 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Primary Amebic Meningoencephalitis --- Georgia, 2002In early September 2002, the Georgia Division of Public Health and CDC were notified about a fatal case of primary amebic meningoencephalitis (PAM) caused by Naegleria fowleri in a boy aged 11 years who had recently swum in a local river. This report summarizes the case investigation. In response to this case, the district health department recommended that local community authorities advise persons to avoid swimming in this river during periods of high temperature and low water depth. In late August, the previously healthy boy was evaluated in a local emergency department for a 2-day history of headache and emesis; he was febrile and lethargic without focal neurologic or meningeal signs. A computerized tomography (CT) scan of the head without contrast was normal. Lumbar puncture was unsuccessful, and the patient was started on intravenous antibiotics for suspected bacterial meningitis. Within several hours of admission, he had spontaneous nonpurposeful movements, was unable to follow verbal commands, and was transferred to a children's hospital intensive care unit (ICU). En route to the ICU, he had a 30-minute right-sided seizure. A CT scan of the head on admission to the ICU showed edema of the midbrain, and cranial magnetic resonance imaging (MRI) demonstrated areas of meningeal enhancement in the brainstem suggestive of meningitis. No organisms were observed on a Gram-stained smear of cerebrospinal fluid (CSF); CSF antigen-detection tests were negative for bacterial pathogens. Fresh preparation of CSF revealed no amebae. CSF red blood cell count was 1,550/mm3 (normal: 0/mm3), white blood cell count was 13,650/mm3 (normal: 0--5/mm3), glucose was <5 mg/dL (normal: 40--70 mg/dL), and protein was 679 mg/dL (normal: 12--60 mg/dL). Follow-up lumbar puncture later the same day revealed motile amebae in a centrifuged CSF specimen. The patient was started on intravenous amphotericin and oral rifampin and ketoconazole. Approximately 12 hours after admission to the ICU, the patient had apneic episodes and anisocoria and was tracheally intubated. Treatment included hyperventilation, hypertonic sodium chloride infusion, mannitol infusion, and the placement of a ventriculostomy. Despite these efforts, the patient's condition worsened, with progressive neurologic deterioration. On the fourth hospital day, the patient died. A postmortem lumbar puncture demonstrated a few motile amebae. Autopsy findings revealed acute PAM caused by N. fowleri identified by immunofluorescence testing with an N. fowleri-specific antibody (Figure). The patient's CSF, which was innoculated on non-nutrient agar plates streaked with Escherichia coli (1), yielded amebae identified by immunofluorescence as N. fowleri. Four days before onset of illness, the patient had attended a social event and had swum in a freshwater river with a group of friends in southern Georgia. An epidemiologic investigation was initiated to evaluate risk factors associated with N. fowleri infection. Interviews were conducted with 13 of 15 children aged 6--12 years who attended the event and their parents. In addition, an extensive environmental investigation of the site was conducted in conjunction with state and district health departments. Laboratory analysis of river water samples was performed at state public health laboratories and at CDC. Of the 15 children who attended the event, 10 had water exposure in the river despite a sign prohibiting swimming, a posting that was not connected to concern for N. fowleri. The maximum exposure time in the water was 2.5 hours (range: 30 minutes--2.5 hours). Water activities included swimming, swimming under water, wrestling in the water, and diving into the water. The patient was one of five children who spent the most time in the water (>2 hours) and engaged in underwater swimming, water wrestling, and diving. He also might have incurred trauma to the face or nose earlier that day during rough play. The environmental investigation revealed a high ambient temperature (>90º F [>32º C]) and water temperature (91º F [33º C]) in the river at the time of the exposure. In addition, because no recent rainfall had occurred in the region, the river level was low, and the river was flowing slowly. Bacteriologic testing of the river water demonstrated that fecal coliform levels were within acceptable limits. N. fowleri was isolated from two of three river water samples tested and from a control sample taken from a local lake. Reported by: T McKee, MD, Memorial Health Univ Medical Center, Savannah; L Davis, MD, South Central Health District, Dublin; P Blake, MD, L Kreckman, S Bialek, MD, Georgia Div of Public Health. MJ Beach, PhD, G Visvesvara, PhD, JH Maguire, MD, Div of Parasitic Diseases, National Center for Infectious Diseases; L Fox, MD, J Amann, MD, EIS officers, CDC. Editorial Note: PAM is a rare but nearly always fatal infection caused by N. fowleri, a thermophilic, free-living ameba that inhabits freshwater ponds, lakes, and rivers, minimally chlorinated pools, and hot springs throughout the world (2). PAM results when amebae-contaminated water incidentally enters the nose during swimming or other aquatic activity, followed by migration of amebae to the brain through the olfactory nerve. Symptoms occur 1 day--2 weeks after exposure, are indistinguishable from fulminant bacterial meningitis and can include headache, fever, stiff neck, anorexia, vomiting, altered mental status, seizures, and coma. Death typically occurs 3--7 days after the onset of symptoms (3). Autopsy findings usually show acute hemorrhagic necrosis of the olfactory bulbs and cerebral cortex (4). The disease is extremely rare despite the millions of persons with exposure to recreational water. During 1989--2000, CDC's waterborne disease outbreak surveillance system documented 24 fatal cases of PAM in the United States (5). The majority of these cases occurred during the summer months and among children. Because of the thermophilic nature of N. fowleri, an increased incidence occurs in areas where temperatures are high (6). The case described in this report is the first case of PAM in Georgia since 1987. In 2002, two cases were reported in Texas, two in Arizona, and two in Florida. Recognition of PAM depends on clinical suspicion based on patient history (Box). CSF findings mimic those of bacterial meningitis, with a predominantly polymorphonuclear leukocytosis and increased protein and decreased glucose concentration. Occasionally, amebae can be observed on Gram-stained smears. If PAM is suspected, a fresh-centrifuged specimen of CSF should be inspected by wet-mount preparation and with fixation and staining (7). Confirmation of N. fowleri infection requires a culture or an indirect fluorescent antibody test, which is performed at a reference laboratory (8). Only three survivors of PAM have been documented (9,10). Successful therapy appeared to be related to early diagnosis and administration of intravenous and intrathecal amphotericin B with intensive supportive care. One surviving patient received intravenous and intrathecal miconazole and oral amphotericin B and rifampin (10). Little is known about the risk factors for infection with PAM. Although these amebae are ubiquitous in freshwater bodies, high water temperatures and decreased precipitation leading to a low river depth might have contributed to proliferation of amebae in this river, subsequently increasing the risk for infection. In response to this case, the district health department recommended that local community authorities advise persons to avoid swimming in this river during periods of high temperature and low water depth. Acknowledgments This report is based on contributions by M Harden, MPA, South Central Health District, Dublin; EA Franko, DrPH, C Daniell, Georgia Div of Public Health. R Sriram, Div of Parasitic Diseases, National Center for Infectious Diseases, CDC. References
Figure 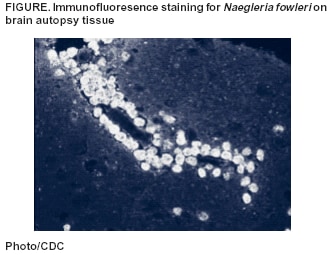 Return to top. Box 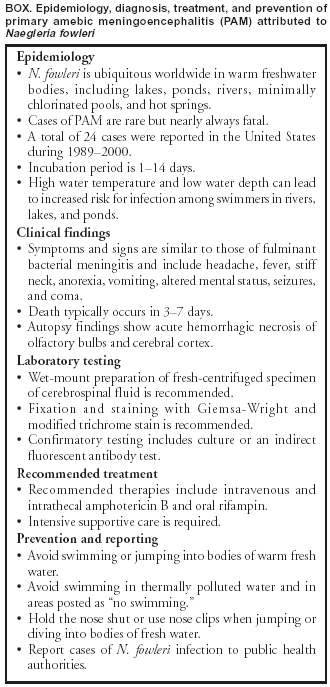 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/9/2003 |
|||||||||
This page last reviewed 10/9/2003
|