Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Wild Poliovirus Type 1 and Type 3 Importations --- 15 Countries, Africa, 2008--2009
The Global Polio Eradication Initiative began in 1988; by 2006, indigenous transmission of wild poliovirus (WPV) type 2 infection had been interrupted globally, and indigenous transmission of type 1 and 3 (WPV1 and WPV3) infection had been interrupted in all but four countries worldwide (Afghanistan, India, Nigeria, and Pakistan) (1). Despite this success in controlling indigenous transmission, during 2002--2006, 20 previously polio-free countries* in Africa and Asia had importations of WPV1 originating from Nigeria (2--4), and three polio-free countries in Africa had WPV1 importations originating from India (1). By the end of 2007, control efforts in all countries except Angola, Chad, Democratic Republic of the Congo (DRC), Niger, and Sudan had stopped transmission of WPV1 caused by these importations. However, during 2008--2009, multiple importations of WPV from countries with ongoing transmission resumed in Africa. This report describes 32 WPV importations into 15 African countries, resulting in 96 polio cases during January 2008--March 2009 and persistent WPV transmission in five previously polio-free African countries (5). As with the 2002--2006 resurgence, all of the importations originated from Nigeria or India, but more rapid WPV identification and response resulted in substantially fewer polio cases than reported during 2002--2006. Sensitive surveillance and continued rapid response supplemental immunization activities (SIAs)† are key to limiting further WPV spread, interrupting the outbreaks, and allowing the polio prevention focus in Africa to return to eradicating polio in countries with persistent WPV transmission.
WPV Importations, 2008--2009
Comprehensive genomic sequencing provided by the global polio laboratory network (6) allows tracing of the origins of virus importations and estimation of the duration of circulation in a chain of transmission.§ An importation event is defined as detection of one or more polio cases in a country resulting from WPV transmission that genetic analysis shows to have first circulated in another country. An outbreak associated with an importation event is defined as two or more polio cases caused by WPV genetically related to the identified imported WPV case with earliest onset.
During January 2008--March 2009, 32 importations of WPV1 and WPV3 resulted in 96 polio cases in 15 African countries (Figure, Table 1). Of these, 29 WPV importations originated from Nigeria, with WPVs either imported directly or after transmission through another country, resulting in 68 polio cases. Three WPV importations originated from India, either imported directly or after transmission through another country, resulting in 28 cases. As of March 24, 2009, multiple outbreaks resulting from importations were ongoing. Three regions of Africa were affected by importations during 2008--2009: West Central Africa, the Horn of Africa, and South Central Africa.
West Central Africa. During 2008--2009, increased circulation of WPV1 in Nigeria resulted in WPV1 importations into eight countries. WPV1 was imported directly from Nigeria or indirectly through neighboring countries into Benin, Burkina Faso, Chad, Côte d'Ivoire, Ghana, Mali, Niger, and Togo (Figure, Table 1). The number of reported polio cases to date resulting from a single importation event in West Central Africa during 2008--2009 ranged from one to seven (Table 1). Niger reported five WPV1 and five WPV3 importation events originating from Nigeria; of these, subsequent spread within Niger was identified for three events. One WPV1 importation into Chad from Nigeria occurred in 2008. In addition, persistent circulation of WPV imported into Chad and Niger from Nigeria before 2008 was detected during 2008--2009 (Table 2).
The Horn of Africa. During 2008, two WPV3 importations into western Sudan occurred. The WPV3 in both cases originated from Nigeria and transmitted through Chad, and resulted in two isolated polio cases with no evidence of further spread (Table 1). Other cases occurred in the Horn of Africa during 2008 that were traceable to previous importations. In 2003, a WPV1 importation event originating in Nigeria caused an outbreak of 51 polio cases in Chad. Transmission was then imported to Sudan in mid--2004, resulting in 147 polio cases in that country during 2004--2005.¶ Subsequent related transmission occurred in seven other countries (Eritrea, Ethiopia, Indonesia, Kenya, Saudi Arabia, Somalia, and Yemen) (2--4). The Sudan outbreak subsided, and no additional related WPV1 polio cases attributable to the 2004 importation into Sudan were detected until April 2008, after which 53 additional cases were detected: three cases in Ethiopia, two in northern Kenya, five in Uganda (Table 1), and 43 in Sudan itself (Table 2). Since 2004, a total of 190 polio cases in Sudan have resulted from the 2004 importation (Table 2), despite multiple SIAs.
South Central Africa. Two WPV1 importation events and one WPV3 event in Angola, all originating from India, resulted in WPV transmission during 2008--2009. An outbreak in Angola that followed WPV1 importation in 2005 ended in 2007 with 19 confirmed cases but led to 58 cases in DRC during 2006--2008** and three cases in the Central African Republic in 2008 (Table 1). A second WPV1 importation into Angola originating from northern India was associated with 15 polio cases in Angola during April 2007--February 2009 (Table 2). A WPV3 importation, also originating from northern India, resulted in 24 polio cases in Angola and one case in DRC in 2008 (Figure, Table 1).
Vaccination Coverage
Vaccination histories of children aged 6--59 months with acute flaccid paralysis (AFP) for which specimen testing has not indicated WPV infection (i.e., nonpolio AFP [NPAFP]) have been used as a surrogate estimate of oral polio vaccine (OPV) coverage with ≥3 total OPV doses of the overall target population. The median percentage of coverage for children aged 6--59 months with NPAFP in countries affected by importations was 74% in 2008 (range: 54%--90%) (Table 1) (2).
Timeliness of Detection and Response
In the 15 countries with 32 importation events during 2008--2009, the median interval from onset of paralysis in the first identified case to laboratory confirmation of polio was 31.5 days (range: 10-61 days) (Table 1), substantially lower than the median of 51 days reported during 2002-2005 polio resurgence (2). Similarly, the median interval from laboratory confirmation to first large-scale vaccination response was 27.5 days (range: 1-91), lower than the median of 37 days reported during 2002-2005 (2). Response SIAs in Africa in 2008 were synchronized among 12 countries. After detection in 2009 of new polio cases in Kenya and Uganda, synchronized SIAs for these and neighboring countries were conducted in March and are planned again for April and May.
Persistent Transmission After Importation
Five previously polio-free countries (Angola, Chad, DRC, Niger, and Sudan) had WPV importation events before 2008 that resulted in persistent transmission for ≥12 months, extending into the period 2008-2009 (Table 2). As an indicator of weaker routine and SIA vaccination in these five countries, the median proportion of children aged 6-59 months with NPAFP and a vaccination history of ≥3 total OPV doses during 2008 was 64%, compared with 75% for all other countries with cases following importation during 2008-2009. Among these five countries, Angola, Chad, and Sudan have been the source of multiple WPV importations to neighboring countries and also have reported polio cases in their own countries since November 2008. Deficiencies in AFP surveillance†† and SIA implementation in certain subnational areas noted in technical reviews have not been corrected in the three countries. Efforts are under way to strengthen AFP surveillance and to enhance SIA implementation in these countries through examination of operations by technical advisory committees, improved oversight by international consultants, and strengthened planning and supervision of SIA and routine vaccination delivery.
Reported by: Global Polio Laboratory Network, Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases, Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note:
During January 2008 to March 2009, four countries in Africa (Angola, Chad, Nigeria, and Sudan) were the source of repeated WPV importation into other countries on the continent. In Angola, Chad, and Sudan, indigenous WPV circulation appeared interrupted before 2002, but ongoing transmission was reestablished during 2004-2008 after WPV importation (2). In Angola, Chad, Nigeria, and Sudan, health infrastructure is weak, routine vaccination coverage in certain areas is low, and multiple SIAs have failed to reach a substantial proportion of children in critical locales because of inadequate planning and implementation. Angola, Chad, and Sudan all have experienced civil war in recent years; Chad and Sudan continued to have civil unrest during 2008-2009.
Nigeria has been the major reservoir of both WPV1 and WPV3 circulation for further spread in Africa during 2008-2009 and of WPV1 during 2002-2006 (2-4). Indigenous WPV transmission has never been interrupted in Nigeria. Chronically weak routine vaccination and SIA implementation were compounded during 2003-2004 by a decrease in vaccine acceptance and an increase in WPV transmission; during that period, misconceptions about the safety of OPV led to loss of public confidence and suspension of SIAs in some northern states (8). The continuing polio prevention challenge in Nigeria is being addressed through a reinvigorated federal government effort to engage local community leadership and enhanced state and local government oversight of SIA implementation (8).
During 2005-2008, Angola received WPV importations on three occasions from India and was the source of polio cases in the DRC during 2006-2008, the Central African Republic in 2008, and Namibia in 2006 (1,9). The exact modes of WPV transmission from India to Angola have not yet been identified, but studies are under way to determine what travel factors might be associated. Although WPV3 is less commonly associated with importation events than WPV1 (which is more likely to cause paralytic disease and have a wide geographic spread), both long-distance importation of WPV3 and transmission across country borders occurred in Africa during 2008-2009.
The outbreaks associated with WPV importations during 2008-2009 have tended to be smaller than those observed during 2002-2005, when 47 importation events originating from Nigeria affected 16 countries in Africa and resulted in 1,335 polio cases (2). The fewer number of polio cases resulting from the more recent importations likely can be attributed to more timely laboratory confirmation (2), more rapid initiation of SIAs, and improved coverage with OPV in the targeted population. A surrogate indicator of OPV coverage is the percentage of children aged 6-59 months with NPAFP who have received ≥3 total doses of OPV; in the 15 countries affected by WPV importation during 2008-2009, the median was 74%, (range: 54%-90%) in 2008 compared with 55% (range: 31%-83%) for these same countries in 2004. Critical to early recognition of WPV importation and timely response is a sensitive AFP surveillance system that meets WHO performance criteria at the lowest subnational level.
Early recognition and response to WPV transmission limit the size of affected areas within a country, and enable more rapid control of an outbreak (10). SIAs are planned to continue in these affected countries and neighboring areas in 2009. WPV importations from reservoir countries into polio-free areas will continue to occur until transmission is interrupted globally. The risk for importation is greatest for countries adjacent to those countries where WPV transmission continues; however, globalized transportation and international migration pose a risk for WPV reintroduction for all countries. Recent findings of WPV in sewage samples in Switzerland and Egypt, where no polio cases have been detected since 1984 and 2004, respectively, confirm that long-distance importations can occur and that high levels of vaccination coverage limit local transmission (5,6). All polio-free countries are advised to maintain sensitive, efficient AFP surveillance systems in all areas to detect importations rapidly and to maintain sufficient levels of immunity against polioviruses through routine vaccination programs or, where necessary, SIAs. National authorities should prepare and update plans for timely, large-scale, high-quality response SIAs should importations occur (10).
References
- CDC. Progress toward interruption of wild poliovirus transmission-worldwide, January 2007-April 2008. MMWR 2008;57:489-94.
- CDC. Resurgence of wild poliovirus type 1 transmission and consequences of importation-21 countries, 2002-2005. MMWR 2006;
55:145-50. - World Health Organization. Outbreak news. Poliomyelitis, Ethiopia and Somalia. Wkly Epidemiol Rec 2006;81:350.
- World Health Organization. Outbreak news. Poliomyelitis, Kenya. Wkly Epidemiol Rec 2006;81:410.
- CDC. Progress toward interruption of wild poliovirus transmission-worldwide, January-December 2008. MMWR 2009;58:308-12.
- CDC. Laboratory surveillance for wild and vaccine-derived polioviruses-worldwide, January 2007-June 2008. MMWR 2008;57:967-70.
- Liu HM, Zheng DP, Zhang LB, Oberste MS, Pallansch MA, Kew OM. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J Virol 2000;74:11153-61.
- CDC. Progress toward poliomyelitis eradication-Nigeria, January 2007-August 12, 2008. MMWR 2008;57:942-6.
- CDC. Outbreak of polio in adults-Namibia, 2006. MMWR 2006;55:1198-201.
- World Health Organization. Advisory Committee on Polio Eradication-standing recommendations for responding to circulating polioviruses in polio-free areas. Wkly Epidemiol Rec 2005;80:330-1.
* Countries with no evidence of indigenous WPV transmission for ≥1 year and subsequent cases determined to be of external origin by genomic sequencing analysis.
† Mass campaigns conducted for a brief period (days to weeks), during which 1 dose of oral poliovirus vaccine is administered to all children aged <5 years, regardless of vaccination history.
§ The sequence of the complete VP1 coding region is determined by using automated cycle-sequencing procedures described previously (7) and by comparing the resulting sequences with those in a database of all recent poliovirus isolates. The origins of virus importation are then derived via phylogenetic analysis.
¶ In Sudan during 2004--2005, transmission of another chain of WPV1 accounted for five additional polio cases, and a previously undetected lineage of WPV3 accounted for three other polio cases.
** Eighteen polio cases resulted from three separate WPV1 importations into DRC from Angola during 2006-2007. A fourth importation of WPV1 into DRC resulted in 40 cases during December 2006-August 2008 (Table 2).
†† AFP surveillance quality is monitored by performance indicators that suggest the ease by which any WPV transmission will be detected. The current World Health Organization (WHO) targets are a NPAFP detection rate of >2 cases per 100,000 population aged <15 years and adequate stool specimen collection from >80% of AFP cases, in which two specimens are collected >24 hours apart, both within 14 days of paralysis onset, and shipped on ice or frozen ice packs to a WHO-accredited laboratory, arriving in good condition. National data might mask surveillance system weaknesses at subnational levels.
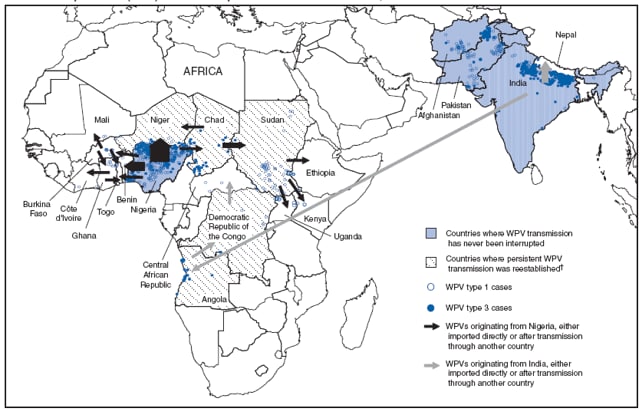
* Importation routes (not each importation event) indicated by arrows. Width of arrow corresponds to number of importation events. Genomic sequencing analysis identified Nigeria as the country of WPV origin in 29 importation events, and India in three events. Importations across the Pakistan-Afghanistan border are not included. Data as of March 24, 2009.
† Countries with no evidence of indigenous WPV transmission for ≥1 year, subsequent cases determined to be of external origin, and reestablished transmission of WPV for ≥12 months.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/15/2009


