting >2 days and without other apparent cause) and is laboratory confirmed (i.e., by a positive IgM test result or positive viral culture) or epidemiologically linked to a confirmed case. A confirmed case can be asymptomatic if a mumps viral culture is positive. Probable: case that meets the clinical case definition but has noncontributory or no serologic or virologic testing and is not epidemiologically linked to a confirmed or probable case. Suspect: case with a positive IgM test result but no confirmation of the clinical definition. ¶ Available at http://www.idph.state.ia.us. Figure 1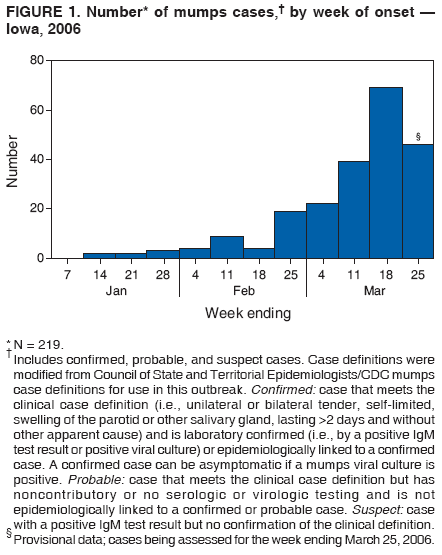 Return to top. Figure 2 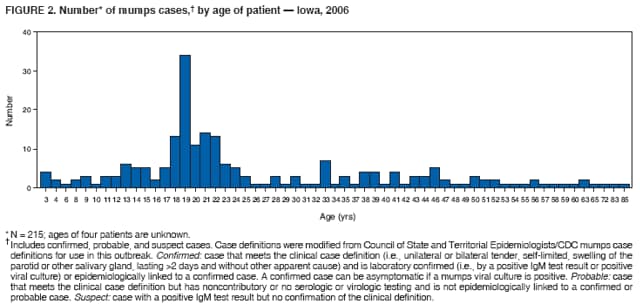 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/30/2006 |
|||||||||
|