 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommendations for Partner Services Programs for HIV Infection, Syphilis, Gonorrhea, and Chlamydial InfectionNOTE: A Continuing Education Activity has been approved for this report and will be included in the print and electronic format on November 07, 2008, in Vol. 57, No. RR-9. The credits awarded are as follows: CME, 3.5; CNE, 3.5; CEU, .3; and CHES, 3.0. Prepared by
Corresponding preparer: Samuel W. Dooley, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC, 1600 Clifton Road, NE, MS D-21, Atlanta, GA 30333; Telephone: 404-639-5229; Fax: 404-639-0897; E-mail: sdooley@cdc.gov. SummaryThis report provides updated, integrated recommendations for services provided to partners of persons with human immunodeficiency virus (HIV) infection and three other sexually transmitted diseases (STDs) (i.e., syphilis, gonorrhea, and chlamydial infection) and replaces the CDC 2001 Program Operations Guidelines for STD Prevention---Partner Services and the 1998 HIV Partner Counseling and Referral Services Guidance (1,2). These recommendations are intended for health department program managers responsible for overseeing partner services programs for HIV infection and the three other STDs at the state and local levels. The recommendations also might be beneficial for HIV prevention community planning groups, STD program advisory bodies, technical assistance providers, community-based organizations, and clinical care providers. The value of partner services in the control of syphilis and gonorrhea is widely accepted. However, such services are underused among partners of persons with HIV infection. On the basis of evidence of effectiveness and cost-effectiveness of these services, CDC strongly recommends that all persons with newly diagnosed or reported HIV infection or early syphilis receive partner services with active health department involvement. Persons with a diagnosis of, or who are reported with, gonorrhea or chlamydial infection also are suitable candidates for partner services; however, resource limitations and the numerous cases of these infections might preclude direct health department involvement in certain instances. Health departments might need to limit direct involvement in partner services for gonorrhea and chlamydial infection to selected high-priority cases and use other strategies for the remainder (e.g., expedited partner therapy). These recommendations highlight the importance of program collaboration and service integration in the provision of partner services. Because coinfection with HIV and one or more other STDs is common, all persons with a diagnosis of HIV should be tested for other types of STDs, and vice versa; rates of coinfection with HIV and syphilis have been particularly high in recent years. Many persons at risk for these infections also are at risk for other infectious diseases, such as tuberculosis and viral hepatitis, as well as various other health conditions. STD and HIV partner services offer STD, HIV, and other public health programs an opportunity for collaboration to deliver comprehensive services to clients, improve program efficiency, and maximize the positive effects on public health. IntroductionInconsistencies in the partner services module of the CDC 2001 Program Operations Guidelines for STD Prevention and the 1998 HIV Partner Counseling and Referral Services Guidance (1,2) have been confusing for providers of partner services for human immunodeficiency virus (HIV) infection and three other sexually transmitted (STDs) for which partner services are often provided: syphilis, gonorrhea, and chlamydial infection. In addition, new information has become available through research and program experience, new technologies are available (e.g., rapid HIV tests), and new challenges have emerged, such as finding sex partners via the Internet and determining the role of expedited partner therapy for partners of patients with gonorrhea or chlamydial infection. To reduce duplication and discrepancies, incorporate new information, and address emerging challenges, this report integrates guidelines for partner services for HIV infection, syphilis, gonorrhea, and chlamydial infection into a single set of recommendations. These updated, integrated recommendations serve as a basis for delivery of partner services and related training and technical assistance. These recommendations are intended for health department program managers responsible for overseeing partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection at the state and local levels and were developed to help program managers plan, implement, and evaluate partner services for infected persons and their partners. The recommendations should be used to help plan and manage prevention measures, target use of resources, establish program priorities, and develop program policies. The recommendations also should influence future training for partner services staff members and should be shared with any staff members who are involved in any aspect of partner services. These recommendations are not intended to provide sufficient detail to be used as an operational or instructional manual for the daily activities of disease intervention specialists (DISs), nor are they intended to be used as a substitute for a training manual or curriculum. Although the recommendations address several legal concerns related to partner services, they do not provide a review of law relevant to partner services and should not be considered legal advice. CDC provides partner services training for public health staff members; future implementation planning (including training) will incorporate these revised recommendations. These recommendations also are not intended to provide specific technical guidance and program requirements for CDC grantees. That information can be found in STD and HIV funding opportunity announcements and related supplemental guidance. These recommendations focus primarily on traditional, health department--based strategies for conducting partner services. Although other models might be used, the goal of partner services is to maximize the number of partners who are notified of their exposure to HIV, syphilis, gonorrhea, or chlamydia and who are treated or linked to medical, prevention, and other services. All partner services programs should be able to demonstrate, through monitoring and evaluation, that their programs are accomplishing this goal. These recommendations support the CDC health protection goal "healthy people in every stage of life" (available at http://www.cdc.gov/osi/goals/people.html). Although health department program managers are the primary intended audience, information in this report might be beneficial for HIV-prevention community planning groups, STD program advisory bodies, trainers and providers of technical assistance, community-based organizations (CBOs), clinical care providers, and others with an interest in partner services. The recommendations in this report focus on partner services for HIV infection and three other STDs: early syphilis (i.e., primary, secondary, and early latent syphilis), gonorrhea, and chlamydial infection. Information and recommendations for HIV infection and these three other STDs are integrated throughout this report, and many of the recommendations apply to all four infections. In certain instances, recommendations are different for one or more of the four infections. Information about partner management for STDs and clinical syndromes other than HIV infection, syphilis, gonorrhea, and chlamydial infection are available in the CDC Sexually Transmitted Disease Treatment Guidelines (3). Published, scientific, evidence-based information on partner services is limited. To the extent possible, the recommendations in this report were based on published evidence. However, when published evidence was lacking or insufficient, recommendations were based on program experience, with input from subject-matter experts. HIV and STD prevention programs exist in highly diverse, complex, and dynamic social and health service settings. Substantial differences exist in disease patterns, availability of resources, and range and extent of services among different health department jurisdictions. The recommendations should be used in conjunction with local area needs, resources, and laws. HIV and STD prevention programs should establish priorities, examine options, calculate resources, evaluate the distribution of the diseases to be prevented and controlled, and adopt strategies appropriate to their specific circumstances. MethodsCDC led a work group that planned and coordinated the process of revising and combining the two existing guideline documents into a single set of recommendations. Simultaneously, numerous organizations and experts with potential interest in partner services were notified that the guidelines were being revised and invited to provide input; approximately 70 stakeholder groups were included in this process. In addition, an extensive review was conducted to identify relevant published research. During 2005--2006, CDC sought input from attendees at five national HIV and STD conferences. Detailed reviews of HIV partner services programs were conducted at eight health departments (six state health departments and two city health departments) to identify current program practices and challenges and to obtain input from persons directly involved in delivering partner services. Discussions with focus groups of potential and actual recipients of HIV partner services were held in five cities to elicit information about experiences with and perceptions of these services. In addition, discussions with focus groups of private clinicians were held in seven cities to assess their level of awareness and understanding of partner services and their perceptions of the importance and effectiveness of such services. Finally, a detailed review was conducted of state laws related to HIV partner services to identify legal concerns and provide a framework of the legal and regulatory environment in which partner services are delivered. A draft of recommendations was developed and in November 2006, a meeting was convened in Atlanta, Georgia, to obtain input. The meeting was attended by approximately 70 participants from 23 states and the District of Columbia (DC). Participants included representatives of other federal agencies; state and local HIV and STD health department directors, program managers, and staff members; academic research experts; ethicists; representatives from legal, medical, and other professional organizations; and representatives from CBOs, correctional facility health organizations, community advocacy groups, and training centers with expertise in partner services. After the meeting, CDC convened seven workgroups, which included CDC staff members and non-CDC participants recruited from the meeting, to revise the draft of the recommendations based on input from meeting participants. In January 2008, a revised draft was distributed for review to federal agencies, health departments, academic and research centers, professional organizations, CBOs, and community advocacy groups. In compliance with requirements of the Office of Management and Budget for influential scientific assessments, CDC also solicited reviews from nonfederal subject-matter experts. The recommendations were revised after reviewer comments were received. How These Recommendations Differ from Previous Partner Services GuidelinesThese recommendations integrate previously separate guidelines for partner services for HIV infection, syphilis, gonorrhea, and chlamydial infection into a single set of recommendations; a complete summary of these new recommendations is provided (Appendix A). These recommendations have increased emphasis on the following:
The 1998 HIV Partner Counseling and Referral Services Guidance used the term partner counseling and referral services rather than contact tracing or partner notification to describe the type and range of public health services recommended for sex and drug-injection partners of HIV-infected persons (2). The 2001 Program Operations Guidelines for STD Prevention used the term partner services to describe similar activities (1). This report uses the term partner services to describe services offered to persons with HIV or other STDs. The term partner services is broad and encompasses services typically included in partner counseling and referral services and other services (e.g., screening for other STDs, screening for chronic infection with hepatitis B virus [HBV] and hepatitis C virus [HCV], and vaccination for hepatitis A virus [HAV] and HBV). In addition, the principles of notifying an exposed person do not differ substantially among diseases, and persons with STDs other than HIV often need the same array of services as persons with HIV infection. Using the same term for partner services for HIV and other STDs emphasizes these points. TerminologyMany terms used in this report are familiar to persons with experience in partner services for HIV and other STDs; however, certain terms might be used differently than they were in previous guidelines, and certain new terms are used. Following are terms used frequently in this report; a glossary and list of abbreviations also are provided (Appendices B and C).
Definition and Overview of Partner ServicesPartner services are a broad array of services that should be offered to persons with HIV infection, syphilis, gonorrhea, or chlamydial infection and their partners. A critical function of partner services is partner notification, a process through which infected persons are interviewed to elicit information about their partners, who can then be confidentially notified of their possible exposure or potential risk. Other functions of partner services include prevention counseling, testing for HIV and other types of STDs (not necessarily limited to syphilis, gonorrhea, and chlamydial infection), hepatitis screening and vaccination, treatment or linkage to medical care, linkage or referral to other prevention services, and linkage or referral to other services (e.g., reproductive health services, prenatal care, substance abuse treatment, social support, housing assistance, legal services, and mental health services). The rationale for use of partner services is that appropriate use of public health resources to identify infected persons, notify their partners of their possible exposure, and provide infected persons and their partners a range of medical, prevention, and psychosocial services can have positive results including 1) positive behavior changes and reduced infectiousness; 2) decreased STD/HIV transmission; and 3) reduced STD/HIV incidence and improved public health (Figure 1). The value of partner notification in the control of syphilis and gonorrhea is widely accepted (3). In recent times, syphilis prevalence peaked in approximately 1990, resulting in a concerted national attempt to apply public health resources, including partner services, toward its reduction and, later, elimination (4). Subsequently, syphilis prevalence decreased to historic lows (approximately 6,000 primary and secondary cases in 2000). Cost data from the early 1990s on syphilis partner services suggest costs per partner treated are commensurate with current costs of other syphilis-elimination strategies in the United States (5). However, recent increases in primary and secondary syphilis cases to approximately 10,000 cases in 2007 indicate that continued vigilance in syphilis control is needed. In New York, notification and referral services for gonorrhea have targeted specific geographic areas with notification services rather than attempting to interview all index patients and notify all partners in person. Evaluation of 10 years of data from the New York program, as well as of other program data, has shown a reduction in gonorrhea prevalence (6,7). Treatment of partners is valuable for control of chlamydial infection and cost-effective in averting sequelae. When used, partner services via provider referral seems to identify enough infected partners to decrease transmission and therefore promote infection-control measures, and more partners are treated through partner services than through other strategies (8--10). However, provider referral coverage for chlamydial infection is low and not a significant contributor to controlling this infection (8,11,12). For example, one survey indicated that only 12% of patients with chlamydial infection were interviewed by health department staff members about their partners (13). Partner services can play an essential role in preventing and controlling HIV in the United States. Of approximately 1--1.2 million persons living with HIV infection in the United States, approximately 25% are not aware of their infection; transmission from persons not aware of their infection accounts for 54%--70% of new infections (14,15). Partner notification, a critical component of partner services, effectively identifies persons with previously undiagnosed HIV infection. A review of the case-finding effectiveness of partner notification found that among partners for whom notification was initiated, the median percentage with newly diagnosed cases was 8%, approximately the same as for syphilis (8); in the reports included in this review, eight index patients were interviewed for partner notification to discover one newly diagnosed case of HIV, on average. A systematic literature review conducted for the Task Force on Community Preventive Services found that among the nine studies included, a range of one to eight partners was identified per index patient with HIV infection, a mean of 67% of partners were notified of their exposure to HIV, a mean of 63% of persons notified of exposure were tested, and a mean of 20% of those tested were newly diagnosed as infected with HIV (range: 14%--26%). On the basis of this review, the task force concluded that sufficient evidence exists to demonstrate that partner services, with partner notification by a public health professional, increases identification of a high-prevalence population for HIV testing and increases the identification of HIV-infected persons (16,17). Although limited, additional data also suggest that HIV partner services are cost-effective (18--22). Despite the potential benefits, HIV partner services are highly underused (23). The services are more frequently provided to persons who receive diagnoses in publicly funded HIV testing sites than outside of public health sites (23). On the basis of evidence of the effectiveness and cost-effectiveness of partner services, CDC strongly recommends that all persons with newly diagnosed or reported HIV infection or early syphilis receive partner services with active health department involvement. All persons who receive a diagnosis of or who are reported with gonorrhea or chlamydial infection also are suitable candidates for partner services; however, high numbers of cases and resource limitations might preclude direct health department involvement in all instances. Health departments might need to limit their direct involvement in partner services for gonorrhea and chlamydial infection to selected high-priority cases and use other strategies for the remainder. Principles of Partner ServicesThe following principles serve as the foundation for providing partner services to persons with HIV infection or other STDs and their partners:
Goals of Partner ServicesThe goals of partner services for infected persons, their partners, and the community are as follows:
Benefits of Partner ServicesPartner services programs offer substantial benefits to three principal groups: persons infected with HIV infection or other STDs, their partners, and the community (Figure 1). A primary benefit for index patients is that DISs can help them ensure that partners are notified of their possible exposure to the infection, while protecting the confidentiality of the patients. For index patients who expect to notify partners themselves, DISs can provide coaching and assistance with this process and provide support if the index patient is unable to complete the notification successfully. In addition, when interviewing index patients, DISs can assess whether they have been adequately treated or linked to appropriate medical and prevention services and, for those who have not, facilitate access to these services. DISs also can assess whether index patients need other services (e.g., reproductive health services or substance abuse treatment) and make appropriate referrals for such services. Finally, when persons are repeatedly reported as index patients for syphilis or gonorrhea or have been previously reported with HIV infection, DISs can provide additional prevention counseling or help them access more intensive risk-reduction interventions. For persons having difficulty achieving and maintaining behavior changes, these services can help develop skills to reduce their risk for repeatedly acquiring new STDs or transmitting HIV to current or future partners. Partners of persons with HIV infection or other types of STDs are at high risk for infection, as indicated by the high prevalence of infection among notified partners (8,16). In the case of HIV, many partners are not aware of their risk and have never been tested for HIV (24). Partner services provide a confidential process for these persons to become aware of their risk and access appropriate diagnostic, treatment, and prevention services. Recently exposed partners of persons with early syphilis and gonorrhea who do not yet have evidence of infection can be treated preventively, and partners with evidence of infection can be treated for cure. All partners can be assessed to determine whether they need other services (e.g., reproductive health services or substance abuse treatment) and receive appropriate referrals. Partner services might also benefit the community by helping reduce transmission rates, reducing effects of disease, and facilitating earlier identification and treatment of previously undiagnosed STDs/HIV infection among its members. Demonstrating that a specific prevention intervention (e.g., comprehensive risk counseling and services) reduces transmission rates at the community level is difficult. Nevertheless, studies have demonstrated that 1) quality prevention counseling can reduce risk for acquiring a new STD, 2) behavioral interventions can reduce transmission risk behaviors, and 3) persons with HIV infection who are aware of their infection have substantially lower levels of transmission risk behaviors than those who are not aware (15,25--31). Thus, by increasing access to prevention counseling and other prevention interventions and by providing counseling and testing to persons at very high risk for infection (i.e., known partners of infected persons), partner services should result in lower transmission rates. In addition, by reducing the viral load in HIV-infected persons to undetectable levels, antiretroviral therapy (ART) likely reduces (but does not eliminate) infectiousness and risk for sex- and injection-related transmission (32--37). Therefore, identifying persons with previously undiagnosed HIV infection and linking them to medical care services, and possibly to ART, also might reduce transmission within the community. Finally, partner services can improve disease surveillance and identify sex and drug-injection networks at high risk for infection that can then be targeted for screening and prevention services (38). Challenges for Partner ServicesChallenges for partner services include whether the services will be accepted by patients, the potential for abuse resulting from partner notification, and potential negative effects on relationships after partner notification. DIS training includes methods to maximize acceptability of partner services among patients. A recent systematic review of the acceptability of HIV partner counseling and referral services found that among participants in the studies reviewed, 1) the majority of surveyed potential clients (i.e., HIV-positive or HIV-negative persons who had no direct experience with HIV partner counseling and referral services) indicated that they would be willing to participate in client referral (i.e., notify a partner themselves); 2) most potential clients would be willing for health department personnel to notify their partners; 3) the majority of HIV-positive clients receiving partner counseling and referral services used provider referral to notify one or more partners; 4) the majority of partners either wanted to be notified or were comfortable with a health-care provider notifying them; and 5) the majority of providers were in favor of partner notification (39). The high level of acceptability of HIV partner services among diverse groups suggests that, when provided appropriately, they are considered a service rather than an imposition by those for whom they are intended. A second challenge is the potential for emotional or physical abuse by or against the index patient as a result of partner notification. Available data suggest that the rate of violence attributable to partner notification is likely low; however, data are limited, and additional study is needed (40--43). A third frequently cited challenge is the potential negative effect of partner notification on relationships (e.g., dissolution of a long-standing relationship) (39,40,44). In one study, the rate of partnership dissolution was 46.8% among partnerships involving syphilis or HIV cases, with no significant difference between the two infections; however, the rate was lower in partnerships for which partner notification was completed than in those for which notification was not completed (24.3% and 75.7%, respectively) (40). A similar study addressing the effect of HIV partner notification on partnership dissolution found that although the rate of partnership dissolution was high (65% at 6 months postinterview), the rate was not increased by partner notification (44). Study design and low enrollment make drawing firm conclusions from these studies difficult; however, the studies suggest that partner notification itself does not increase rates of partnership dissolution. Legal and Ethical ConcernsWell-implemented partner services balance the interests of infected persons, their partners, and the community. Describing a single plan for successfully balancing the interests of all involved parties is difficult because the legal context within which partner services programs operate varies among states and jurisdictions. Nonetheless, recognition of and adherence to certain principles is essential for all partner services programs. This report does not include a comprehensive discussion of all areas of law relevant to partner services. Program managers should consult with the legal counsel of their agency to gain a thorough understanding of the legal framework in which their specific programs operate, including their own legal authorities and those of other agencies (e.g., law enforcement) with whom they might interact. These CDC recommendations should not be taken as legal advice or as CDC interpretation of the laws of any jurisdiction. Legal AuthoritiesStates hold the legal authority for the notification and referral of partners of persons with HIV infection and other types of STDs. One federal law specifically addresses HIV partner notification services for spouses: the Ryan White CARE Act Amendments of 1996 (Pub. L. No. 104-146 [May, 2, 1996]) require that states receiving funds under part B of title XXVI of the Public Health Service Act (42 U.S.C. Sect. 300ff-27a [1996]) take "administrative or legislative action to require that a good faith effort be made to notify a spouse of a known HIV-infected patient that such spouse might have been exposed to the human immunodeficiency virus and should seek testing." A spouse is defined as any person who is the marriage partner of an HIV-infected patient or has been the marriage partner of that patient at any time within the 10-year period before the diagnosis of HIV infection. Voluntary and Informed Nature of Participation in Partner ServicesParticipation in partner services is voluntary only if it is informed and not coerced. The effectiveness of partner services as a public health intervention relies on the voluntary cooperation and participation of index patients, partners, social contacts, and associates. These persons voluntarily choose to 1) provide information about themselves and others in response to questions and requests from a DIS; 2) notify others of their possible exposure to HIV, syphilis, gonorrhea, or chlamydia; 3) accept STD/HIV testing and treatment; and 4) engage in behaviors that promote health and reduce risk for transmission or acquisition of HIV infection and all other types of STDs. Ethically, for a public health official or health-care provider to coerce, deceive, or withhold information from persons to influence them to take any of these actions is inappropriate. In addition, persons who believe that they are being coerced might lie or withhold information. These considerations do not preclude use of persuasive reasoning to gain the cooperation of index patients and others and to motivate them to participate actively in partner services. However, for partner services to be truly voluntary, all persons should be clearly informed of the known benefits and risks for themselves and others that might result from their participation. ConfidentialityIn the context of partner services, confidentiality refers to keeping information obtained from or about index patients, partners, social contacts, and associates in confidence; information is not divulged to others or obtained or maintained in a way that makes it accessible to others. The concept of confidentiality is related to privacy, which might be a legal right in certain instances. That is, laws might prohibit forcing persons to reveal certain types of information, and persons who decline to provide certain types of information are not prevented from receiving services. When a person agrees to disclose private information, especially in the context of a service aimed at helping others, such information should be held in strict confidence, both because of its private nature and as a sign of respect for the person who is volunteering to share the information. Research has demonstrated that the degree to which confidentiality is maintained by partner services programs is an important determinant of the acceptability of those services to clients and client willingness to participate in partner services (39,45--47). Real or perceived breaches of confidentiality can endanger persons being served, who might face stereotyping; social isolation; loss of social or financial support; barriers to accessing housing, employment, and various social and medical services; and physical or emotional abuse (48,49). Such breaches also can undermine community trust in and access to essential public health programs and services. For these reasons, policies and procedures for protecting confidentiality are critical. State laws generally protect the confidentiality of all STD information, including information related to HIV and acquired immunodeficiency syndrome (AIDS). In certain states, specific laws or regulations prescribe the parameters of information to be kept confidential and establish penalties for confidentiality breaches. Although confidentiality is a central principle of partner services, it is subject to legal exceptions such as those stipulated in certain duty-to-warn laws, which in certain situations require medical or public health officials to notify known partners who are at risk for infection, even against the specific wishes of the index patient. Confidentiality also is subject to practical limits, including the possibility that partners might guess the identity of the index patient at any point during the process. Because partner services programs cannot absolutely guarantee patient or partner anonymity, health officials must make all reasonable attempts to ensure that the confidential nature of communication with a DIS is respected and protected to the fullest extent allowed by law. Duty and Privilege to WarnThe legal duty to warn has its foundation in a 1976 case, Tarasoff v. Regents of the University of California, in which the family of a murdered woman sued because the killer's therapist did not warn their daughter that his patient planned to kill her (49). The Tarasoff decision indicates that a patient's intention to seriously harm another person could result in a provider's duty to warn. The Tarasoff decision does not overshadow the importance of confidentiality and trust in a therapeutic relationship but emphasizes that the threatened harm must be serious, imminent, targeted at an identified (or identifiable) person, and articulated in the context of an existing therapeutic relationship. At the state level, the legal concept of the duty to warn is complex; consultation with legal counsel is necessary. Certain states have laws requiring practitioners (directly or with the assistance of public health authorities) to warn persons they know to be at risk for infection with a communicable disease, an STD, or HIV by their patients. Many other states have laws permitting but not requiring practitioners to warn persons that they are at risk (i.e., privilege to warn). DISs generally must avoid disclosing the name of an index patient. However, because cases involving duty to warn require the health-care providers to provide sufficient information for partners to protect themselves, situations involving a duty to warn might require a provider to reveal the name of an index patient to at-risk partners, thereby breaching the confidential relationship between the provider and the patient (50). Programs that too readily assume that the duty to warn is applicable in a specific case and alert partners against the will of or without the knowledge of an index patient might find future patients reluctant to be honest about sexual or drug-sharing activities or unwilling to accept testing or medical care. In such situations, important opportunities for counseling, support for disclosure, and prevention education might be lost. Accordingly, health-care providers and public health program managers should proceed cautiously and seek legal counsel before assuming that a duty to warn has been triggered or that they have a privilege to warn. Criminal Transmission and ExposureDespite extensive education and counseling to prevent transmission and acquisition of HIV infection and other types of STDs, certain persons persistently engage in behaviors that put themselves and others at risk for infection. Certain criminal laws of general application, such as assault, battery, or reckless endangerment laws, might be used to prosecute a person who intentionally exposes another person to infection. However, many states have enacted criminal laws focusing either specifically on HIV transmission or generally on transmission of sexually transmitted infections. These laws vary according to several factors, including 1) which types of conduct are considered criminal (e.g., with HIV, most states proscribe engaging in conduct that exposes someone else to HIV rather than limiting liability to situations in which transmission has occurred) (51); 2) the specificity with which the proscribed conduct is described (e.g., most statutes that consider exposing someone to HIV to be a criminal act do not define exposure, although certain statutes specifically proscribe exposure by transfer of body fluids or tissues, engaging in sexual activities, or needle sharing) (51); and 3) the knowledge required (e.g., for exposure to be considered criminal, almost all states require that infected persons who expose another person to HIV must have had knowledge of being infected with HIV) (51). Laws might also vary depending on whether disclosure of HIV status before engaging in the conduct 1) means that no crime has been committed, 2) is an affirmative defense that can be raised by a person charged with criminal transmission or exposure, or 3) means that the person is not legally liable. Depending on the unique circumstances of each case, options available to partner services program managers in cases involving persons who persistently engage in behaviors that put themselves and others at risk might include 1) initiating increasingly intensive prevention interventions (e.g., comprehensive risk counseling and services); 2) facilitating access to HIV primary care; 3) arranging linkage to substance abuse treatment, mental health services, or other relevant services; 4) initiating epidemiologic investigation of situations involving possible exposure of persons to infection; and 5) seeking legal advice when public health interventions are not sufficient or appropriate. Determining the most appropriate course of action requires consideration of the details of the specific situation; every case must be managed carefully and confidentially. Recommendations for Legal and Ethical Concerns
Elements of Partner ServicesPartner services include several essential elements (Figure 1). In general, these elements are relevant for partner services for HIV, early syphilis, gonorrhea, and chlamydial infection, although differences in how they are implemented vary by infection. Program managers should ensure that policies and procedures adequately address each of these elements. Index Patients
Partners
Identifying Index PatientsIdentifying persons who are candidates for partner services (i.e., index patients) is a critical step in the partner services process. For early syphilis and, in certain instances, gonorrhea, standard identification of index patients occurs 1) when persons seek care with no prompting (i.e., volunteers) and 2) when persons receive screening or testing and their case reports are provided to STD programs for treatment, case management, and partner services. For early syphilis, public health records indicate that since the 1940s, index patients routinely have been interviewed and their partners followed. In modern times, a survey of partner notification for STDs/HIV found that 89% of syphilis-infected persons in high-morbidity geographic areas were interviewed (13). The same survey found that a markedly lower proportion (17%) of persons with gonorrhea were interviewed, although certain jurisdictions still attempt to interview all patients with gonorrhea. Other jurisdictions that lack resources to interview all patients with gonorrhea have focused their interviews on patients in high-morbidity areas (i.e., core areas) (7). Interview strategies for chlamydial infection tend to be similar to those for gonorrhea, although interviews are generally considered lower priority than interviews for gonorrhea. Among high-morbidity jurisdictions in a survey of STD/HIV partner services, only 12% of persons with chlamydial infection were interviewed (13). The workload for health departments is related to the number of cases reported, which is an essential factor affecting approaches to partner services for early syphilis, gonorrhea, and chlamydial infection. During 2000--2007, fewer than 50,000 cases of early syphilis (i.e., primary, secondary, and early latent) were diagnosed each year. In contrast, estimates of annual prevalence of gonorrhea and chlamydial infection are one to two orders of magnitude higher (52,53), far too many patients for public health staff members, at the current staffing level, to interview directly. Available evidence suggests that the majority of HIV-infected persons are not interviewed for HIV partner services. A survey found that in 22 jurisdictions with HIV reporting, health departments interviewed 32% of 20,353 persons with newly reported HIV infection (23). Active strategies for identifying more candidates for partner services are needed. Because an extensive literature search did not identify any published studies or program evaluations that examined this topic, recommendations in this report for identifying HIV index patients were based on input from consultants with partner services expertise. For HIV, although the main emphasis of partner services programs should be on persons with newly diagnosed or reported infection, partner services also might be appropriate for persons with previously diagnosed infection on an as-needed basis (54). Persons with Newly Diagnosed HIV Infection, Syphilis, Gonorrhea, or Chlamydial InfectionDiagnoses Received in STD or Other Health Department Clinics Partner services are provided almost exclusively by health departments, often by STD program staff members. When all partner services are provided by STD program staff members, persons with an STD diagnosis, including HIV, in health department STD clinics can easily be linked to partner services. However, when HIV and STD programs are separate, some or all HIV partner services might be provided exclusively by HIV program staff members. In these situations, managers of both programs should establish policies and procedures to ensure that persons with a diagnosis of HIV infection, syphilis, gonorrhea, or chlamydial infection by either program receive appropriate partner services. Systems also are needed to ensure that persons with a diagnosis of HIV infection or any of these three other STDs in other health department clinics (e.g., tuberculosis [TB] or reproductive health clinics) are linked to the partner services program. Certain patients receive a diagnosis of HIV infection and of another STD simultaneously. Policies and procedures are needed to ensure that these patients and their partners receive partner services for both infections from only one DIS to improve services for the patients and partners and maximize program resources. Identification of syphilis cases can be complicated because treated and noninfectious persons can have reactive syphilis tests indefinitely. Titration of the rapid plasma reagin (RPR) test can yield elevated RPR titers for persons who have already been treated and clinically cured of syphilis. Therefore, CDC encourages programs to use syphilis treatment registries and algorithms for prioritizing follow-up investigations of persons with reactive syphilis tests (i.e., reactors). A syphilis reactor grid is constructed from a combination of quantitative test results, age, and sex to identify which persons with reactive tests are most likely to be both untreated and infectious. Individual programs vary in precisely how they use a reactor grid but generally investigate all persons with RPR titers higher than a specified level, all persons younger than a certain age, and persons most at risk for negative outcomes (e.g., pregnant women). A recent evaluation of syphilis reactor grids suggested that most missed cases of early syphilis were among men aged 30--50 years and women aged 20--40 years with low RPR titers (55). Diagnoses Received in Settings Other than Health Department Clinics Most types of STDs are frequently diagnosed in settings other than health departments (56), such as public hospitals and clinics, private hospitals and medical practices, community health centers, Veterans Administration health-care facilities, Indian Health Service and tribal health-care facilities, correctional facilities, CBOs, reproductive health service organizations, substance abuse treatment centers, and student health centers. In particular, chlamydial infection and gonorrhea are more frequently diagnosed in private care settings. Reporting delays, especially for cases diagnosed when patients are the most infectious, diminish the effectiveness of partner services in infection control. Approximately 90% of all HIV tests and 70% of positive HIV tests are performed in settings other than health department clinics (57). Persons diagnosed in settings other than health department clinics might not be directly linked to partner services if the provider does not notify the partner services program; therefore, program managers should establish strategies for rapidly identifying these persons and offering them partner services. This can be accomplished by linking disease reporting systems and partner services programs, conducting active outreach to service providers (e.g., physicians and health-care facilities that frequently diagnose STDs/HIV infection, HIV counseling and testing providers, and case managers) and diagnostic laboratories, or using a combination of these strategies. Each strategy has potential advantages and disadvantages. For example, linking disease reporting activities and partner services programs might maximize the number of newly diagnosed persons identified for partner services, but reporting delays might reduce the timeliness with which partner services are initiated. In contrast, active outreach to health-care providers might improve the timeliness of partner services but result in more missed cases because reaching all providers is difficult. For most programs, a combination of these two strategies will likely be most effective. Program managers might also develop other strategies for identifying persons with newly diagnosed infection. Strategies should be monitored for how effectively they identify index patients and the timeliness with which they provide services. Linkage with Disease Reporting. For surveillance purposes, cases of HIV/AIDS and other STDs might be reported to health departments by service providers (e.g., clinicians or CBOs providing testing services), diagnostic laboratories, or both. Data collected through HIV/AIDS and STD surveillance systems are used for many complementary public health purposes at the national, state, and local levels. Examples of such uses include disease monitoring, estimating incidence of infection, identifying changing trends in transmission, targeting and evaluating prevention interventions, and allocating funds for care and prevention services. Certain states and territories also use case reports to initiate partner services for infected persons and offer referrals for prevention, medical care, and supportive services. In 2007, the Council of State and Territorial Epidemiologists (CSTE) conducted a national assessment of HIV/AIDS surveillance capacity and training by surveying HIV surveillance coordinators in 65 state, large city, and territorial health departments. Several questions assessed current practices regarding use of HIV surveillance data to support partner services. Seventy-one percent of respondents (30 of 42 respondents to the question) reported sharing data in some form with partner services programs; 43% (24 of 56 respondents to the question) reported sharing individual-level data that included personal identifiers with partner services (CSTE, unpublished data, 2007). Sharing information between HIV/AIDS and STD surveillance programs and partner services programs is important for comprehensive disease intervention and offers many potential mutual benefits, including the following:
Before using surveillance data to identify candidates for partner services, health departments should consider staffing and resources, relevant state and local laws and regulations, and level of community awareness and acceptance. The organizational structure of the health department also affects the way surveillance and partner services programs interact. For example, health departments in which surveillance and partner services programs are integrated often share staff members, have similar missions, have programmatic and administrative commonalities, and receive oversight from a shared overall responsible party (ORP, an official who has overall responsibility for implementing and enforcing HIV/AIDS and STD surveillance security standards), all of which might facilitate information sharing for partner services purposes. Potential barriers to sharing surveillance data include a negative impact on provider reporting because of concerns about confidentiality of information, increased workload for surveillance staff members, and, for HIV, perceived negative effects on HIV-testing behaviors of providers or persons at risk for infection. For most STDs, data from a physician survey suggest that although physicians might be reluctant to collect partner services data themselves, they are willing to report cases to health departments to ensure that their patients receive partner services (58). Although the data from this survey do not include HIV, other surveys have found that the majority of health-care providers favor HIV partner notification (39). To facilitate information sharing between partner services and surveillance programs, health departments should review state and local laws and regulations that might apply to data sharing. Engaging key stakeholders such as medical providers, community advocates, and CPGs in the design and implementation of surveillance and partner services data linkage processes can result in support of and success in these measures. Clear, well-defined security and confidentiality policies and procedures that are followed by both surveillance and partner services program staff members increase the likelihood that surveillance data will be kept secure and patient information confidential, leading to patient and medical provider trust and cooperation with partner services programs. Historically, certain programs have limited the sharing of HIV/AIDS surveillance data with partner services programs. In certain situations, programs imposed these limits after collaboration with communities and medical providers on implementation of named-based HIV reporting, which resulted in use of reporting methods that separate surveillance and partner services. When considering changes in data-sharing policies, programs should use the same careful collaboration and deliberation with medical providers and affected communities to prevent erosion of the public trust and of the integrity of the systems already in place. Levels of Surveillance Information. Three levels of surveillance data can support partner services: 1) individual, 2) provider, and 3) aggregate. These range from very sensitive data requiring high levels of security and confidentiality (individual level) to substantially less sensitive data (aggregate level). Individual-level data are the most valuable for immediate provision of partner services, although provider- and aggregate-level data also can be useful.
CDC and CSTE have published technical guidance describing minimum standards for HIV/AIDS data security and confidentiality that should be met by surveillance programs; these standards reflect best practices for protecting HIV/AIDS surveillance data (59). With minor adjustments to accommodate practical realities encountered in many health departments, the same standards should be upheld by any partner services program with which HIV/AIDS surveillance programs share individual-level data (Appendix D). To ensure that appropriate policies and procedures are developed and followed, HIV/AIDS surveillance programs designate an ORP, who is responsible for security of the program's information collection and management systems, including processes, data, information, software, and hardware. Although this guidance was developed specifically for HIV/AIDS surveillance activities, it might be useful for data and information collected and used by all programs conducting partner services. Outreach to Service Providers and Diagnostic Laboratories. Persons might receive a diagnosis of HIV or other STDs from various service providers outside of health department clinics. In addition to using disease reporting systems to identify potential candidates for partner services, programs can collaborate with service providers and diagnostic laboratories to help ensure that persons who receive a diagnosis of STDs/HIV are linked rapidly to health department partner services programs. Although reaching all service providers is unlikely to be feasible, a small number of providers or laboratories might account for a large proportion of new diagnoses. In this case, health department partner services program managers can collaborate with surveillance coordinators to identify these providers and laboratories to establish procedures for partner services referrals. Certain partner services programs have identified health-care facilities that diagnose large numbers of cases and have placed DISs in those facilities to meet with persons with new diagnoses. This strategy might reduce the need for extensive field work to locate individual index patients. However, such strategies should be monitored closely to assess their effectiveness and cost-effectiveness; no systematic evaluations of these strategies have been published. CDC recommends that in all health-care settings, voluntary screening for HIV infection should be performed routinely for all patients aged 13--64 years unless a patient declines HIV testing or has been tested recently (60). These recommendations might produce a substantial increase in new HIV diagnoses. Therefore, program managers responsible for HIV partner services should work with health-care providers who implement the screening recommendations and diagnose numerous HIV-infected persons to help ensure that those persons are linked to partner services. Anonymous HIV Testing Anonymous testing accounts for a small but significant proportion of all HIV tests and might reach a subset of persons who might not otherwise be tested (61,62). Persons who test positive for HIV anonymously should be strongly encouraged to transfer to a confidential system; however, if they decline, HIV partner services can still be offered and performed. Partner services might be more difficult to provide for persons using anonymous testing than for those using confidential testing. A study in Colorado assessed provider-referral partner notification for persons who tested HIV positive during October 1990--March 1992 at a single anonymous test site in Denver and 13 confidential test sites throughout the state (63). The average number of named, notified, and counseled partners was 30%--50% greater among index patients tested at sites offering confidential testing than among those tested at sites offering anonymous testing. A North Carolina study found that the number of partners notified and counseled per index patient interviewed was 2.7 times greater for index patients tested confidentially compared with those tested anonymously (64). A literature review of this topic indicated that two to three times more partners are notified when persons are tested confidentially than when they are tested anonymously (8). However, one study, conducted by the Multistate Evaluation of Surveillance for HIV Study Group in five states with name-based HIV reporting, found no difference in the number of notified partners between persons who were tested anonymously and those tested confidentially (65). Therefore, program managers who are responsible for HIV partner services should work with providers who offer anonymous HIV testing to develop strategies for offering and providing partner services to persons who test positive anonymously and elect not to enter a confidential system. Persons with Previously Diagnosed HIV Infection, Syphilis, Gonorrhea, or Chlamydial InfectionRecurrent Infections Acquisition of a new STD of any type by persons with previous infections, including HIV, indicates ongoing sexual risk behaviors and a need for additional partner services, prevention counseling, and other prevention interventions, such as comprehensive risk counseling and services. Identifying HIV-infected persons who have new STDs is particularly important because infection with other STDs facilitates transmission and acquisition of HIV (66). Persons with recurrent STDs of any type might be identified in STD clinics, other care and service venues, or STD/HIV reporting systems. Partner services programs should have systems in place to identify these persons, counsel them, offer them partner services, and link them to more intensive prevention interventions, as indicated. Ongoing Partner Services for Persons with HIV Infection Certain persons who received a previous diagnosis of HIV might have declined partner services at the time of diagnosis, might have partially participated but subsequently become interested in participating fully, or might have new partners. These persons can be reached through outreach to HIV care providers or case managers. CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medical Association of the Infectious Diseases Society of America collaborated to create recommendations for incorporating HIV prevention into the medical care of persons living with HIV infection (54). These recommendations urge HIV clinical care providers to 1) ask patients at the initial visit whether all their partners have been informed of their exposure to HIV; 2) regularly screen patients for HIV transmission risk behaviors, STDs, and pregnancy; 3) inquire at routine follow-up visits whether patients have had any new sex or drug-injection partners who have not been informed of their exposure; and 4) refer patients to the appropriate health department to discuss partners who have not been informed of their exposure and arrange for their notification and referral for HIV counseling and testing. Program managers responsible for HIV partner services can work actively with HIV clinical care providers and case managers to engage them in identifying patients who need partner services, offering them these services, and linking them to health department DISs when indicated. Persons who previously received a diagnosis of HIV also might be named as partners in the course of conducting partner services with other index patients. These persons should be interviewed to assess behavioral risk, provided partner services, and referred for more intensive prevention interventions, when indicated. Recommendations for Identifying Index PatientsGeneral
HIV Infection
Prioritizing Index PatientsAll persons with newly diagnosed or reported HIV infection or early syphilis should be offered partner services and prioritized for interview, although some of these patients have a higher priority than others. Because of the high incidence of gonorrhea and chlamydial infection in many jurisdictions, attempts to reach and interview all patients might be hampered by various factors, including insufficient funds and staffing. Therefore, for these infections, programs might need to use partner services strategies that do not require interviews by DISs, focusing their interviewing on specific subsets of patients. To maximize available resources, programs should establish criteria for determining which index patients are prioritized for interview. In general, these criteria should include behavioral and clinical factors that affect the likelihood of additional transmission and, thus, increase the epidemiologic consequences of delayed receipt of partner services. This information might not be known until the index patient is interviewed; however, it might be available from the diagnosing clinician or counselor or through record review. Criteria for prioritizing index patients vary somewhat according to the infection involved. Program effectiveness and efficiency can be improved by periodically reviewing and adjusting criteria for prioritizing index patients for partner services. The following categories of persons are considered high-priority index patients for partner services, regardless of the infection involved:
Syphilis, Gonorrhea, and Chlamydial InfectionThe following categories of persons also are considered high-priority index patients for partner services for syphilis, gonorrhea, and chlamydial infection.
HIV InfectionThe following categories of persons also are considered high-priority index patients for partner services for HIV.
Recommendations for Prioritizing Index PatientsGeneral
Syphilis
Interviewing Index PatientsWith the exception of interview period and timing of interviews, the following information is applicable to partner services for HIV infection, early syphilis, gonorrhea, and chlamydial infection. The success of partner services depends on the cooperation of index patients. If index patients do not provide complete, accurate information about partners, partner services are not effective. Obtaining accurate information largely depends on treating index patients with respect and gaining their trust. Withholding relevant information is likely to generate mistrust. When offering partner services, public health personnel should delicately balance the need to provide these important services with the knowledge that index patients can choose whether to participate. Index patients should have the following types of information explained to them:
The amount of information an index patient needs about each of these topics varies. Regardless, all patients should be offered ample opportunities to ask questions and voice concerns. Types of InterviewsInterviewing index patients to elicit partner information is a cornerstone of partner services. Two types of interviews are used: the original interview and the reinterview. A supplementary approach, clustering, also is used by certain programs to obtain information about the index patient's social network. Original Interviews and Reinterviews The purpose of the original interview is to gather information from index patients about partners they have had within a defined period (i.e., the interview period). In addition to eliciting as many partner names as possible, the interviewer attempts to obtain enough information about the partners so that they can be located and notified of their possible exposure. Most programs conduct a subsequent interview, the reinterview. The reinterview has several purposes: 1) to gather additional location information on partners identified by index patients in the original interview, if sufficient information was not initially obtained; 2) to follow up on the status of partners that index patients initially elected to notify themselves; 3) to elicit additional partners index patients might not have recalled in the original interview; and 4) to verify that index patients have received adequate treatment or additional tests. Frequently, more than one reinterview is conducted. Few studies have been conducted regarding the yield of reinterviewing or the relative yield of the original interview compared with the reinterview. Second interviews of 1,000 persons with STDs in a clinic in Berlin, Germany, in 1976 resulted in elicitation of 9% more partner names (D Brewer, unpublished manuscript, 2003). A subsequent sample of 110 persons from the same clinic were interviewed before diagnosis with gonorrhea then reinterviewed twice after diagnosis. The second and third interviews together elicited 12% more partner names compared with the first interview, resulting in an 11% increase in the total number of partners located and a 13% increase in the number of infected partners identified. In a study of forgetfulness as a cause of incomplete reporting of partner names, patients recalled roughly equal numbers of partners in the first and second interview; however, the sets of partners recalled in the two interviews tended to differ (71). Finally, in a randomized trial of supplementary techniques used during contact interviews for chlamydial infection, gonorrhea, and early syphilis, use of a combination of five sets of recall cues increased the number of partner names elicited by approximately 23% and the number of partners notified by approximately 11% per index patient. This approach was approximately two to three times as effective in eliciting additional partners as a second interview (72). Reinterviews are recommended for all early syphilis investigations and are standard practice in most partner services programs. Persons who have just been informed that they are infected with HIV are often willing to provide partner information and might address the topic of partners without being prompted. Other patients might be too overwhelmed by the new diagnosis to focus on identifying partners effectively or to hear and understand messages regarding prevention or linkage to care during the original interview. In these instances, using the first interaction with an index patient to help with various challenges arising from the HIV diagnosis and addressing partner elicitation in a subsequent interview might increase the likelihood that the index patient will identify partners. This approach must be weighed against the possibility that the index patient might not be available for a second interview. Clustering A technique known as clustering has been recommended for use when interviewing index patients (1). Clustering involves eliciting information from index patients about persons in their social networks, other than partners, who might benefit from counseling, testing, and other services. These persons, referred to as social contacts (and referred to as suspects in previous guidelines), might include persons with symptoms suggestive of disease, partners of other persons known to be infected, or others who might benefit from examination (e.g., pregnant females). Clustering also might include eliciting information about venues in which the index patients and their social contacts interact socially (e.g., bars or clubs). Clustering differs from cluster interviewing, which involves asking uninfected partners or social contacts of index patients about their own social networks. Cluster interviewing is addressed in more detail in the section on partners. Data on the effectiveness of clustering for case finding are limited. A 6-month pilot project was conducted in which a network approach was used for routine syphilis partner notification in an Atlanta, Georgia, zip code with a high rate of early syphilis (260 cases per 100,000 population) (73). Among sex partners of syphilis index patients, 23.1% were infected with syphilis, whereas 5.9% of nonsexual contacts were infected. Another study included an analysis of 1993--1996 partner notification data for 12,927 patients with syphilis throughout Louisiana (74). Of 19,188 partners located and examined, 19% were newly identified as having syphilis; of 3,121 social contacts of index patients, 11% were newly identified as infected. A review of the effectiveness of partner notification and cluster investigations for identifying HIV and other STD cases indicated that, based on a small number of studies from the previous 20 years, the yield of cluster investigation for cases early syphilis was substantially less than the yield from partner notification for early syphilis (mean number of newly diagnosed cases found from interviewing one index patient was 0.002--0.11 for cluster investigation compared with 0.05--0.46 for partner notification) (8). Furthermore, the same review found that, compared with a few reports from previous years of syphilis control, the yield of cluster investigation has declined considerably, possibly related to the marked decline in early syphilis prevalence. In summary, data from a small number of reported studies suggest that the case-finding yield of clustering for early syphilis is substantially lower than that of partner notification. However, the yield is highly variable, and clustering might be more productive in areas with relatively high early syphilis case rates. Clustering and cluster investigation might be particularly useful during an outbreak. Published data on the case-finding yield of clustering for HIV are not available. Although data regarding the effectiveness of clustering for case finding are limited, information obtained through clustering has potential epidemiologic value. By obtaining and analyzing information about social contacts and venues of index patients, programs can gain insight into how and where infection is being transmitted in the community and develop strategies for conducting screening or other prevention interventions (e.g., social marketing campaigns) at the community level. Interview EnvironmentsBecause of confidentiality concerns, interviews generally should be conducted in environments that are private and comfortable enough that clients do not feel afraid or coerced. Public health clinics provide a safer environment for partner services staff members and a more confidential setting for interviewing and counseling than field settings. However, interviews conducted in settings other than the clinic might allow index patients to feel more comfortable discussing highly personal information. Interviews have traditionally been conducted in person; however, this approach is time and labor intensive and not always possible. The next most common method (and the most common in certain settings) is interview by telephone. Other interview methods, such as use of self-administered questionnaires and audio computer-assisted self-interviews have been suggested as alternatives or supplements to in-person interviews; however, little research has been done on this topic (D Brewer, unpublished manuscript, 2003). Although self-administered questionnaires are frequently used in medical care settings to obtain information from patients before they are seen by a clinician, no studies of this approach for partner services have been published. Likewise, little data are available on telephone interviews and partner services. Previous CDC recommendations for STD partner services suggested that a telephone interview might be considered if attempts to meet with an index patient in person are unsuccessful or the index patient is in a different geographic location than the interviewer. One study, in which STD and community clinic attendees responded to varying hypothetical partner services providers and interview and notification conditions, showed that interview settings that were not in clinics were less favorably viewed than clinic interviews (75); telephone notification and letters by partner services providers were also less favorably viewed, although not significantly so. Although the available evidence suggests that audio computer-assisted self-interviews might effectively elicit partner information from index patients, no studies examining this particular use were found (76--85). Interview TechniquesIncomplete reporting of partner names might stem from various factors, such as concern about potential negative consequences (e.g., fear of partner violence), perceived social undesirability of acknowledging participation in stigmatized or illegal activities, and forgetting or memory errors (71). However, although partner elicitation can be challenging, its effectiveness can be improved through systematic use of simple techniques. In a study of forgetting as a cause of incomplete reporting of partner names, using simple and nonspecific prompting (e.g., "Who else have you had sex with in the last 12 months?") and reading back the list of partners already named improved recall substantially (71). On average, these methods accounted for 10% of all partners recalled during an interview. In a randomized trial of interviewing techniques for persons at high risk for HIV infection, administering a set of five types of cues to participants, after they had freely recalled their partners, increased the number of sex and drug-injection partners elicited by an average of 40% and 123%, respectively (86). Eliciting partners in reverse chronological order, as suggested in previous CDC recommendations, was no more effective than using free recall. In a subsequent study of supplementary interview techniques for eliciting partners from index patients with chlamydial infection, gonorrhea, and early syphilis, patients were asked to freely recall partners, were prompted nonspecifically, and had the list of elicited partners read back (72). They were then administered three sets of cues, which increased the number of new cases found by 12% and identified network branches not previously recognized. Using supplementary interview techniques might be an efficient strategy for increasing the number of partners elicited and located. This approach seems to be effective for persons at risk for HIV as well as those with other STDs, suggesting that comprehensive, systematic use of such techniques during the original interview might enhance the yield of new partners identified. Interview PeriodThe interview period is the time interval for which index patients are asked to recall their partners. Ideally, the interview period covers the time from the earliest date an index patient could have been infected up to the date of treatment (i.e., the time period during which the patient could have become infected or transmitted the infection to others). Anyone who had sex with or, for those with HIV infection, shared drug-injection paraphernalia with the index patient during this interval is at high risk for infection. Because of differences in biological factors and natural history, the interview periods for HIV, early syphilis, gonorrhea, and chlamydial infection differ (Table 1). Syphilis, Gonorrhea, and Chlamydial Infection The interview period for early syphilis varies according to the stage of disease (primary, secondary, or early latent) because stages develop within well-defined timelines. Thus, the interview is used not only to find early syphilis cases but also to estimate which partners are most likely to have infected the index patient (i.e., the source) and which partners the index patient is most likely to have infected (i.e., spread). Source and spread information aid in defining the epidemiology of the infection and can be used to identify networks of infection. To some extent, source and spread can be estimated for gonorrhea and chlamydial infection but almost exclusively on the basis of interview results and generally not on the basis of stage of disease. The interview period for syphilis is based on the disease stage at the time of diagnosis and incorporates all maximum time periods for incubation and stage of disease. The interview period for a person with a diagnosis of primary syphilis is 4 months and 1 week, based on a 90-day maximum incubation plus 5 weeks (35-day maximum duration of lesion). The interview period for a person with a diagnosis of secondary syphilis is 8 months (34 weeks), based on a maximum 90-day incubation period and 5-week duration of primary syphilis, 10-week primary-secondary latency, and 6-week maximum duration of secondary symptoms. The maximum interview period is 12 months for early latent cases unless a credible primary or secondary history can be established and for cases of unknown duration. Unlike syphilis, which has multiple increases and decreases in level of infectivity, infectivity for gonorrhea and chlamydial infection increases to a single peak and then decreases. The standard interview period is 60 days before the date of specimen collection and should be extended through the date of treatment if the patient was not treated at the time the specimen was collected. For persons who seek treatment at a clinic because of signs or symptoms of gonorrhea and chlamydial infection, incubation is almost entirely covered within this 60-day period (although a few programs use 90 days). For asymptomatic cases of either STD (e.g., cases discovered during screening), the number of cases identified from partner notification can decrease substantially. This is especially relevant for chlamydial infection, which is most likely to be asymptomatic and for which widespread screening is most likely. In such instances, attempts to ascertain source and spread would have to be established on behavioral reports during interviews. However, data are useful for describing networks and the epidemiology of infection. HIV Infection Determining when an index patient was infected with HIV often is difficult. Therefore, HIV programs frequently establish an interview period based on the estimated likelihood of being able to locate and contact the partners (e.g., 1--2 years before HIV diagnosis). The recommended interview period in a particular jurisdiction might subsequently be modified based on analysis of local data or availability of resources. Additional considerations might influence the interview period for specific index patients. Previous Negative HIV Test. A confirmed history of a negative HIV antibody test might be useful for defining the appropriate interview period for a specific index patient. Knowing the date of the patient's most recent negative test and considering the window period (i.e., the time interval following infection during which an HIV test might be negative because antibodies have not yet developed to a detectable level), the interviewer can establish how far back in time the interview period should extend. An estimated 95% of persons infected with HIV develop detectable HIV antibody within 6 months of infection, suggesting 6 months might be an appropriate interval to use as a window period; however, these estimates were developed using previous, less sensitive versions of the HIV enzyme immunosorbent assay (EIA) (87,88). More recent EIA tests (e.g., second-generation immunoglobulin G [IgG] tests and third-generation IgG/immunoglobulin M [IgM] tests) are considerably more sensitive than the previous tests and might allow the estimated window period to be shortened to 3 months (89,90). Evidence of Recent Infection. For certain patients, recent infection might be suggested by a clear history of an acute retroviral syndrome, which might result in the interview period being shortened for these specific patients (91). Recently, HIV RNA testing has been used to screen pooled, HIV antibody--negative specimens to identify persons with acute or very recent infections (i.e., HIV RNA--positive and HIV antibody--negative) (70,90,92--98). For these index patients, the likely period of the date of infection can be narrowed considerably, and the interview period can be adjusted accordingly. Spouses. As noted previously, the Ryan White CARE Act Amendments of 1996 require that states receiving funds under part B of title XXVI of the Public Health Service Act ensure that a good-faith effort is made to notify the current spouse of an HIV-infected person or persons who have been legal spouses of that person during the 10 years before the diagnosis that such spouse might have been exposed to HIV and should seek testing. The 10-year interview period might be modified if a confirmed history of a negative HIV test or other laboratory testing indicates that the index patient was infected more recently; however, states should consult with their legal counsel to determine whether their laws allow them to make this modification. Timing of InterviewsThe time in which partner services should be offered to reduce the disease transmission rate and the likelihood of reinfection might vary somewhat by disease. However, in general, partner services should be offered as soon as possible. Syphilis, Gonorrhea, and Chlamydial Infection The pattern of infectiousness for early syphilis requires rapid notification and treatment of sex partners to have an effect on subsequent disease progression or transmission; therefore, partners should be notified quickly. For gonorrhea and chlamydial infection, the vast majority of additional transmission (i.e., by infected and untreated partners of the index patient) occurs quickly, and a delay in the interview is inadvisable. The morbidity for these two infections, especially chlamydial infection, have led to investigation of various notification and treatment options that are not widely advocated for syphilis (e.g., contact slips for patients to deliver to partners or patient delivery of oral medications). HIV Infection Persons who test positive for HIV should be contacted and offered partner services as soon as possible after being identified by the partner services program, ideally within a few days. Rapid identification, notification, and testing of partners can reduce risk for additional transmission. A rapid interview allows partners to be identified and notified of possible exposure as soon as possible so that they can 1) obtain HIV counseling and testing; 2) take steps to avoid becoming infected or, if already infected, to avoid infecting others; and 3) access medical care and other services as soon after infection as possible. Patient reactions to learning about their HIV infection vary, and personal circumstances differ among patients. Partner services providers should recognize and, within reason, accommodate index patients who need particular concerns resolved before feeling ready to participate fully in partner services. For index patients who decline or are not ready to participate in an initial partner services interview at the time of first contact, a follow-up appointment should be arranged to discuss partner services concerns more thoroughly, preferably no later than 2 weeks after the initial contact. No studies are available related to introducing partner services to persons with reactive rapid HIV tests and interviewing them to elicit partner information. Partner services providers might consider conducting an initial interview and eliciting partner information when the reactive rapid test result is obtained and before results of confirmatory testing are available if the index patient accepts partner services at that time. However, persons with considerable partner services experience have suggested that partners not be contacted and notified until a positive confirmatory test is obtained. If a confirmatory test is not performed at the time the rapid test is found to be reactive, attempts can be made to locate the patient and obtain confirmatory testing. If the patient cannot be located or declines confirmatory testing, and the rapid test was performed on a blood specimen, the DIS can then contact the partners and notify them about their possible exposure to HIV through someone who had a reactive rapid test result. This suggestion is based on the high predictive value of a reactive rapid test, in most circumstances, when performed on a blood specimen. Local policies might preclude use of this approach. InterviewersTraditionally, index patients have been interviewed by health department DISs. Certain evidence indicates that health department specialists might elicit more partner names from index patients with gonorrhea or chlamydial infection than other, presumably untrained, interviewers (99). A cohort study of the efficacy of partner notification for HIV infection found that although patients counseled by health department specialists reported more locatable partners than those counseled by physicians, the number of partners per index patient interviewed who were then identified as having a new diagnosis of HIV infection was similar for both groups of health-care providers (100). No such comparative data exist for gonorrhea or chlamydial infection, although the frequency with which both STDs are diagnosed outside public settings (especially STD clinics) suggests that collaboration with physicians is appropriate. Health departments might be able to expand access to and coverage of partner services by developing agreements with providers who are not in health departments to elicit information about partners and provide that information to a health department DIS. The DIS can then notify the partners. Existing relationships and rapport between these providers and their patients might facilitate partner elicitation; providers must inform patients that the information will be shared with health department DISs. Documented experience with this strategy is scarce; however, such approaches have been used by health departments in several jurisdictions, with anecdotal reports of success. Other than DISs, types of providers who might elicit partner information from index patients on behalf of partner services programs include the following:
If other types of providers participate in this way, roles and responsibilities should be clearly defined. Unique Circumstances of Index PatientsUnique characteristics of index patients and their individual circumstances might affect the partner services process. In certain instances, the mental health status and decision-making capabilities of an index patient might affect the approach to providing partner services. Guardians or others might be charged with making legal and health-care decisions for such persons. Local laws, regulations, and policies might address these concerns. Programs should develop policies and procedures that describe how to work with index patients who might have limited comprehension or other mitigating circumstances; this might require consultation with persons who have expertise in these areas. Examples of concerns that should be considered when developing protocols include age and developmental level, literacy level, language barriers, cultural concerns, hearing or visual impairments, alcoholism or abuse of other substances, mental health concerns, or potential violence (either on the part of index patients or their partners). Recommendations for Interviewing Index PatientsGeneral
Syphilis, Gonorrhea, and Chlamydial Infection
HIV Infection
Risk-Reduction Interventions for Index PatientsMany HIV-infected persons are knowledgeable about STD/HIV transmission and prevention; however, misconceptions and inadequate information about transmission and methods for reducing transmission risk are common (102--105). All index patients likely can benefit from receiving information and brief prevention messages about adopting and maintaining safer behaviors to protect their own health and that of their partners (25,106). In the case of HIV infection, this includes discussing the index patients' responsibility for disclosing their HIV serostatus to current and future partners. These messages can be integrated easily into the activities of DISs. In addition to provision of information and brief prevention messages, prevention counseling can be relevant for all infected persons, regardless of the STD diagnosis. Project RESPECT, a risk-reduction intervention trial conducted during the mid-1990s, was aimed at preventing HIV and other STDs in HIV-negative, heterosexual STD clinic patients (25). Approximately one third of participants had an STD at the time of enrollment. Participants were randomly assigned into three groups and received either two sessions of interactive risk-reduction counseling; four sessions of enhanced interactive, theory-based counseling; or two brief sessions of didactic information. Compared with baseline, participants in all three groups reported higher levels of condom use at 3, 6, 9, and 12 months follow-up. At 3 and 6 months, participants receiving either of the two counseling interventions reported significantly higher levels of condom use than those receiving only didactic information. At 9 and 12 months, participants in all three groups continued to report higher levels of condom use than at baseline, but the differences between those in the two counseling groups and those in the didactic information group were no longer significant. In addition, compared with participants in the didactic information group, 30% fewer participants in the two counseling groups had new STDs after 6 months, and 20% fewer participants in either counseling group had new STDs through the 12-month interval. The relative effectiveness of counseling was greater among participants who had an STD diagnosis at enrollment than among those with no STD. Interactive and individually tailored counseling is likely similar to the communication between many DISs and patients regarding partner services and future behavior. However, use of the intervention in STD clinics, with at least two sessions, has been limited (107). Another study of counseling to prevent STDs and HIV infection in STD clinic patients compared the effectiveness of two 20-minute individual counseling sessions with four 1-hour group sessions with a follow-up session 2 months later. After 12 months, both groups had similar and significant increases in condom use, decreases in number of partners, and decreases in numbers of new infections with gonorrhea (14%), chlamydia (10%), or syphilis (2%) (108). Syphilis, Gonorrhea, and Chlamydial InfectionAlthough prevention counseling is relevant for persons with early syphilis, gonorrhea, or chlamydial infection, prevention counseling other than individualized attempts during an interview is typically composed of brief prevention messages delivered once. With early syphilis patients, repeated contact with DISs during the course of an investigation is common enough that they can establish a record of behavioral change or reinforce previous counseling. Except for repeat cases, health department--mediated prevention counseling with gonorrhea or chlamydial infection is almost certain to be a one-time session. The gap between demonstrated efficacy and implementation merits additional examination. Certain aspects of counseling for patients with syphilis, gonorrhea, and chlamydial infections are devoted to promoting rescreening at 3 months, which CDC recommends, given frequently high rates of reinfection among STD clinic attendees (3,109). In one study of STD clinics in Los Angeles County, California, and Maryland, telephone reminders and point-of-care interviews (approximately 20 minutes) to reinforce the importance of rescreening to the patient were both associated with increased rates of return at 3 months (110). Subsequent efficacy and cost-effectiveness analyses suggested the telephone reminder alone would be most efficient and most cost-effective, although the point-of-care interviews should be used in situations in which telephone contact is unlikely (110,111). Another condition tested in the study, a $20 incentive with brief instructions, was associated with higher return rates for men but not women. Although programs might consider incentives to improve return rates, offering them only to men would have ethical implications. Data on interventions to promote follow-up testing for syphilis recurrence would broaden the scope of evidence available for making program decisions about this disease. HIV InfectionMany persons substantially reduce HIV transmission risk behaviors after learning they are infected (28,30,112--114). A metaanalysis of high-risk sexual behavior in HIV-infected persons aware and not aware of their infection found that the prevalence of unprotected anal or vaginal intercourse with any partner was an average of 53% lower for HIV-infected persons aware of their infection compared with HIV-infected persons not aware of their infection and 68% lower after adjusting data to focus on unprotected anal or vaginal intercourse with partners who were not already HIV infected (29). However, a considerable percentage (range: 10%--60%) do not consistently practice safer behaviors and might transmit infection to others or put themselves at risk for acquiring other STDs (26). Thus, although certain HIV-infected index patients might already have reduced their level of risky behaviors by the time they are interviewed for partner services, others are continuing risky behaviors and require additional prevention counseling or other more intensive prevention intervention. Index patients who need additional counseling or other risk-reduction interventions can be identified through brief behavioral risk screening that can be integrated easily into the interview process (54). Questions used for behavioral risk screening need to be broad enough to identify most index patients engaging in risky behaviors. This includes index patients currently or recently engaging in risky sex or drug-injection practices, those who have a current or recently diagnosed STD, those who might be pregnant or at risk for unintended pregnancy, those with other characteristics associated with risky behaviors (e.g., alcohol or other noninjection drug use), and those who were previously identified and are now named as partners by other index patients, which suggests ongoing risky sex or drug-injection behavior. Index patients whose risk screening indicates continuing risky behavior should be informed of the risks involved in continuing the behavior. They should also be provided prevention counseling or referred for counseling or more intensive prevention services. Several risk-reduction interventions designed specifically for HIV-infected persons have been demonstrated to be effective. Most of these interventions involve multiple sessions provided over time, usually in a group format (26,27,31). Most do not focus only on reducing transmission risk; rather, they address multiple life concerns faced by HIV-infected persons, which might increase the likelihood that patients can make and sustain behavioral changes. These interventions are not feasible to provide through partner services but might be available through CBOs in the area or as part of ongoing prevention activities incorporated into the medical care of persons living with HIV infection (54). Certain partner services programs might have adequate resources to assess and provide prevention counseling to index patients whose screenings indicate continued risky behaviors. However, health department DISs often have limited numbers of interactions with index patients. Furthermore, the time available for DISs to provide prevention counseling to index patients might be limited, especially if health departments expand their partner services activities to ensure that all persons with newly diagnosed or reported HIV infection receive adequate partner services. Consequently, in certain programs, health department DISs might have difficulty providing prevention counseling to all index patients for whom it is indicated. In these situations, and for index patients requiring more intensive prevention interventions, referral or linkage to agencies that provide these services or to case managers who can arrange them is appropriate. Recommendations for Risk-Reduction Interventions for Index Patients
Treatment for Index PatientsSyphilis, Gonorrhea, and Chlamydial InfectionThe CDC 2006 STD treatment guidelines provide preferred and alternative treatments for syphilis, gonorrhea, and chlamydial infection (3). DISs should verify that index patients have been treated appropriately. Because each of these STDs is curable, linkage to additional medical care (in the absence of coinfection with HIV) generally is not needed, although recommendations for follow-up testing are appropriate. HIV InfectionEffective and timely medical evaluation, initiation of currently recommended combination ART, and provision of appropriate vaccinations and other preventive health interventions have led to substantial reductions in HIV-related morbidity and mortality (115,116). HIV-infected persons who begin receiving (or reestablishing) medical care not only can benefit from ART but also can receive screening for other STDs and bloodborne infections (e.g., HBV and HCV), appropriate vaccinations for vaccine-preventable infections, and other medical services. In addition, through medical care and HIV case management, patients can be evaluated and receive referrals for a wide range of other medical and psychosocial services, and the medical care setting offers an opportunity for patients to be more completely assessed for HIV transmission risk and provided or referred for appropriate HIV prevention services (54). Furthermore, ART might decrease infectiousness and reduce risk for transmission to others by reducing the patient's viral load (32,117,118). However, delays in accessing medical care and inadequate use of care are common among persons who receive an HIV diagnosis (14,119,120). Linking HIV-infected persons to medical care and ongoing HIV case management as soon as possible after diagnosis is essential. Brief HIV case management for persons with newly diagnosed HIV infection increased attendance at HIV care facilities. The Antiretroviral Treatment Access Study, a multisite, randomized control intervention for persons with newly diagnosed HIV infection, directly compared passive referral (i.e., giving patients a list of medical providers) with brief HIV case management and found that those who received HIV case management were significantly more likely to be linked to and attend clinic visits over a 12-month period (121,122). In certain situations, these brief HIV case-management services were provided by DISs. This underscores the importance of DISs actively helping index patients with newly diagnosed or newly reported HIV infection to access medical care either directly or by linking them to HIV case managers. DISs also might be able to facilitate reestablishment of reentry into HIV case management and medical care for HIV-infected persons who are not currently receiving medical care but have in the past. Recommendations for Treatment for Index PatientsSyphilis, Gonorrhea, and Chlamydial Infection
HIV Infection
Referring Index Patients to Other ServicesMany index patients have underlying problems that impede their ability to access medical care or adopt and maintain safer behaviors and would benefit from referrals to various psychosocial services. Because of the numerous U.S. cases of gonorrhea and chlamydial infection, and because medical management of syphilis, gonorrhea, and chlamydial infection does not generally require an ongoing care relationship with partners, the process of referral to other services for index patients with these STDs is less intense than it is for index patients with HIV infection. Nevertheless, many jurisdictions offer referrals for care on request or if the need for other services is ascertained during the course of interviewing the index patient. For index patients whose infections are likely related to their living conditions (e.g., homelessness or partner violence), attention to need for supportive services might reduce the likelihood of reinfection and contribute to infection control. Program collaboration and service integration facilitate this process. Index patients might need a range of services, such as the following:
DISs usually do not have the time or skills to address these concerns; they can best be addressed through referral. For HIV-infected index patients, these services most likely will be available through linkage to medical care and HIV case management. At a minimum, program managers should identify updated referral resources and develop procedures for making successful referrals. Recommendations for Referring Index Patients to Other Services
Notifying Partners of ExposureNotification StrategiesAfter index patients have identified partners, the partners should be notified of the exposure as soon as possible. Traditionally, four strategies have been used to accomplish this: provider referral, self-referral, contract referral, and dual referral. Provider referral notification involves a partner being notified of their possible exposure by a health department specialist who has been specifically trained to locate and notify partners. The specialists then link the partners to medical, prevention, and support services while protecting the confidentiality of the index patient. The term provider referral has sometimes led to confusion, because health-care providers other than health department specialists might conduct some or all steps in the partner services process, especially for index patients who receive a diagnosis in a setting other than the health department. Therefore, these recommendations use the term provider referral to specifically describe notification carried out by health department staff members and the term third-party referral to describe partner notification carried out by other professionals (e.g., HIV counselors and clinicians who are not in health departments). Self-referral notification (also called client or patient referral notification) gives the index patient full responsibility for informing partners of exposure and referring them to appropriate services. Contract referral notification involves index patients selecting specific partners they prefer to notify themselves and agreeing to a specific time frame in which they will do so. Patients agree that if they do not notify the selected partners within the established time frame, the DIS will notify the partners. Dual referral notification involves an index patient and a provider (a DIS or third party) jointly notifying a partner of exposure. The notification strategies primarily differ in the degree of responsibility assumed by the DISs. Variations in the extent of DIS involvement, in turn, contribute to differences among the strategies in terms of effectiveness, intensity of resource use, and acceptability to index patients and partners (Table 2). The limited available data suggest the following:
Provider Referral Notification STDs Other than HIV. A 1977 study comparing provider referral with self-referral for gonorrhea found that similar proportions of partners were evaluated and treated, although provider referral follow-up was required for a small number of partners who originally had been randomly assigned to the self-referral group (123). In a study of partner notification for syphilis, for which provider referral is most strongly emphasized of all the STDs, a comparison of three referral approaches (two groups with provider referral, one with contract referral) revealed no clear evidence of increased effectiveness or cost-effectiveness for any strategy compared with another (5). Because the majority of spread of infection of primary and secondary syphilis is likely to occur near the same time as the interview, infection control requires almost immediate partner notification and referral. Such swift notification is most reliably accomplished through provider referral (although notification of Internet partners might be an exception). The effectiveness of provider referral (or third-party referral) depends on the ability and willingness of index patients to provide sufficient identifying and locating information. Index patients often cannot provide sufficient information to conduct provider referral for all partners, and other strategies might be needed. For example, during syphilis outbreaks in several U.S. cities during 2002--2005 among men who have sex with men (MSM) (124,125), program staff members considered using Internet-based notification when index patients could provide only e-mail addresses or chat-room nicknames as identifiers. Gonorrhea and chlamydial cases are frequently too numerous to permit provider referral. The basis for notification in such instances should be self-referral, although basic instructions can be supplemented with brief oral counseling, written instructions, and contact information for patients to give to partners (most commonly known as contact slips or referral cards) (126,127). Circumstances in which index patients also are provided with medications or prescriptions to deliver to partners are known as patient-delivered partner therapy, a form of expedited partner therapy. HIV Infection. Provider referral has been found to be an effective means of identifying new cases of HIV. In nine studies that qualified for inclusion in the Guide to Community Preventive Services review, a range of one to eight partners were identified per index case. A mean of 67% of named partners were found and notified of their exposure to HIV (range: 44%--89%), a mean of 63% of those notified were tested, and of those tested, a mean of 20% were newly identified as HIV infected (range: 14%--26%) (16). Only two U.S. studies comparing provider referral with other referral strategies for HIV partner notification have been published; both were included in the Guide to Community Preventive Services review. In one study comparing the effectiveness of provider referral and self-referral (i.e., patient referral) notification in three health departments in North Carolina, index patients were randomly assigned to provider referral or patient referral groups (128). In the provider referral group, index patients were given the option of selecting between provider referral conducted by a health department counselor and contract referral. With contract referral, they were given 2 weeks to notify a partner themselves, after which time a counselor attempted to notify any partners who had not been notified by the index patient. In the patient referral group, index patients were asked notify all of their partners themselves and were not given the option of requesting provider referral for any partners. Patients were given 1 month for notification, after which time the counselors attempted to notify any partners who had not been notified by the index patient. In the provider referral group, counselors notified 70 (45%) of 157 partners. In the patient referral group, index patients notified only 10 (7%) of 153 partners. Thus, in this study, provider referral was approximately 6.5 times more effective than patient referral. Of the 143 partners who were not notified by index patients in the patient referral group, counselors were able to notify only 40 (28%) partners. A second study analyzed results of HIV partner notification services provided by the Colorado Department of Health in 1988 (129). Of 84 partners for whom provider referral was intended, 71 (85%) were notified by providers. Of 30 partners for whom patient referral was intended, 17 (57%) were notified by index patients. Thus, in this analysis, provider referral was approximately 1.5 times more effective than patient referral. Self-Referral Notification Syphilis, Gonorrhea, and Chlamydial Infection. Although provider referral is favored and generally expected for notifying partners of persons with syphilis, self-referral is the typical form of partner notification for persons with chlamydial infection. Successful public health involvement with partner notification for chlamydial infection is likely to be limited to improving self-referral effectiveness through interventions provided at the time of diagnosis or treatment (e.g., brief counseling) and possibly through increased monitoring of the proportion of those seeking care who have been referred by a partner (11). Gonorrhea is somewhat more likely than chlamydial infection to be targeted for provider referral in public clinic settings; nonetheless, strategies for chlamydial infection often are applicable for gonorrhea (especially outside public clinic settings); basic instructions can be supplemented with brief verbal counseling. A randomized, controlled trial in Brooklyn, New York, showed male notification rates of partners could be improved with a brief counseling session (approximately 20 minutes) aimed at identifying and reducing barriers (130). Index patients' intentions, skills, and belief in their ability to notify (i.e., self-efficacy) have been associated with more successful referrals, including among adolescents, and interventions to increase the effectiveness of self-referral typically have incorporated approaches aimed at improving self-efficacy (131). Written instructions for index patients to deliver to partners are known as contact slips or referral cards. Referral cards are used to add legitimacy to the index patient's notification of the partner, provide information to the partner, and provide information and a short history of exposure to any clinician from whom the partner seeks evaluation. This ensures that the clinician has sufficient, accurate information to guide appropriate evaluation and management of the partner. In ideal circumstances, the referral card with treatment notes from the evaluating clinician is returned to a public health program, but this situation rarely occurs. A referral card can include the specific type of exposure, where to go for timely evaluation, what to expect in an evaluation, the recommended treatment, and what to do until treatment begins (e.g., abstain from sexual activity). For confidentiality reasons, no jurisdictions permit names on a referral card, and many jurisdictions have policies prohibiting naming the type of infection. One British study showed that partners of index patients with chlamydial infection were much more likely to seek evaluation if their referral card specifically referred to chlamydial infection (84% versus 33%, p<0.01) (132). Among program evaluations in the United States and other industrialized nations, use of referral cards typically has been associated with improved notification and treatment rates. In one trial, their use was associated with reduction in reinfection of index patients but not improved notification (11,126). HIV Infection. As noted previously, a randomized, controlled trial in North Carolina and an analysis of program data in Colorado both found self-referral notification strategies to be less effective than provider referral for notifying partners of exposure, especially when index patients were required to notify their own partners and given no other options (128,129). However, in the North Carolina study, patients notified 14% of all partners who were eventually notified, and in the Colorado report, patients notified 20% of all partners who were eventually notified. Research of HIV disclosure practices and attitudes toward partner notification might offer insight into index patient and partner characteristics associated with higher likelihood of disclosure or self-referral. Disclosure or self-referral is more likely for partners described by the patient as primary, regular, or main partners than for partners described as nonmain, casual, or one-time partners, regardless of patient age or risk behaviors (133--137). Intention to notify also is associated with a higher likelihood of disclosure. In turn, intention is related to factors such as sense of duty or responsibility to the partner and an HIV-infected person's perceived self-efficacy for disclosing serostatus (138--143). Increasing number of partners is inversely related to likelihood of disclosure; as the number of partners increases, the likelihood that the index patient will notify any of them decreases (134,144--147). Contract Referral Notification Syphilis, Gonorrhea, and Chlamydial Infection. Contract referral has not been widely evaluated for syphilis, gonorrhea, or chlamydial infection. A trial including provider and contract referral notification for syphilis infection revealed no clear advantages to either method (5). DISs must balance the efficiency gained by having to notify fewer partners in the short term (i.e., partners who are notified by the index patient and also seek evaluation) with the efficiency lost by conducting additional interviews with index patients who did not notify partners as intended and with the potential for additional transmission because of delayed notification. HIV Infection. No published study has assessed directly the notification and case-finding effectiveness of contract referral for HIV partner notification. However, some insight might be gained from the previously discussed North Carolina and Colorado studies (128,129). In the North Carolina study, 128 (41%) of 310 partners were notified by a combination of providers and patients, whereas of the 292 partners who providers attempted to notify alone, 110 (38%) were notified. In the Colorado study, 104 (91%) of 114 partners were notified by a combination of providers and patient, whereas of the 91 partners who providers attempted to notify alone, 81 (89%) were notified. These findings suggest that including index patients in the notification process might be as effective as relying solely on providers to carry out all notifications and that the strategy certainly is efficient. Dual Referral Notification Dual referral notification involves an index patient and provider, together, notifying a partner of exposure. Dual referral provides the index patient direct support in the notification process and might decrease the possibility of negative consequences such as violence or severe emotional reactions. The DIS is available to offer immediate counseling, provide accurate information, answer questions, address concerns, and provide referrals to other services. At the same time, participation by index patients might help patients begin to think about their infection status, increase the likelihood that partners are located and notified, and increase the acceptability of the partner services process to partners. In theory, dual referral has substantial advantages over other approaches; however, the frequency with which this approach is used and its effectiveness (either absolute or relative to other notification methods) are not known. Third-Party Referral Notification Third-party referral notification involves partners being notified by providers who are not with health departments (e.g., private physicians). The frequency with which this strategy is used, its feasibility and effectiveness, and its acceptability to index patients, providers, and partners are not known. In general, the most appropriate roles for third parties in partner services are likely interviewing index patients to elicit partner information and possibly participating in partner notification when dual referral strategies are used. Because no data are available on the effectiveness and safety of third parties conducting field notification, the level of training and skill needed for third-party referral is unclear. State and local laws might have specific requirements related to duty to warn for third-party providers. Prioritizing Partners for NotificationAll identified partners should be notified of their possible exposure as soon as possible, unless partner violence resulting from the notification is a concern. However, prioritizing certain partners for the most immediate notification is appropriate. In general, criteria for prioritizing partners for more immediate notification include behavioral and clinical factors that increase the likelihood of the partner having been infected as a result of exposure or of transmitting infection to others if the partners are infected. Criteria vary somewhat according to the infection involved. Program effectiveness can be improved by periodically reviewing and adjusting prioritization criteria. HIV Infection, Syphilis, Gonorrhea, and Chlamydial Infection Following are categories of partners who are considered to have the highest priority for notification of exposure, regardless of the infection involved:
HIV Infection Following are examples of other categories of partners who are considered to have the highest priority for notification of exposure to HIV:
ConfidentialityWhen notifying partners of exposure, the identity of the index patient must never be revealed. Partners might correctly guess the identity of the index patient and pressure health department staff members to confirm their suspicions, but well-trained DISs avoid such confirmations, either orally or through body language. In addition, information about partners should not be reported back to the index patient. Steps can be taken to reduce the likelihood that neighbors, family, friends, or others are able to discern the purpose of health department staff members in the field looking for index patients or named partners, such as not wearing identification badges, not using marked vehicles, and not explaining to others the reason a particular person is being sought. Screening for Potential Partner ViolenceThe potential for violence initiated either by a partner or by an index patient during the process of partner notification is an important concern. Published data on violence associated with partner notification are limited. A study conducted in New Orleans, Louisiana, examining the effect of HIV and syphilis partner notification on partnerships found that, at baseline, 42.3% of index patients reported having experienced emotional abuse from a partner in the 3 months before interview and 23.6% reported having experienced physical violence from a partner during the same interval (40). No difference between HIV and syphilis partnerships was found in terms of the proportion of participants reporting either emotional abuse or physical abuse at baseline; during the 6 months after partner notification, emotional abuse and physical abuse decreased significantly among both HIV and syphilis partnerships, with no difference between the two. However, this study did not determine whether any of the abuse or violence was directly related to partner notification. A study of Mexican-American and African-American women with nonviral STDs examined factors related to whether the women had notified their male partners or intended to do so (43). Of 775 women in the study, 63% reported having ever been physically or sexually abused, but history of abuse was not associated with notification status. The women reported having experienced abusive behavior in relationships with 19% of the male partners; however, this also was not associated with notification status, and only 4% of the women cited concern about violence as a reason for not notifying a partner. Additional insight into the topic of notification-associated violence might be gained through studies of partner violence associated with disclosure of positive HIV serostatus, although the findings are less likely to apply to other STDs. A small number of surveys of HIV-infected women have indicated that rates of disclosure-associated partner violence might range from 0.5% to 4% (41). Interviews with 336 HIV-positive and 298 HIV-negative pregnant women in Brooklyn, New York; Connecticut; Miami, Florida; and North Carolina found that the proportion of women reporting violence was not higher among 142 HIV-positive women who received the HIV diagnosis during the current pregnancy (5.8%) than among seronegative women (10.7%) or HIV-positive women who previously received the diagnosis (9.4%) (42). Of 260 HIV-positive women with main male partners, 206 (79.2%) said their partner knew their serostatus; of these, one (0.5%) reported being physically assaulted when her partner found out she was infected. Thus, this study indicates that disclosure-associated partner violence was rare. However, 21% of the women had not disclosed their serostatus to their partners; the estimated risk for violence might have been higher had all these women disclosed their status Although the rate of violence directly attributable to partner notification is likely low, the available data are limited, and additional study is needed. The prevalence of partner violence among the populations studied in the few published reports is of substantial concern, regardless of whether the violence was precipitated by partner notification or was coincidental. Therefore, screening for potential risk for partner violence before notifying partners is important. Recommendations for Notifying Partners of ExposurePartners
Social Contacts General. In general, notification of partners should have a higher priority than notification of individual social contacts identified through clustering. Routine follow-up of social contacts should be carried out only after the program is successfully interviewing most new patients with cases and locating and notifying most partners and only after carefully considering the potential case-finding yield and resource implications. If this strategy is used, the number of cases identified should be carefully monitored, and the approach should be continued only if its effectiveness and cost-effectiveness equal or exceed those of other case-finding strategies. Notification of social contacts might be given higher priority during an outbreak. HIV Infection. For persons with HIV infection, information about social contacts should be used as an aid to understanding transmission dynamics in the community and to help guide additional prevention interventions at the community level (e.g., screening and social marketing). In general, if individual social contacts are to be recruited for HIV testing, a self-referral approach rather than provider referral should be used. A provider referral approach should be used only after careful consideration of potential individual and community concerns about privacy and confidentiality. Provider referral might be appropriate during an outbreak. Risk-Reduction Interventions for PartnersProviding Information, Brief Prevention Messages, or Interactive Prevention CounselingMisconceptions and inadequate information about STD/HIV transmission and methods for reducing transmission risk are common; all partners likely can benefit from receiving information and brief prevention messages about adopting and maintaining safer behaviors to reduce their risk for acquiring or transmitting STDs/HIV (25,106). These messages can be integrated easily into DIS activities. Previous CDC guidelines for HIV partner counseling and referral services and STD partner services have recommended interactive, client-centered prevention counseling for partners (1,2). No published studies are available regarding the effectiveness of prevention counseling specifically in the context of partner services. Some reduction in risk behavior after partner notification has been reported; however, overall, data are too limited to allow any conclusions to be drawn (44,151). A metaanalysis of HIV counseling and testing research published during 1985--1997 concluded that HIV counseling and testing did not seem to reduce risk behaviors among HIV-negative persons (112). However, the studies included generally provided little or no detail about the type of counseling used. Subsequently, the previously mentioned Project RESPECT trial (25) demonstrated that heterosexual STD clinic patients who tested negative for HIV and received either two sessions of brief, interactive, client-centered prevention counseling intervention or four sessions of enhanced, interactive, theory-based prevention counseling reported higher levels of condom use at 3- and 6-month follow-up than those who received two sessions of didactic information only; all three groups continued to report higher levels of condom use at 9 and 12 months than at baseline, but the difference between the two counseling groups and the didactic information group was no longer significant. Compared with participants in the didactic information group, 30% fewer participants in the two counseling groups had new STDs during the first 6 months following enrollment, and 20% fewer had new STDs during the entire 12-month follow-up period. A later study examined the effect of adding a follow-up counseling session 6 months after the initial 2-session counseling intervention (152). Participants who received the follow-up counseling session and those who did not had similar rates of new STDs during the subsequent 6 months. At the 9-month follow-up visit (3 months after the follow-up counseling session), participants who received the follow-up counseling session reported significantly less sexual risk behavior than those who did not receive the follow-up counseling; however, at the 12-month follow-up, this difference was no longer significant. Another study examined the relative efficacy of a single prevention counseling session in conjunction with rapid HIV testing compared with two prevention counseling sessions in conjunction with standard HIV testing (153). The incidence of new STDs among participants in the two groups during the subsequent 12 months was not significantly different (19.1% among rapid testers vs. 17.1% among standard testers). Brief group-level counseling of STD clinic patients also has been found to be effective (154--156). The possibility that prevention counseling might be more effective for notified partners than for persons in a more general population, given that the exposure risk for partners is personal and certain rather than hypothetical, has not been studied. As previously mentioned, many persons testing positive for HIV reduce transmission risk behaviors after learning they are infected (30,112,114). Other Prevention InterventionsFor certain partners, more intensive prevention interventions might be appropriate. Behavioral risk screening might be useful for identifying these persons. Several more intensive risk-reduction interventions have been demonstrated to be effective (26,27,31,157). As mentioned previously, these interventions cannot reasonably be delivered through partner services activities but might be available through other service providers in the area (e.g., CBOs) or as part of ongoing prevention activities incorporated into the medical care of persons living with HIV infection (54). DISs can play an important role in referring partners to these services. Many partners who are notified of exposure to HIV do not receive counseling and testing. In one review, only 63% of notified partners were known to have been counseled and tested (16). One reason for this might be that partner services programs are unaware when partners are counseled and tested by another provider or receive counseling and testing at a later date. Recommendations for Risk-Reduction Interventions for Partners
Cluster Interviewing PartnersPrevious CDC guidelines for STD partner services have recommended the use of cluster interviews with partners (1). Cluster interviews involve eliciting information from uninfected partners about their own partners and other persons in their social networks who might benefit from counseling and testing. These persons, referred to as associates, might include persons with symptoms suggestive of disease, partners of other persons known to be infected, or others who might benefit from examination (e.g., pregnant females). Cluster interviewing might also include eliciting information about venues in which partners and their associates interact socially (e.g., bars or clubs). As with clustering of index patients, cluster interviews of partners can be used for identifying additional cases or for epidemiologic purposes. Data on the effectiveness of cluster interviewing for case finding are limited. In one study, a network approach was used to notify partners of persons with syphilis in an Atlanta, Georgia, zip code with a high syphilis rate. Among sex partners of uninfected partners, social contacts, and associates, 5.7% were infected with syphilis, whereas 5.3% of nonsexual contacts were infected (73). Another study analyzed partner notification for syphilis in Louisiana and found that a total of 29 (6%) of 503 associates who were located and examined had newly diagnosed cases of syphilis (74). As previously mentioned, a review of the case-finding effectiveness of cluster investigation for HIV and other STDs found that the number of cases identified through cluster investigations for syphilis is substantially less than the number identified from syphilis partner notification (8). Finally, during an outbreak of syphilis in a suburban Atlanta, Georgia, community, interview of social contacts and associates facilitated identification of an extensive sexual network that might otherwise have gone undetected (158). Data from a small number of reported studies suggest that the case-finding yield of cluster interviews for syphilis is substantially lower than that of partner notification, that this approach might be more productive in areas with relatively high syphilis case rates, and that it might be particularly useful during an outbreak. Published data on the case-finding yield of cluster interviews for HIV are not available. As with clustering of index patients, information obtained through cluster interviews has potential value for providing insight into how and where infection is being propagated in the community and might help guide screening or other prevention interventions (e.g., social marketing campaigns) at the community level. Recommendations for Cluster Interviewing PartnersGeneral
HIV Infection
Testing PartnersAfter partners are notified of possible exposure to STDs/HIV, they must have access to appropriate diagnostic testing and treatment as soon as possible. Many partners who are notified of possible exposure to HIV do not receive counseling and testing. The number of partners who are examined and receive counseling and testing might be increased if testing is performed at the time of notification, whether this occurs at the clinic or another health-care facility or in the field. SyphilisSerologic testing remains the standard for syphilis testing and requires a blood sample (159). Blood can be drawn easily in a clinical setting; certain DISs are trained in phlebotomy and can draw blood in the field. Rapid tests have been developed but are not yet approved by the Food and Drug Administration for use. Moreover, rapid tests do not indicate stage of disease like reagin-based tests (i.e., through measuring titers). Whether partners are interviewed or have blood drawn in the field, they should be referred for evaluation and possible treatment. Gonorrhea and Chlamydial InfectionGonorrhea and chlamydial infection both can be detected via culture; however, chlamydia cultures are demanding and lack sensitivity, and transport of both types of organisms require careful attention to ambient conditions. However, nucleic acid hybridization tests, and, increasingly, nucleic acid amplification tests (NAATs) have been used more frequently in recent years. Non-NAATs are less sensitive than NAATs, and NAATs can be used with urine samples as well as urethral (men) and endocervical (women) samples (160,161 ). Testing of samples in the field is not feasible; therefore, partners tested at the point of notification can only be referred for evaluation or dispensed medication on a prophylactic basis (i.e., via field-delivered therapy). For those who are notified via telephone, follow-up evaluation can be conducted by obtaining urine samples via mailed kits; kits can be mailed to the partners and returned in person or by mail. No data are available on the application of mailed kits for testing, but use of this option for program-level rescreening was moderately successful (i.e., a 22% response rate and 3% positivity) in one study with women (162). Although 22% is not a strong response rate in many settings, public health agents who are rescreening per CDC guidelines have 22% fewer patients (3). A similar approach was used for chlamydial screening (not rescreening) of men in a managed care organization; 7.8% of men who received a kit were tested, although this rate was higher than the rate achieved by a letter alone (3.6%) (163). However, testing rates might rise if tests were conducted in conjunction with notification, because partners might be more concerned about being infected. HIV InfectionTesting in clinic settings can be conducted with conventional test procedures or with rapid tests using oral fluid or blood. If notification is carried out in the field, a rapid test can be performed, an oral fluid specimen can be obtained or blood drawn for conventional testing, or the partner can be escorted or referred to a public health clinic or other test provider. Ensuring that partners who are tested, especially those who test positive, receive their test results is critical. At publicly funded counseling and testing sites in 2004, only 84% of persons testing positive and 78% of those testing negative received their test results (61). Research has shown that rapid testing is acceptable and feasible in various settings and that more persons might get tested and learn their results if they are tested with rapid rather than conventional tests (164--170). Rapid testing has also been found to promote earlier initiation of care compared with conventional testing (167). Although the use of rapid testing in partner services has not been well studied, in one survey of health departments, 16 (37.2%) of 43 departments that responded reported using rapid tests in their partner services programs (171). Partners might be infected with HIV but test negative because of the window period between infection and development of detectable levels of HIV antibodies. With recent EIA tests (e.g., second-generation IgG-sensitive tests and third-generation IgG/IgM-sensitive tests), most infected persons develop detectable antibody within 3 months of infection (89,90). Therefore, partners who test negative but whose last date of exposure is unknown might ordinarily be advised to be retested 3 months later; those known to have been exposed recently might be advised to be retested 3 months after the date of last known exposure. In partner services, suggestions for retesting are complicated because reference to any date might compromise the index patient's identity. For this reason, routinely suggesting that partners be tested at the time of notification and retested 3 months later might be the best course of action. Persons with acute or recent HIV infection might test negative because of the window period. HIV RNA testing has been used to screen pooled, HIV antibody--negative specimens to identify persons with acute or very recent infection (i.e., HIV RNA positive and HIV antibody negative) (70,90,92--98). Given the high prevalence of previously undiagnosed HIV infection among partners and the possibility that partner notification might lead to earlier detection of HIV than other strategies, HIV RNA testing might also be useful in this context. However, prospective use of testing for acute or recent infection in the context of partner services has not been reported. Screening for Concomitant InfectionsAlthough rates of coinfection vary considerably in different areas and settings, partners who are notified about exposure to one STD often are at risk for other STDs, including HBV. Drug-injection partners are at risk for both HBV and HCV (172). Consequently, partners being notified of exposure to any STD, including HIV, might benefit from 1) screening and treatment for other STDs and 2) HBV vaccination (and HAV vaccination for MSM) (3). Those with a history of injection drug use should be screened for both HBV and HCV. Screening for HIV, syphilis, chronic HBV, and chlamydial infection is currently recommended for all pregnant women, as is screening for gonorrhea and HCV in pregnant women at risk (3). For sexually active MSM, current screening recommendations include serologic tests for HIV and syphilis, tests for urethral gonorrhea and chlamydial infection in men who have had insertive intercourse in the preceding year, tests for rectal gonorrhea and chlamydial infection in men who have had receptive anal intercourse in the preceding year, and a test for pharyngeal gonorrhea in men who have had receptive oral intercourse in the preceding year (3). HBV vaccine is recommended for all nonvaccinated, uninfected persons being evaluated for an STD (3,173,174). HAV vaccine is recommended for MSM and users of illicit drugs (both injection and noninjection) (3,175). Specific details about hepatitis vaccination, including prevaccination serologic testing, are available at http://www.cdc.gov/hepatitis. Partner services provide an opportunity to integrate these services at the client level. Although integration might be difficult for logistical reasons (e.g., testing being done in the field by a person not authorized to administer vaccines) or because of limited resources, partner services and other health department program managers might be able to collaborate to make these services available to partners. Recommendations for Testing PartnersGeneral
Syphilis
Gonorrhea and Chlamydial Infection
HIV Infection
Treatment for PartnersSyphilis, Gonorrhea, and Chlamydial InfectionThe principal goal for syphilis, gonorrhea, and chlamydial infection is immediate treatment, whether curative for infected partners or preventive if a partner tests negative or has an unknown status. Timely treatment of partners serves as a primary means of minimizing subsequent transmission. Expedited partner therapy (EPT) is a process through which treatment for partners of persons with a diagnosis of gonorrhea or chlamydial infection is administered before the clinical evaluation occurs. Most uses of EPT involve patient-delivered partner therapy (PDPT), or delivery of medications or prescriptions via the index patient. EPT is recommended as a clinical option for heterosexual men and women, especially for partners who are not likely to seek evaluation (3,176). On an individual basis, clinicians and patients decide whether to use EPT; at the program level, no evidence suggests that partners of persons with either gonorrhea or chlamydial infection seek care in sufficient proportions to stem transmission. Randomized, controlled trials of single-dose oral therapy for both STDs have shown reduced rates of reinfection among index patients exposed to EPT compared with controls; approximately 20% for chlamydial infection and 50% for gonorrhea (126,177,178). A 2007 metaanalysis of trials revealed that these were statistically significant overall reductions (179). Use of EPT also was associated with increased rates of index patient notification of partners and of partner treatment. However, EPT was not associated with reduced reinfections among women with trichomoniasis; in addition, EPT with MSM should be used cautiously because of lack of data showing efficacy of EPT for MSM, and because the risk of potential comorbidity with HIV is higher among MSM with STDs than among heterosexual males or females (3,127). Ensuring that EPT is accompanied by written instructions is important, including instructions for the medication, for the length of time to avoid sexual activity, and advice to seek evaluation. Essentially, instructions are equivalent to a referral card. Single-dose therapy with EPT is the most likely to result in treatment being administered appropriately and completely, just as with therapy prescribed to a patient. EPT with multidose regimens has not been evaluated (e.g., doxycycline for chlamydial infection). Other general treatment recommendations relevant to EPT include cotreatment for chlamydial infection in persons with a diagnosis of gonorrhea, but not vice versa. Although the high caseload of gonorrhea and chlamydial infections have inhibited provider referral, a few programs have used EPT through DIS delivery of medications, or field-delivered therapy (FDT). The DIS (or public health nurse) delivering FDT should be licensed to do so under a protocol for standing orders or another similar arrangement. In 1999, the San Francisco Department of Public Health used FDT for partners of patients with gonorrhea and chlamydial infection (180). By 2000, the proportion of partners completing treatment increased from 62% to 81%. The advantage of FDT over PDPT is that DISs can be trained to watch for immediate adverse reactions (e.g., allergic reactions) and can verify treatment and deliver prevention messages directly, an approach similar to directly observed therapy for TB infections. The disadvantage of FDT is that a public health staff person is required to trace and notify partners; therefore, resources remain a vital consideration. Although unevaluated, FDT is a possible strategy for treating syphilis if 1) a person licensed to administer injections and monitor and respond to adverse reactions accompanies the DIS and 2) the partner's stage of disease and coinfection can be adequately addressed during the contact interview. Although treatment on the basis of exposure is a well-known public health strategy, the absence of a clear physician-patient relationship places EPT (especially in the form of PDPT) in an uncertain legal status in many jurisdictions. To aid programs in establishing formal programs that are clinically useful and legally defensible, CDC has a website (available at http://www.cdc.gov/std/ept) with CDC guidance, guidance models from states in which EPT is specifically permitted, and a state-by-state analysis of the legal landscape of EPT, based on a recent survey of laws, regulations, court decisions, and policies (181). HIV InfectionAs mentioned previously, effective and timely medical evaluation, initiation of currently recommended combination ART, provision of appropriate vaccinations and other preventive health interventions, and referrals for a wide range of other medical and psychosocial services are critical for persons with a new diagnosis of HIV infection. Linking partners who test positive for HIV to medical care and HIV case management is essential as soon after diagnosis as possible. The importance of linking HIV-infected partners to medical care and verifying that they have had a medical evaluation or received HIV case management at least once cannot be overemphasized. Accumulated data from animal and human clinical and observational studies suggest that PEP for sexual, injection-drug use, and other substantial nonoccupational HIV exposure might prevent HIV infection and that potential risks from PEP (e.g., increase of risky sexual behavior, adverse reactions to medications, and selection of resistant virus) might be minimal (182). PEP is intended to be initiated within 72 hours of exposure to HIV, and antiretroviral medications must be taken for 28 days. Partners who have been exposed and can be notified within this time frame might be candidates for PEP (3). Because PEP is only intended for persons who are HIV negative and because partners might not be aware of their HIV status, access to rapid testing is necessary for this option to be offered. Incorporating PEP into partner services programs poses substantial logistical and resource challenges; however, certain health departments have developed program recommendations for PEP that might be useful for jurisdictions considering implementing such a program (183). Although PEP might be useful in certain partner services contexts (e.g., with new partners of the index patient), health departments will ultimately need to evaluate whether integrating PEP into their partner services programs is feasible and consistent with program objectives. Recommendations for Treatment for PartnersSyphilis, Gonorrhea, and Chlamydial Infection
HIV Infection
Referring Partners to Other ServicesWhether partners test positive or negative for a particular disease, underlying factors might impede their ability to access medical care effectively or adopt and maintain safer behaviors, and they might benefit from referral to various psychosocial services. Considerations regarding referrals for partners are essentially the same as those for referrals for index patients. Recommendations for Referring Partners to Other Services
Specific PopulationsThe recommendations in this report generally apply to all clients with HIV infection or other STDs regardless of setting, population, or disease. However, certain populations such as youths, migrant and immigrant populations, and persons in correctional facilities have unique characteristics and circumstances. YouthsThe rates of many STDs are highest among young populations; each year in the United States, approximately 19 million new STD infections occur, almost half of them among persons aged 15--24 years (53). Recent national HIV/AIDS surveillance data indicate that the estimated number of prevalent HIV/AIDS cases increased from 1,465 to 2,478 among youths aged 15--19 years and from 3,910 to 5,367 among those aged 20--24 years during 2002--2006 (184). Youths are at higher risk for HIV infection and other types of STDs because they frequently have unprotected intercourse, are biologically more susceptible to infection (especially females), are engaged in sexual partnerships of limited duration, and face multiple obstacles to using health care (185--189). The unique biologic and cognitive developmental concerns associated with youths require that services for them be developmentally appropriate and as comprehensive and seamless as possible. Partner Elicitation Approaching youths who have received a recent diagnosis of HIV or any other type of STD can be challenging. Before broaching the subject of partner elicitation with a young index patient, assessing immediate needs is important, especially for patients in need of housing or food. Youths might have many misperceptions and information gaps about partner services that need to be addressed, such as understanding that partner services are voluntary and that they have the right to decline participation. They also should understand the concept of confidentiality and that it includes not reporting to their parents unless the youth requests parent or guardian involvement. In addition, specific counseling skills might be necessary, especially for youths with a recent diagnosis of HIV, to ensure that they understand the ramifications of the diagnosis and how to prevent future acquisition of HIV and other types of STDs and transmission to others (190). Youths who fear losing partners and friends might find it especially difficult disclosing information about sexual or injection-drug partners and other social contacts (191). In addition, youths might be reluctant to provide information about adult partners because of fear of legal repercussions related to sex between an adult and a youth. In addition, fear of partner violence might prevent partner identification; therefore, assessing the potential for partner violence is essential for each partner identified. Assessing other potential violence or maltreatment situations that might occur involving parents, guardians, or friends also is critical. Finally, DISs providing services to youths should be sure to discuss the topic of sexual abuse with their clients; if sexual abuse is suspected, they should notify the appropriate authorities (e.g., child protective services agency) in accordance with applicable laws and regulations. Notifying, Counseling, and Testing Partners Although the process of notifying partners named by youths and named by adults is the same, legal concerns might exist in situations with youths, especially when an adult partner is named. Knowledge of state laws is essential; if sexual abuse or statutory rape is suspected, staff members must be prepared to report to the appropriate agency. Counseling skills of partner services providers are especially important when partners are very young or immature. Developing simple and clear messages regarding the STD and HIV notification process is needed to ensure that youths are able to understand the purpose of notification and the urgency of getting tested if testing is not provided in the field (190). Being able to assess the maturity of the partner is a fundamental skill for DISs so that they can ensure that an appropriate plan of action is developed. Young partners might also require specific types of assistance to obtain testing. For example, partner services staff members should be prepared to call previously identified testing sites, make an appointment for testing, and then follow up with the youths to verify that they received the test. Youths might be reluctant to access services because of financial and transportation limitations and because of fears that parents must give permission or be informed. Youths must understand that, with a few exceptions, all adolescents in the United States can legally consent to receiving a confidential diagnosis and treatment of STDs (3) In all 50 states and DC, medical care for STDs can be provided to adolescents without parental consent or knowledge. In addition, in the majority of states, adolescents can consent to HIV counseling and testing. Consent laws for vaccination of adolescents differ by state. Several states consider provision of vaccine similar to treatment of STDs and provide vaccination services without parental consent. Because confidentiality is crucial, health-care providers should follow policies that provide confidentiality and comply with state laws for STD services. Partner services staff members should remain knowledgeable and updated on related state and local laws and regulations. Treatment for Partners Because youths often are a medically underserved population compared with persons in other age groups, they might be less likely to receive office-based medical care or to use primary care services (192,193). Reasons for this include concerns about confidentiality, lack of health insurance, lack of adolescent-specific services including health-care providers with adolescent health experience, and appointment times that conflict with school schedules (185,194--198). HIV-infected youths might face additional challenges, and health care providers serving HIV-infected youths report that acceptance of HIV diagnosis and return for care and treatment can take many months. Programs might be able to increase the likelihood of successfully linking adolescents to care and treatment by developing relationships with medical facilities that are sensitive to youth concerns and that have a strong case-management component (199,200). Confidentiality and Privacy Although confidentiality is a basic principle for all steps of the partner services process, careful attention must be paid to providing a private and safe place for the interview and notification process for young index patients and their partners. However, ensuring confidentiality in cases involving suspected child or sexual abuse is not always possible. Local laws, statutes, and regulations define the limits of confidentiality in these cases. Recommendations for Youths
Immigrants and MigrantsData on the prevalence of HIV infection or other STDs among immigrant and migrant populations in the United States are limited. However, certain immigrant and migrant populations in the United States might be particularly vulnerable to HIV and other STDs because of inadequate knowledge about the infections, lack of information about and access to prevention and related health-care services, and delays in accessing HIV and other STD testing, treatment, and care (201,202). Immigrant and migrant women also might experience concerns related to power and economic disparities between men and women that make women more vulnerable to sexual abuse or domestic violence and decrease their ability to protect themselves from sexual exposures to infection (203). All of these concerns might contribute to HIV and other STD acquisition and transmission among these populations. Partner services programs can be an effective way to identify and reach members of immigrant and migrant populations who might not otherwise access HIV and other STD testing services. Partner Elicitation Concerns affecting partner elicitation among migrants and immigrants might include difficulty in locating such persons because of their transient movement within the United States or across international borders (e.g., U.S.-Mexico border) (204). Interviews might be difficult because of language and cultural barriers. Index patients might be reluctant to provide information if translators are family members or are from their own communities. A lack of understanding about the voluntary and confidential nature of partner services makes it essential that simple and clear messages are provided to encourage participation and gain the trust of index patients. Partner elicitation might be hindered by concerns that named partners could be deported (205). Concern about individual stigma related to STDs or HIV infection and activities related to transmission (e.g., male-to-male sex or injection drug use) also might be a barrier to full participation in partner services. Because of fears of partner violence, which might be substantial among immigrant and migrant women, DISs must be able to adequately assess the potential of partner violence before initiating partner notification (206). Notifying, Counseling, and Testing Partners Locating and notifying partners among immigrants and migrants might be difficult for the same reasons that partner elicitation is challenging. In addition, the usual counseling models might not be culturally appropriate because of cultural norms regarding discussion of sex and sexual behaviors. These concerns can make risk assessments or HIV and STD prevention counseling especially difficult. Treatment for Partners Treatment and care services might not be available or easily accessible to immigrant and migrant populations because of a lack of financial resources, transportation, and child care resources. Concerns about confidentiality, loss of employment, and fear of deportation or other legal consequences also might make immigrant and migrant populations reluctant to access care. If they do access care, medical care providers might lack linguistically and culturally appropriate services. Recommendations for Immigrants and Migrants
Incarcerated PopulationsThe majority of the 2.2 million adults and juveniles residing in jails, detention centers, and state and federal prisons eventually will be released and rejoin the larger community. Persons in prisons are generally housed for longer periods of time than persons in other correctional facilities, such as jails. Certain persons in city and county jails and juvenile facilities are released in less than 24 hours, with the majority (93%) of jail inmates staying less than 1 year (207). Multiple studies and surveillance projects have demonstrated high rates of sexual risk and STD prevalence among persons entering prisons, jails, and juvenile correctional facilities (208--210). Data from the Bureau of Justice Statistics indicate that, as of December 31, 2005, a total of 22,480 (1.8%) state and federal prison inmates were infected with HIV or had confirmed AIDS. The prevalence was higher in state prisons (20,888 inmates, 1.8%) than in federal prisons (1,592 inmates, 1.0%) and was higher among female than male inmates (2.3% and 1.7%, respectively) (211). A study of syphilis cases during 1997--2002 in Indianapolis, Indiana, and Nashville, Tennessee, found that 19% of cases in women and 27% of cases in men were identified through jail screening; in certain situations, the case-finding yield of jail screening approached that for STD clinics (212). Many persons who are arrested are at high risk for STD and HIV infection because of high-risk behaviors (e.g., multiple sex partners or injection and other drug use) and poor health-care--seeking behaviors while in the community. Therefore, providing routine screening for HIV and other types of STDs during the correctional facility intake process offers an opportunity to identify infections, prevent complications, and reduce further transmission by improving access to treatment, care, and prevention. Many correctional facilities provide screening for HIV and other types of STDs. Conducting partner services for persons in correctional facilities who test positive presents a unique opportunity to access possibly hard-to-reach partners at high risk for infection both in the facility and in the community. Conducting partner services might lead to a better understanding of risk behaviors and prevention needs of inmates, help programs better target resources and evaluate prevention program performance, and possibly lead to a better understanding of disease transmission dynamics in the broader community. The extent to which partner services are conducted in correctional facilities varies with program resources and individual facilities. When public health staff members conduct these services in correctional facilities, formal collaboration mechanisms between the health department and the correctional facility are essential to help coordinate activities and ensure that all parties understand what is needed to conduct services within the facilities. Following are factors to consider when developing partner services programs for incarcerated populations. Partner ElicitationInmates who receive a diagnosis of HIV infection or another STD while incarcerated might not want to identify sex or injection-drug partners that reside in the community or the facility. Partner services providers should be aware that partners might include other inmates, correctional facility staff members, or visitors. Reasons for not wanting to identify partners might include fears of partnership dissolution, loss of privileges within the facility, and concerns about revealing possible illegal activities. Before partner services providers ask inmates for information about partners, the providers should ensure that the inmates understand the confidential and voluntary nature of partner services and the limits of confidentiality related to disclosing information about sex partners who reside within the facility. In all states, sex with another inmate or with correctional facility staff members is prohibited and might be required to be reported (213). Therefore, partner services programs should have a full understanding of these laws and regulations as well as of individual facility policies before initiating any partner services activities. Inmates have a right to privacy and confidentiality of their medical information, and DISs have a duty to protect all confidential information. However, maintaining the confidentiality of inmate health information might be challenging in correctional facilities. Partner services programs should work with medical staff members within the facilities to ensure that procedures are in place to reduce possible breaches of confidentiality. Breaches of confidentiality for inmates with HIV infection or other STDs might result in increased discrimination, stigmatization, and violence. Because incarcerated populations often are moved about within correctional systems, locating or accessing an index patient might be difficult. Partner services providers should work with correctional facility staff members to determine how best to locate and access inmates. In addition, other challenges might arise if a particular inmate has already been released, because released inmates are often transient, use aliases, or do not have a permanent address. If the inmate has already been released, DISs should obtain contact information from the correctional system to assist with partner services activities. Having a private space to conduct partner elicitation in correctional facilities might be a challenge. Correctional health-care clinics often are busy, and space is not always available. In addition, security concerns often require that custodial staff members are able to see staff members and inmates at all times to ensure safety. Thus, clinic layout and proximity of non--health-care staff members can create an impression of lack of privacy or confidentiality. Partner services staff members must work with correctional facility staff members to identify a private area, whether in the clinic or in the housing area, to elicit partner names without compromising safety. The Prison Rape Elimination Act of 2003 (PREA) (Pub. L. 108--79, Stat 117.972 4 [September 2003]) might affect the delivery of timely partner services. Under PREA, allegations of sexual assaults in correctional facilities are to be treated as criminal acts and investigated as such. The criminal investigation might take precedence over partner services activities and might cause partner services and notification to be delayed because of pending criminal charges. Notifying, Counseling, and Testing PartnersFor named partners who are located in the community (i.e., not in the correctional facility), the notification process is no different than for partners named by persons outside correctional facilities. However, legal concerns might exist related to named partners who are located within the correctional system (e.g., other inmates or correctional facility staff members). Knowledge of state laws and possible reporting requirements are essential, and partner services staff members must be prepared to adhere to these regulations and should consult with program managers or legal counsel when questions arise regarding specific situation. Treatment for PartnersEnsuring medical care for partners who are inmates is the responsibility of the correctional facility. Facilities that release inmates before adequate care or treatment can be provided should provide referrals before the release. However, when program resources are available, partner services staff members can provide follow-up for recently released persons to verify that they are adequately treated or are linked to care. Correctional facilities or the health department also should consider partnering with local service providers, including CBOs, that provide transitional services. These agencies might be able to provide follow-up and possibly HIV case-management services especially for those who are HIV infected. Recommendations for Incarcerated Populations
Strategies to Enhance Case Finding and Partner NotificationCore AreasA core area is a specific, typically geographically defined area, such as a neighborhood or census tract, that has a relatively high concentration of STDs and likely accounts for a large proportion of disease transmission in a community. Infected persons in a core area might not have any social or sexual connections; their only relationship might be geographic. An example of a core area is a zip code in which >50% of the gonorrhea cases in the county are identified. Core areas are different from core groups, which are socially defined groups of persons who are likely to be a source of continued disease transmission (i.e., core transmitters). In certain circumstances, programs might maximize resource use and prevention effectiveness by concentrating on specific core areas. For example, in New York state, targeting 100% of provider-referral partner-notification measures for gonorrhea in core areas (as defined by endemic prevalence, or census tracts containing 50% of reported annual gonorrhea cases) was associated with a substantial decline in incidence, even compared with a control area in which a larger proportion (60%--70%) of gonorrhea-infected persons were actually interviewed (7). In Colorado, partner notification services for gonorrhea that focused on a military base (the putative core area) and the surrounding civilian community produced a 13% decrease in overall gonorrhea cases and a 20% decrease in the civilian community (214). Military incidence was largely unchanged. Similar large-scale measures in the United Kingdom (i.e., the Tyneside scheme) have been associated with reductions in gonorrhea morbidity (215). Similar data for chlamydial infection are lacking, and whether core areas play a substantial role in chlamydial infections is uncertain (216). In general, syphilis is so geographically concentrated that syphilis infection-control measures, by definition, involve a core-area approach. Recommendation for Core Areas
Social NetworksA social network is a group of persons connected by various types of social relationships, such as family, work, and recreational relationships; sexual partnerships; and drug-use relationships. A social network also can include the venues in which interactions among the members of a social network occur. The persons in a social network might share social, economic, cultural, or behavioral characteristics that influence their risk for various health conditions, including HIV and other STDs (217). Consequently, members of the social network of a person with HIV infection or other STDs might also be at increased risk for these infections, even though they might not have a sexual or drug-injection relationship specifically with the infected person. By exploring the social, sexual, and drug-use connections among persons and places and diagramming these links, HIV/STD prevention programs might uncover more cases than by relying on partner notification and testing alone. This approach also can provide helpful information about a disease in a core area by integrating epidemiologic, geographic, and sociodemographic information. Using social network methods to identify persons with HIV infection can help bring previously undiagnosed HIV-infected persons into the partner services process and might also be used to identify persons who previously tested positive and left care or never received care. A program that uses this approach can track networks at several levels, first assessing persons and places and then possibly going further to look at geographically defined sociodemographic data. Although this approach can seem intimidating and labor intensive, DISs often collect much of these data during patient interviews and from field records, and certain programs use network approaches on a de facto basis. Other data can be added as resources permit. An established and periodically updated network diagram might aid in the investigation of outbreaks as they occur (rather than as a retrospective tool to explain why they occurred). Programs might also conduct more formal network analyses, which involve calculating various statistics that describe characteristics of a network (e.g., components, degree, betweenness, information centrality, and k-core) (158). However, the capacity to perform these analyses is not available in many health departments and might not be performed quickly enough to affect outbreaks as they occur. Nevertheless, analyses of outbreaks and endemicity can reveal details not otherwise identified and can therefore inform program needs and future actions (218,219). Peer referral is one type of social-network approach that has been used to identify HIV cases; clients are enlisted as recruiters and coached to refer persons from their social networks (peer referral) for counseling and testing. Those referred also can be enlisted to recruit others, creating a peer-driven cluster approach. With the peer-referral approach, virtually all contact with program staff members is at the point of care, and extensive field work is not needed. The approach can be refined by assessing which persons are more effective at referring infected persons or those at high risk for infection and concentrating on the persons who are the most effective. In a demonstration project conducted in seven U.S. cities, nine CBOs enrolled HIV-positive persons and HIV-negative persons at high risk for infection to serve as peer recruiters. These persons agreed to refer persons in their networks who they thought to be at risk for HIV infection for counseling, testing, and referral services (220). The 6% prevalence of newly identified HIV infection found among social network associates tested in this project was five times the average prevalence reported by publicly funded HIV counseling and testing sites. An evaluation project conducted in King County, Washington, enlisted and trained MSM who had received a diagnosis of HIV or other STDs to become peer recruiters and yielded similar results (221). Of the 438 recruited peers who had not previously received a diagnosis of HIV, 22 received a new diagnosis of HIV, an 8% increase in the health department's total HIV case-finding yield. The approach was particularly useful for identifying non-white MSM with HIV infection, increasing the health department's total case-finding yield for this group by 19%. This peer-referral approach was a more cost-effective strategy for identifying HIV cases among MSM in this area than other outreach approaches. (Additional information on implementing a social networks strategy for HIV case is available at http://www.cdc.gov/hiv/resources/guidelines/snt.) Use of a network approach should not replace partner notification; instead, the approach should be used to enhance existing partner services practices. Initiation of a network approach can be labor intensive, and a full-scale network approach to describing disease in a given program area requires analytic capacity that not all programs possess. Nevertheless, basic network data are often already collected by DISs and other program staff members, and a program could link these data to produce a more complete representation of STDs/HIV than previously possible. Additional research on the use of social networks for disease prevention is needed. Studies analyzing the use of social networks to enhance partner services and assess disease transmission for a particular area or population have produced encouraging results, but these results might not be generalizable. Peer-driven cluster referral has been most effective for finding cases of both HIV and HCV. As with cluster interviewing and clustering, the effectiveness of the approach in detecting cases is affected by the prevalence of the disease. For example, in Seattle, where the prevalences of gonorrhea and chlamydial infection are relatively low, peer-driven referral among MSM detected minimal numbers of cases of gonorrhea and chlamydial infections (221). The approach needs to be tested among groups with higher prevalence of bacterial STDs. Recommendations for Social Networks
The InternetThe Internet is used to facilitate formation of sexual partnerships and is a potential contributing factor in situations involving high-risk behaviors and transmission of HIV and other STDs (222--225). Although most of the published research evaluating links between sexual risk behaviors and Internet use has focused on MSM, findings from studies of heterosexual male and female groups indicate trends that are similar to those identified among MSM; seeking sex partners online is associated with high-risk behaviors and acquisition of HIV and other types of STDs (222,226--230). Certain partner services programs have used the Internet for partner notification when the only contact information available for a partner is an e-mail address or Internet screen name. Two studies have documented outcomes for HIV Internet partner notification, and the rate of response (i.e., number of partners who responded to contact attempts) differed substantially between the two studies. Public health staff members who conducted a cluster investigation among MSM in Minnesota used the Internet to contact 50 persons who had been exposed to HIV or other STDs but for whom the only available contact information was an e-mail address or screen name; responses were received from 30 (60%) (231). In Los Angeles, California, an HIV-infected index patient had 111 sex partners for whom he could provide only an e-mail address; of these, 29 (26%) responded to e-mails sent by staff members at the Los Angeles County Department of Health Services (LACDHS) (232). None of these partners would have been notified without Internet partner notification. In a survey of 1,848 U.S. MSM recruited by a banner advertisement on an MSM-targeted website for meeting sexual partners, acceptance of Internet partner notification was high: >92% of respondents indicated that they would use Internet partner notification in some way (i.e., use the health department to notify sex partners via e-mail, notify sex partners themselves via e-mail, or do both) to inform their sex partners if infected with an STD in the future (233). Available data regarding use of the Internet to notify partners exposed to other STDs such as syphilis are sparse but encouraging. In 1999, the San Francisco Department of Public Health (SFDPH) conducted a case-control study of seven early syphilis cases in persons that were associated with an online chat room (234). The mean number of partners per index patients was 5.9, and the only locating information for the sex partners was online screen names. Using the Internet to notify the partners of exposure resulted in 42% of the named partners being notified and confirmed as having been tested. In a review of early syphilis cases among MSM interviewed for partner management during January--April 2003, SFDPH identified 67 men who reported meeting sex partners through the Internet; 14 of these men provided information about 44 sex partners for whom the only locating information was an Internet e-mail address (235). Health department staff members were able to locate 15 (34%) of the Internet partners and ensure that they were evaluated and treated appropriately. In addition, LACDHS reported a case of recently diagnosed syphilis in an index patient who reported having met 16 sex partners through the Internet during his infectious period (232). The patient contacted 13 of these partners via e-mail; seven replied and made arrangements for evaluation. Finally, the Austin/Travis County Health Department sent e-mail messages to sex partners of persons infected with syphilis or HIV when e-mail contact was all that was available to DISs (236). Fifty percent of partners responded, and 81% of those (40% of all partners e-mailed) were evaluated. Thus, although response rates and overall proportion of partners evaluated were substantially lower than for in-person provider referral from the same health department, e-mail provider referral resulted in numerous partner notifications and evaluations when in-person notification was not possible. Internet-based partner notification services are available online for several U.S. cities and states (http://www.inspot.org). Website users can learn the individual- and community-level rationales for partner notification, find locations for testing resources, and send a notification card via e-mail (an e-card) to each partner exposed to an STD (of any type) through sexual contact. E-cards come in several designs and may be sent anonymously or with sender information attached; senders can tailor personal messages. All cards provide information on how to get tested. Both the site and cards provide the basic information that should be shared through any other form of patient-led referral: 1) that the recipient has been exposed to an STD; 2) to seek medical evaluation and where to do so; and 3) the importance of seeking medical evaluation. Use of Internet-based partner services programs is not necessarily restricted to health departments; health departments in areas where these services are available on the Internet generally facilitate access to them (e.g., by providing an index patient access to an on-site computer). The nature of the program makes the effectiveness difficult to evaluate, and no effectiveness data are available. Partner notification programs recognize that the Internet is a potential route for partner notification in certain situations and the only route in others. Nevertheless, certain programs face specific challenges when conducting partner notification using the Internet. Certain challenges are structural or bureaucratic, such as lack of access to computers in clinics or computer firewalls on agency computers meant to bar employees from visiting websites with sexual content (237). Other challenges include program staff members who need training regarding appropriate use of Internet partner notification or health department staff members who have difficulty reaching index patients' partners who rarely enter chat rooms during typical business hours. Compared with in-person notification, e-mail contact presents certain unique challenges for DISs. Ensuring that an e-mailed notification or a chat request is received only by the person for whom it is intended can be difficult. In addition, as with letters and telephone messages, confidentiality constraints often lead to nonspecific initial contacts; this nonspecific contact might increase the likelihood of a recipient deleting the notification e-mail or ignoring a chat request, especially when the sender is unknown. One study aimed at assessing the acceptability of various forms of electronically mediated interventions found that only 45% of 4,601 interviewed persons indicated that they would open an e-mail providing information on HIV and other STDs, and even fewer (30%) indicated that they would chat about the topic (226). Although the study was not directly related to online partner notification, the reasons provided by those surveyed are likely relevant. A substantial proportion of study respondents indicated that they were generally unwilling to open e-mail from unknown senders (40%); a smaller proportion (10%) also considered health department attempts to reach them through e-mail or chat venues to be an invasion of privacy. Strategies to increase the likelihood of a response have not been formally evaluated. However, e-mails that contain the name, occupation, and, particularly, contact numbers of DISs provide a channel for communication and might increase the likelihood of a response. Similar techniques might be used with persons contacted in chat rooms through instant messaging. Patient-led Internet-based partner notification mitigates certain structural and confidentiality concerns related to provider referral, although the approach has some drawbacks. First, malicious notification is a concern (i.e., using the notification process inappropriately, such as to frighten a partner who has not actually been exposed). However, the likelihood might decrease if the website posts injunctions against such use and incorporates protection against automated programs that attempt to use the site for mass e-mailing. Massachusetts offers a verification step that allows the e-mail recipient to contact the customer service department of the website and confirm the validity of the public health account used for partner notification. Second, because Internet-based approaches are easy to use and require less time and resources than the provider referral approach, DISs might use them instead of provider referral; however, verifying that the partner has actually been notified is easier with provider referral. Recommendations for the Internet
Program Collaboration and Service IntegrationHIV and other STDs often occur simultaneously, and many populations at risk for these diseases are at risk for other infections (e.g., TB and viral hepatitis). Program collaboration and service integration is a method of organizing related health concerns, activities, and services to maximize the public health impact and facilitate comprehensive delivery of services. Improving collaboration, coordination, and communication can increase program efficiency, reduce duplication of services, and result in fewer missed opportunities for providing comprehensive prevention services to individual clients. Through linkages with other programs, greater flexibility and responsiveness to changes in the epidemics of HIV infection and other STDs can occur. Finally, by using local information from surveillance and essential monitoring and evaluation data among multiple programs, prevention services can be more comprehensive. The extent to which a state or local program can effectively coordinate and integrate STD/HIV partner services activities could substantially influence the success of the services. Service integration might best be achieved through program integration; however, program collaboration, if effective, can achieve the same goal. At a minimum, addressing all elements of partner services, especially for persons with more than one STD, requires collaboration among health department units responsible for conducting HIV and other STD reporting and surveillance, as well as among HIV and STD prevention programs (if any of these programs operate separately from one another). Ideally, program collaboration and service integration includes TB and hepatitis. Regardless of the way a health department is organized, the HIV and STD programs should be functionally arranged to ensure that the following occur:
Barriers to program collaboration and service integration exist. Separate funding mechanisms for HIV prevention, HIV care, STD services, substance abuse treatment, mental health care, hepatitis prevention, and TB prevention and treatment can present challenges to service integration. Other challenges include ideological differences in approaches to service delivery; distrust among the various entities involved; concern about loss of program identity; political, legislative, or regulatory obstacles; staff member resistance; and lack of staff member training. Despite these challenges, the potential benefits of program collaboration and service integration are substantial enough that program managers should attempt to align partner services programs with other health department units and service entities. Service alignment can lead to increased efficiency in program administration, service delivery, and use of resources and to more knowledgeable staff members (i.e., through training), increased flexibility in providing interventions for both HIV infection and other STDs, and more efficient data collection and analysis. Coordination and Collaboration Within Health DepartmentsThe organization of HIV and STD prevention programs determine the mechanisms used to ensure a coordinated approach to partner services. To facilitate this process within HIV and STD programs, including disease reporting, surveillance, and other health department units (e.g., TB, hepatitis, vaccination, and reproductive health), programs might need to develop shared policies, memoranda of agreement, shared information systems, shared staffing plans and cross-training of staff. For example, staff members who conduct surveillance and staff members who conduct partner services might each have or develop information important to the other person's function. Having information-sharing agreements that encourage timely, accurate, and secure exchange of information can ensure early identification of potential index patients and more complete surveillance data. For STD programs that provide all partner services, defining how the STD program and the HIV program coordinate services for index patients, partners, social contacts, and associates is important. The level of coordination needed with other health department programs depends partly on local epidemiologic factors and needs of populations at high risk for infection. Coordination and Collaboration Among JurisdictionsSecure and confidential sharing of information on patients, partners, and other social contacts among jurisdictions facilitates disease prevention. Index patients often name partners who live in a location other than the state or jurisdiction where the diagnosis was made. In addition, a person who tests positive for HIV or other STDs might move to another state or jurisdiction before the test result can be delivered or an interview conducted. Both situations require communication of confidential information from one state or jurisdiction to another; success depends on the willingness of each program manager to take the steps necessary to ensure that security and confidentiality standards are upheld and to hold others accountable when breaches occur. Trust, mutual cooperation, and shared professional ethics are essential. Coordination and Collaboration with Medical ProvidersOrganizations and agencies that are not part of a health department but are involved in particular aspects of partner services must collaborate to maximize results. HIV partner services program managers should work actively with health-care providers who provide testing for HIV and other STDs, HIV care providers and case managers, and other social service agencies who provide services to HIV-infected persons to identify patients who have not received HIV partner services or who need additional services. In addition, educating private medical providers about the public health benefits of partner services might lead to increased referrals for partner services. Following are important topics to consider when conducting outreach and education activities with medical providers:
When medical providers want to provide any aspects of partner services themselves (e.g., partner elicitation, partner notification via dual referral, or EPT), the health department should collaborate with them to provide training and support. However, evidence suggests such collaboration is rare (238). For example, certain providers might be willing and able to elicit partner information that can then be provided to the health department, but most do not have the time or training needed to perform partner notification services for clients. These medical providers should be encouraged to use partner services provided by local health departments. In addition, program managers should consider any applicable legal or regulatory limits on medical care providers being involved in partner services. Coordination and Collaboration with Other Agencies and OrganizationsMany CBOs and other health and human services organizations (e.g., community health centers) provide HIV prevention services, including HIV counseling and testing, to populations that are hard to reach and at high risk for transmitting or acquiring HIV. Therefore, CBOs can act as a partner services entry point for clients who might not otherwise be offered these services, and staff members can promote partner services to the communities. CBOs also might be adept at gaining trust and establishing rapport with wary, untrusting, and fearful clients and their partners. CBO staff members might effectively elicit partner information from HIV-infected clients and provide counseling and testing to partners who come to the CBOs for services. (Additional information is available from CDC's Provisional Procedural Guidance for Community Based Organizations [239]). Before partner services program managers determine how best to use CBOs in the partner services process, they should consider local laws and regulations. In certain jurisdictions, health departments and medical providers are the only entities with legal authority to notify persons of their exposure to HIV and other types of STDs. Because many index patients and their partners have multiple referral needs that cannot be solely addressed by the health department or CBOs, partner services program managers should coordinate and collaborate with other service organizations. Such needs include violence prevention programs, drug treatment programs, mental health agencies, reproductive health programs, community health centers, parole and probation agencies, faith-based organizations, agencies funded by the Ryan White CARE Act, homeless shelters, legal services, and homeless support services. Collaboration can be used to promote partner services, identify referral resources, establish formalized referral mechanisms, and minimize duplication of effort in the jurisdiction. Communication with Communities and Community Planning GroupsAlthough data indicate that clients are generally accepting of partner services, misperceptions still exist, especially regarding concerns about breaches in confidentiality (39). Program managers should consider developing educational campaigns directed to members of affected communities, advocacy groups, and medical care providers to address concerns and misperceptions about the partner services process. In addition, partner services programs should keep their respective HIV community planning groups (CPGs) informed of partner services activities and ensure that CPGs have access to analyses of current data, including potential implications for HIV prevention in the jurisdiction. Given the comorbidity of HIV and other STDs, as well as relationships among these conditions and various social behavioral risk factors, communication also is warranted among health departments, CPGs, and CBOs about early syphilis, gonorrhea, and chlamydial infection, even if the community groups are primarily focused on HIV. Recommendations for Program Collaboration and Service Integration
Program Monitoring, Evaluation, and Quality ImprovementPartner services programs should be monitored to assess program performance and identify areas that need improvement. Additional information is available from the CDC's Practical Use of Program Evaluation Among Sexually Transmitted Disease (STD) Programs and CDC's Framework for Program Evaluation in Public Health (240,241). Specific performance measures for CDC-funded HIV and STD programs are published in CDC funding opportunity announcements. Recommendations in this section are intended for assessment of programs and not of individual staff members. Program monitoring includes the following:
Monitoring Program Processes and OutcomesProgram monitoring should be designed to address specific questions about program performance, both processes and outcomes; all data collected should be clearly related to answering these questions. Program monitoring data should be accessible to and used by program staff members and all levels of management to improve program effectiveness and efficiency. Program managers should review the data at least quarterly, and more frequently (e.g., monthly) for 1) new programs; 2) programs that are introducing substantial changes in policies, procedures, or program design; and 3) programs that have identified potential problems with any of their processes or outcomes. Essential Questions The following four questions and measures that can be used to assess them must be addressed by managers of partner services programs. These questions were developed by identifying the steps involved in conducting partner services programs and then identifying essential processes and outcomes that can provide measures of program performance (Figure 2). For curable STDs such as syphilis, chlamydial infection, and gonorrhea, the term index case (question number 1) refers to individual episodes of infection. If an individual patient has recurrent episodes of an infection during the defined time period, each episode is counted as a separate index case; an index case does not represent an individual person. For example, a person who has three reported episodes of gonorrhea over a 1-year time period represents three index cases during that year. In contrast, once a person is infected with HIV, the person remains infected. Therefore, once identified as having an index case of HIV infection, the person does not count as another index case in the future (i.e., each index case of HIV infection represents a unique person). Named partners (question number 2) are partners for whom the index patient provides sufficient identifying and other information that the partner can reasonably be considered locatable. Identifying information includes an actual name, an alias, a specific e-mail address or chat-room screen name, or enough other descriptive information that the person can reasonably be considered identifiable. 1. How completely is the program identifying newly reported cases and interviewing patients for partner services? Assess cases of HIV infection, syphilis, gonorrhea, and chlamydial infection separately, for a defined time period [e.g., past month, past quarter, or past year]):
2. How effectively is the program identifying partners, notifying them of their risk, and examining or testing them for infection? Assess cases of syphilis, gonorrhea, and chlamydial infection separately, for a defined time period [e.g., past month, past quarter, or past year]):
Assess cases of HIV infection for a defined time period [e.g., past month, past quarter, or past year]):
3. How effectively is the program identifying new cases of syphilis, gonorrhea, and chlamydial infection through partner services? How effectively is the program treating patients through partner services? How effectively is the program identifying new cases of HIV infection and linking the patients to care services through partner services? Assess cases of syphilis, gonorrhea, and chlamydial infection separately, for a defined time period [e.g., past month, past quarter, or past year]):
Assess cases of HIV infection for a defined time period [e.g., past month, past quarter, or past year]):
4. Do any of the preceding measures indicate variations by index patient age, race/ethnicity, sex, or risk behavior? (Demographic and behavioral risk characteristics might not be known for partners who are not notified.) Additional Assessments In addition to addressing the previous four questions, most programs benefit from more detailed monitoring. For example, by considering how successfully the program is performing each step throughout the partner services process, program managers can identify specific steps that need improvement to enhance overall program performance. Qualitative information can be collected to identify factors contributing to areas of concern and aid in improvement. Stratifying the analysis by demographic and behavioral risk characteristics might provide information that allows services to be tailored to the needs of particular subpopulations. The timeliness with which various steps of the process are completed also can be examined. Following is an example:
Following are examples of stepwise process monitoring questions that programs should address for index patients, based on the detailed steps in the partner services process (Figure 2):
The step process (Figure 2) can be used to create a similar series of stepwise questions for locatable partners that are identified from index patient interviews, such as, "Among identified partners of HIV index patients, how many are already known via record review to be HIV infected? Of these, what proportion is contacted and provided HIV prevention counseling?" Another important consideration for program managers is how the success of provider referral compares with that of self-referral (or third-party referral) for notifying partners of their risk. Outcomes for partners designated to be notified through self-referral usually are challenging to measure, because verifying that the partners have been notified and tested, what their test results are, and whether they have been linked to medical services, HIV case management, or prevention services are difficult. Several strategies have been used in attempts to obtain this information, but none have been adequately tested for reliability. Examples of such strategies include the following:
Finally, similar monitoring can be conducted to assess outcomes of clustering and cluster interviewing (e.g., assessing the relative number of new cases of syphilis and gonorrhea identified or of newly identified HIV-positive partners, social contacts, and associates). Monitoring Program ObjectivesIn addition to monitoring program processes and outcomes, program managers should monitor achievement of program objectives. Annually, programs should establish clear, specific, realistic, time-phased, measurable objectives for each key step or process in the program, as well as expected program outcomes. Progress toward achieving these objectives should be tracked continuously. If progress on one or more processes is unsatisfactory, possible reasons should be considered and processes modified accordingly. Certain originally established objectives might later be determined to be unrealistic and also can be modified. Monitoring Use of Staff Members and Other ResourcesProgram managers also should monitor program staff members and resource use to identify and quantify activities being performed by staff members, the number of staff members and amount of time required to perform each activity, the types and level of other resources required to implement and maintain the program, and the overall cost of the program. Using a combination of qualitative and quantitative data, this information can be used to adjust use of staff members and resources and plan future program activities. Program EvaluationIn general, evaluations require more rigorous design, analysis, and interpretation than monitoring and frequently require more resources. In certain situations, programs might need to consult with experts in evaluation. Following are examples of questions that might be addressed through evaluation:
Quality ImprovementProgram managers should implement quality improvement systems to help ensure that services are delivered as intended, programs are responsive and accountable to the funders and consumers of the services, and program performance and quality of care are continuously improved. Quality improvement activities typically focus on the following areas:
Various methods can be used to help improve program quality, including the following:
Recommendations for Program Monitoring, Evaluation, and Quality Improvement
Support of Staff MembersStaff Development and AssessmentStaff assessment and staff development, training, and support have an important association: staff members who are not adequately prepared for and supported while performing their jobs have difficulty performing satisfactorily. Staff development and support begins with a clear description of staff roles and responsibilities, as well as of the knowledge and skills required for the job. This information is used to recruit staff members and identify an appropriate training curriculum to follow initially and at periodic intervals. In addition, assessment of individual strengths and weaknesses of staff members allows supervisors to help them design specific training plans for building their skills. All staff members conducting partner services activities need in-depth training on partner services goals and principles, methods of partner services, and any specific concerns related to specific infections. Training can be obtained through the CDC-supported Prevention Training Centers. After the initial training, updates should occur periodically; close supervision, observation, and mentoring of staff members is critical, especially for those new to the job. In addition, staff members should have easy access to all materials, tools (e.g., cellular telephones), and resources needed to perform the job efficiently and effectively; this is not the responsibility of individual staff members. Staff assessments should include both qualitative and quantitative outcome measures that are constructive and not punitive. These types of assessments are more likely to result in improvement of staff skills and performance than using a single, quantitative outcome measure. For example, the number of partners tested per index patient interviewed can be used as a single measure of staff proficiency; however, an assessment of each essential step in the process (e.g., proportion of index patients located and interviewed, number of partners elicited per index patient interviewed, proportion of partners located and notified, and proportion of located partners counseled, examined, and tested), supplemented with qualitative information, would provide a better assessment of the staff. Qualitative assessments can begin with supervisors routinely meeting with individual DISs to review the timeliness and completeness of specific cases, with a focus on barriers encountered in managing the case and strategies for overcoming these barriers. These one-on-one meetings provide supervisors an opportunity to review the quantitative measures of important steps with the staff member, discuss the validity of the measures, consider potential factors contributing to the performance of the staff member, and discuss strategies for improving certain skills. These meetings also provide an opportunity to assess staff member awareness of and adherence to program guidelines, protocols, and performance standards. Routine, periodic supervisor observation of DISs in all aspects of activities, with immediate feedback, can be very useful. Direct observation can be an important tool in assessing whether staff members have the necessary skills and knowledge to conduct interviews, provide referrals, and satisfy other client needs (242). For example, successful staff-client interactions, in which staff members demonstrate sensitivity to and interest in the client, as well as adherence to current policies and procedures, are essential for effective partner services. Observation and feedback should be structured and constructive and not punitive. Supervisors should reinforce positive performance and provide specific, constructive comments regarding areas that need improvement. A reasonable initial time frame for supervisor observation of DISs is twice monthly for the first 6 months, monthly for the second 6 months, and quarterly for staff members with more than 1 year of experience, depending on individual performance. This schedule might need to be modified depending on program experience. Case conferences also can be very useful for staff support as well as for quality improvement. Regularly scheduled group DIS meetings allow supervisors to understand the skills and areas that need improvement among staff members and provide an opportunity for staff members to learn from one another. Case conferences are a valuable forum for staff members to discuss specific concerns, address difficult situations, and share resources. Case conferences also give supervisors an opportunity to emphasize that conducting partner services is a team effort and that competitive behavior interferes with collaboration and sharing of valuable information and resources. Frequency of case conferences should be balanced with workload, with attempts to conduct such conferences at least monthly. Finally, although staff member assessments often focus on DISs, ensuring that supervisors and program managers themselves are adequately trained, supported, and assessed is equally if not more important. Staff SafetyCertain field activities can include unsafe situations for DISs. Program managers should develop and maintain detailed guidelines for ensuring staff safety. Examples of safety procedures that are often used by partner services programs include the following:
The primary way staff members can avoid unsafe situations is to have knowledge of the community; consequently, spending time establishing personal rapport with members of the community is important. This can be accomplished while performing health department outreach activities, organizing field screenings, and participating with CBOs in outreach activities. Other safety concerns involve occupational infections in the workplace, particularly for programs that use DISs to draw blood or collect other specimens in the field. These programs should review all relevant state and local health and safety codes and local public health protocols to determine required training and certification procedures before performing these activities. They also must have in place an Occupational Infections in the Workplace policy that is at least as restrictive as applicable Occupational Safety and Health Administration (OSHA) policies in their areas. Policies and procedures should specifically address management of occupational exposure to HBV, HCV, and HIV, including PEP (243). DISs who might be collecting specimens in the field are strongly encouraged to receive an orientation to state or local Occupational Infections in the Workplace policies and supporting procedural manuals. Recommendations for Support for Staff Members
References
Partner Services Work Group Samuel W. Dooley, MD, Odessa T. Dubose, Joel Franklin Fletcher, Jr., Dorothy Gunter, MPH, Matthew Hogben, PhD, Laurie Reid, MS, Amy C. Stuckey, MPH, Abigail Viall, MA, Cedric D. Whitfield, MPH, Tracie Wright-Schnapp, MPH, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Partner Services Steering Committee Partner organization representatives: Don Clarke, MA, National Coalition of STD Directors, Washington, DC; Theresa L. Henry, Virginia Department of Health, Richmond, Virginia, and National Coalition of STD Directors, Washington, DC; David Kern, National Alliance of State and Territorial AIDS Directors, Washington, DC; Peter Kerndt, MD, Los Angeles County Department of Public Health, Los Angeles, California; Eve Mokotoff, MPH, Michigan Department of Community Health, Detroit, Michigan; Douglas Morgan, MPA, Health Resources and Services Administration, Rockville, Maryland; Israel Nieves-Rivera, The Urban Coalition of HIV/AIDS Prevention Services and the San Francisco Department of Public Health, San Francisco, California; Liisa M. Randall, PhD, National Alliance of State and Territorial AIDS Directors, Washington, DC; Cynthia J. Tucker, MS, AIDS Foundation of Chicago, Chicago, Illinois. CDC participants: April Bankston-Nickson, Rheta Barnes, MSN, MPH, Elin Begley, MPH, Rashad Burgess, MA, David Byrum, MPH, Tonji Durant, PhD, Cindy Getty, Bob Kohmescher, MS, Wendy Lyon, MPH, Kevin O'Connor, MA, Tim Quinn, MPA, Sam Taveras, MEd, MPH, Kimberly R. Thomas, MPH, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC.
Consultants to Partner Services Work Group External Consultants James Albino, National Minority AIDS Council, Washington, DC; Bebe Anderson, JD, Lambda Legal, New York, New York; Expedito Aponte, Harlem United Community AIDS Center, New York, New York; Richard Armor, Texas Department of State Health Services, Austin, Texas; Jennifer Augustine, MPH, Advocates for Youth, Washington, DC; Ronald Bayer, PhD, Mailman School of Public Health, Columbia University, New York, New York; Nanette Benbow, MAS, Chicago Department of Public Health, Chicago, Illinois; Dena Bensen, MPH, Virginia Department of Health, Richmond, Virginia; Jeanne Bergman, PhD, Center for HIV Law and Policy, New York, New York; Benjamin E. Berkman, JD, Georgetown University Law Center, Washington, DC; Christopher Borges, MA, California Department of Public Health, Sacramento, California; Melissa Boyette, Alaska Department of Health and Social Services, Anchorage, Alaska; Devon Brewer, PhD, Interdisciplinary Scientific Research, Seattle, Washington; Carol Brown, MS, Pine Belt Mental Healthcare Resources, Hattiesburg, Mississippi; Oscar Cabrera, JD, Georgetown University Law Center, Washington, DC; Carol Carp, MS, Maryland Department of Health and Mental Hygiene, Baltimore, Maryland; Alvaro Carrascal, MD, New York State Department of Health, Albany, New York; Jyaphia Christos-Rodgers, MA, Louisiana Office of Public Health, New Orleans, Louisiana; Kevin Cranston, MDiv, Massachusetts Department of Health, Boston, Massachusetts; Joan Crawford, Oregon Department of Human Services, Portland, Oregon; Wendy Craytor, MBA, MPH, Alaska Department of Health and Social Services, Anchorage, Alaska; Tyloria Crenshaw, Franklin Primary Health Center, Mobile, Alabama; Daniel J. Daltry, MSW, Vermont Department of Health, Burlington, Vermont; Drew De Los Reyes, Gay Men's Health Crisis, New York, New York; Ingrid Denney, MS, Missouri Department of Health and Senior Services, Springfield, Missouri; Carmen Ana Diaz-Aponte, MS, Puerto Rico Department of Health, San Juan, Puerto Rico; Beth Dillon, MSW, MSPH, Colorado Department of Health and the Environment, Denver, Colorado; Brenda Doucette, Pennsylvania Department of Health, Harrisburg, Pennsylvania; Raymond Duke, MDiv, Our Common Welfare, Decatur, Georgia; Kevin Farrell, MSW, California Department of Public Health, Office of AIDS, Sacramento, California; Colin P. Flynn, ScM, Maryland Department of Health and Mental Hygiene, Baltimore, Maryland; Lily Foster, MSPH, New Mexico Department of Health, Santa Fe, New Mexico; Evelyn Foust, MPH, North Carolina Department of Health and Human Services, Raleigh, North Carolina; Marie-Jose Francois, MD, Center for Multicultural Wellness and Prevention, Orlando, Florida; Douglas M. Frye, MD, Los Angeles County Department of Public Health, Los Angeles, California; Andrew Gans, MPH, New Mexico Department of Health, Santa Fe, New Mexico; Ann Gardner, Arizona Department of Health Services, Phoenix, Arizona; Matthew Golden, MD, University of Washington, Seattle, Washington; Elizabeth Gondles, PhD, American Correctional Association, Alexandria, Virginia; Lawrence O. Gostin, JD, Georgetown University Law Center, Washington, DC; Felicia Guest, MPH, Southeast AIDS Training and Education Center, Emory University, Atlanta, Georgia; Catherine Hanssens, JD, Center for HIV Law and Policy, New York, New York; Mary Hayes, Los Angeles County Department of Public Health, Los Angeles, California; Shelley D. Hayes, JD, American Bar Association, Washington, DC; Carlos Hernandez, PROCEED, Elizabeth, New Jersey; Brain Hujdich, American Academy of HIV Medicine, Washington, DC; Anthony Hyunh, Asian and Pacific Islander Wellness Center, San Francisco, California; Jeffrey F. Jenne, City of Philadelphia Department of Public Health, Philadelphia, Pennsylvania; Jeffrey Klausner, MD, San Francisco Department of Public Health, San Francisco, California; Susan Klein, MS, New York State Department of Health, Albany, New York; Cynthia Knox, JD, HIV Law Project, New York, New York; Robert LaChance, New Hampshire Department of Health and Human Services, Concord, New Hampshire; Linda M. Lesondak, PhD, Chicago Department of Public Health, Chicago, Illinois; Lissette Marrero, Children's Hospital at Montifiore, New York, New York; Marsha Martin, DSW, District of Columbia Department of Health, Washington, DC; Jaime Martinez, MD, Division of Adolescent and Young Adult Medicine, Stroger Hospital of Cook County, Chicago, Illinois; Marlene McNeese-Ward, Houston Department of Health and Human Services, Houston, Texas; Alison Mehlman, JD, Center for HIV Law and Policy, New York, New York; Ronald Merrick, MA, Los Angeles County Department of Public Health, Los Angeles, California; Beau Mitts, MPH, Houston Department of Health and Human Services, Houston, Texas; April Richardson Moore, MPH, New York State Department of Health, Albany, New York; David Ernesto Munar, AIDS Foundation of Chicago, Chicago, Illinois; David Novak, MSW, Massachusetts Department of Public Health, Jamaica Plain, Massachusetts; Dan O'Connell, New York State Department of Health, Albany, New York; Frank Oldham, Jr., National Association of People with AIDS, Silver Spring, Maryland; Fern Orenstein, MEd, California Department of Public Health, Richmond, California; Sue Anne Payette, MS, New York State STD/HIV Prevention Training Center, Albany, New York; Ligia Peralta, MD, University of Maryland Medical Center, Baltimore, Maryland; John Peppert, Washington State Department of Health, Olympia, Washington; Orlando Pile, MD, Los Angeles County Sheriff's Department, Los Angeles, California; Pam Pitts, MPH, Tennessee Department of Health, Nashville, Tennessee; John Potterat, Colorado Springs, Colorado; Charlie Rabins, MPH, Illinois Department of Public Health, Springfield, Illinois; Rebeca Ramos, MA, MPH, United States--Mexico Border Association, El Paso, Texas; Deborah Reardon-Maynard, MPH, Arizona Department of Health Services, Phoenix, Arizona; Paul Reyes, New York State STD/HIV Prevention Training Center, Albany, New York; Sam Rivera, The Fortune Society, New York, New York; Ann Robbins, Texas Department of State Health Services, Austin, Texas; Nestor Rocha, District of Columbia Department of Health, Washington, DC; Ricky Rosales, County of Los Angeles Office of AIDS Programs and Policy, Los Angeles, California; Steven Rubin, New York City Department of Health and Mental Hygiene, New York, New York; Mara San Antonio-Gaddy, MSN, New York State Department of Health, Albany, New York; Walt Senterfitt, PhD, Community HIV/AIDS Mobilization Project, Los Angeles, California; Stephen Schletty, Minnesota Department of Health, St. Paul, Minnesota; Luke Shouse, MD, Georgia Division of Public Health, Atlanta, Georgia; Aaron Smee, MPH, Pennsylvania Department of Health, Harrisburg, Pennsylvania; James M. Sosman, MD, University of Wisconsin School of Medicine and Public Health; Madison, Wisconsin; Denise Tafoya, MPA, California STD/HIV Prevention Training Center, Long Beach, California; Lisa Tallin, MSW, Boston Medical Center, Boston, Massachusetts; Litjen (L.J.) Tan, PhD, American Medical Association, Chicago, Illinois; Tamalee Taylor, Yellowstone City County Health Department, Billings, Montana; Rosalind Thomas, MPH, New York State Department of Health, Albany, New York; Evelyn Tomaszewski, MSW, National Association of Social Workers, Washington, DC; Lucia Torian, PhD, New York City Department of Health and Mental Hygiene, New York, New York; Anita Vaughn, MD, HIV Medical Association, Plainfield, New Jersey; Jim Vergeront, MD, Wisconsin Division of Public Health, Madison, Wisconsin; Kathy Watt, Van Ness Recovery House Programs, Los Angeles, California; Jim Welch, MSN, Delaware Department of Corrections, Dover, Delaware; Delbert Williams, PhD, North Carolina Department of Health and Human Services, Raleigh, North Carolina; Lester N. Wright, MD, New York State Department of Correctional Services, Albany, New York. Federal Consultants CDC: Glenn W. Acham, Gustavo Aquino, MPH, Jim Beall, MA, Cady Berkel, PhD, David Block, Vickie Boazman-Holmes, Jeff Bosshart, MSW, MPH, Michael Brown, Rashad Burgess, MA, Dayne Collins, Stewart Coulter, Steven J. Davis, Sr., Sheila Dooley-Edwards, Denise Duran, MPH, Agatha Eke, PhD, Everett Expose, MA, Deymon X. Fleming, MPH, Marvin J. Fleming, Sr., Dan George, Judith K. Griffith, MS, Harneyca Hooper, MSPH, Angela Hutchinson, PhD, Priya Jakhmola, MS, MBA, David Johnson, Jennie Johnston, Lisa W. Kimbrough, MS, Christopher J. Kissler, MPH, Tamara Lamia, MPH, Chang Lee, Jim Lee, Samuel Martinez-Cardona, MD, Anita McLees, MA, MPH, Laura McElroy, Martha Miller, Andrew J. Mitsch, MPH, Anthony D. Moulton, PhD, Karen Mumford, PhD, Janice M.J. Norwood, Leslie Page-Taylor, Warren Passin, Thomas A. Peterman, MD, LaShaun Polk, MPH, Montrece McNeill Ransom, JD, Dawne Rekas, MPA, Wendy Riser, MEd, Steven J. Shapiro, Arun Skaria, MPH, Patricia Sweeney, MPH, Kimberly R. Thomas, MPH, Ronald Turski, MPA, Cindy Weinbaum, MD. Department of Veteran Affairs: Donna Wells-Taylor. Indian Health Service: Scott Giberson, MPH. Office of HIV/AIDS Policy, U.S. Department of Health and Human Services: Christopher Bates, MPA. Office of Population Affairs, U.S. Department of Health and Human Services: Susan Moskosky, MS. Substance Abuse and Mental Health Services Administration: Warren Hewitt, MS. Peer reviewers: Jonathan M. Ellen, MD, Johns Hopkins University, Baltimore, Maryland; Peter Leone, MD, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; Patricia Kissinger, PhD, Tulane University, New Orleans, Louisiana.
Table 1 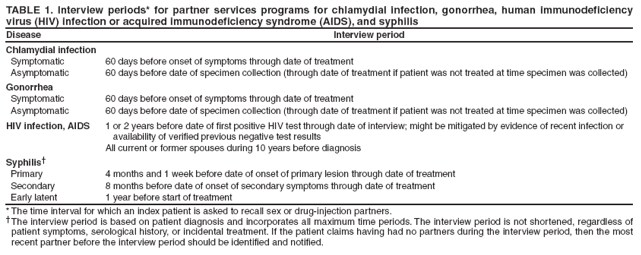 Return to top. Figure 1 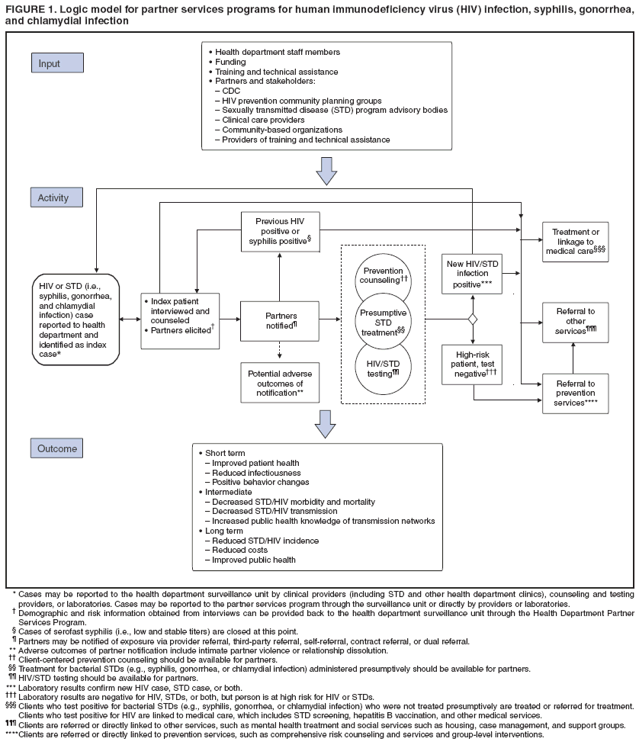 Return to top. Table 2 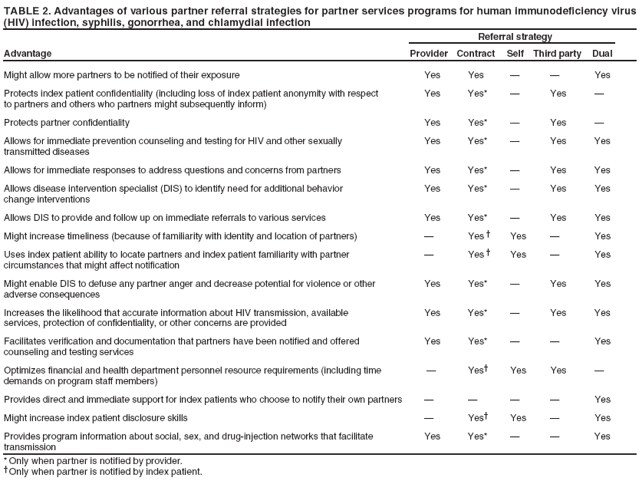 Return to top. Figure 2 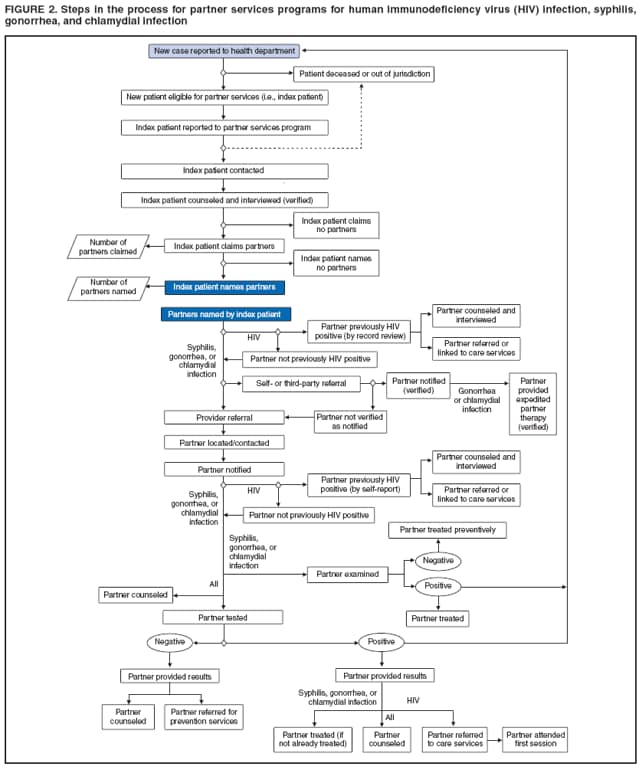 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/23/2008 |
|||||||||
|