 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant RecipientsRecommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow TransplantationAn erratum has been published for this article. To view the erratum, please click here. Abbreviations Used in This Publication
The following CDC staff members prepared this report:Clare A. Dykewicz, M.D., M.P.H. Jonathan E. Kaplan, M.D. in collaboration with the Guidelines Working Group Members from CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow TransplantationClare A. Dykewicz, M.D., M.P.H., Chair William R. Jarvis, M.D. Jonathan E. Kaplan, M.D. Brian R. Edlin, M.D. Robert T. Chen, M.D., M.A. Raleigh A. Bowden, M.D. David Emanuel, M.B.Ch.B. David L. Longworth, M.D. Philip A. Rowlings, M.B.B.S., M.S. Robert H. Rubin, M.D. Kent A. Sepkowitz, M.D. John R. Wingard, M.D. Additional ContributorsJohn F. Modlin, M.D. Donna M. Ambrosino, M.D. Norman W. Baylor, Ph.D. Albert D. Donnenberg, Ph.D. Pierce Gardner, M.D. Roger H. Giller, M.D. Neal A. Halsey, M.D. Chinh T. Le, M.D. Deborah C. Molrine, M.D. Keith M. Sullivan, M.D. SummaryCDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation have cosponsored these guidelines for preventing opportunistic infections (OIs) among hematopoietic stem cell transplant (HSCT) recipients. The guidelines were drafted with the assistance of a working group of experts in infectious diseases, transplantation, and public health. For the purposes of this report, HSCT is defined as any transplantation of blood- or marrow-derived hematopoietic stem cells, regardless of transplant type (i.e., allogeneic or autologous) or cell source (i.e., bone marrow, peripheral blood, or placental or umbilical cord blood). Such OIs as bacterial, viral, fungal, protozoal, and helminth infections occur with increased frequency or severity among HSCT recipients. These evidence-based guidelines contain information regarding preventing OIs, hospital infection control, strategies for safe living after transplantation, vaccinations, and hematopoietic stem cell safety. The disease-specific sections address preventing exposure and disease for pediatric and adult and autologous and allogeneic HSCT recipients. The goal of these guidelines is twofold: to summarize current data and provide evidence-based recommendations regarding preventing OIs among HSCT patients. The guidelines were developed for use by HSCT recipients, their household and close contacts, transplant and infectious diseases physicians, HSCT center personnel, and public health professionals. For all recommendations, prevention strategies are rated by the strength of the recommendation and the quality of the evidence supporting the recommendation. Adhering to these guidelines should reduce the number and severity of OIs among HSCT recipients. INTRODUCTIONIn 1992, the Institute of Medicine (1) recommended that CDC lead a global effort to detect and control emerging infectious agents. In response, CDC published a plan (2) that outlined national disease prevention priorities, including the development of guidelines for preventing opportunistic infections (OIs) among immunosuppressed persons. During 1995, CDC published guidelines for preventing OIs among persons infected with human immunodeficiency virus (HIV) and revised those guidelines during 1997 and 1999 (3--5). Because of the success of those guidelines, CDC sought to determine the need for expanding OI prevention activities to other immunosuppressed populations. An informal survey of hematology, oncology, and infectious disease specialists at transplant centers and a working group formed by CDC determined that guidelines were needed to help prevent OIs among hematopoietic stem cell transplant (HSCT)* recipients. The working group defined OIs as infections that occur with increased frequency or severity among HSCT recipients, and they drafted evidence-based recommendations for preventing exposure to and disease caused by bacterial, fungal, viral, protozoal, or helminthic pathogens. During March 1997, the working group presented the first draft of these guidelines at a meeting of representatives from public and private health organizations. After review by that group and other experts, these guidelines were revised and made available during September 1999 for a 45-day public comment period after notification in the Federal Register. Public comments were added when feasible, and the report was approved by CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. The pediatric content of these guidelines has been endorsed also by the American Academy of Pediatrics. The hematopoietic stem cell safety section was endorsed by the International Society of Hematotherapy and Graft Engineering. The first recommendations presented in this report are followed by recommendations for hospital infection control, strategies for safe living, vaccinations, and hematopoietic stem cell safety. Unless otherwise noted, these recommendations address allogeneic and autologous and pediatric and adult HSCT recipients. Additionally, these recommendations are intended for use by the recipients, their household and other close contacts, transplant and infectious diseases specialists, HSCT center personnel, and public health professionals. Using These GuidelinesFor all recommendations, prevention strategies are rated by the strength of the recommendation (Table 1) and the quality of the evidence (Table 2) supporting the recommendation. The principles of this rating system were developed by the Infectious Disease Society of America and the U.S. Public Health Service for use in the guidelines for preventing OIs among HIV-infected persons (3--6). This rating system allows assessments of recommendations to which adherence is critical. BACKGROUNDHSCT is the infusion of hematopoietic stem cells from a donor into a patient who has received chemotherapy, which is usually marrow-ablative. Increasingly, HSCT has been used to treat neoplastic diseases, hematologic disorders, immunodeficiency syndromes, congenital enzyme deficiencies, and autoimmune disorders (e.g., systemic lupus erythematosus or multiple sclerosis) (7--10). Moreover, HSCT has become standard treatment for selected conditions (7,11,12). Data from the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry indicate that approximately 20,000 HSCTs were performed in North America during 1998 (Statistical Center of the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry, unpublished data, 1998). HSCTs are classified as either allogeneic or autologous on the basis of the source of the transplanted hematopoietic progenitor cells. Cells used in allogeneic HSCTs are harvested from a donor other than the transplant recipient. Such transplants are the most effective treatment for persons with severe aplastic anemia (13) and offer the only curative therapy for persons with chronic myelogenous leukemia (12). Allogeneic donors might be a blood relative or an unrelated donor. Allogeneic transplants are usually most successful when the donor is a human lymphocyte antigen (HLA)-identical twin or matched sibling. However, for allogeneic candidates who lack such a donor, registry organizations (e.g., the National Marrow Donor Program) maintain computerized databases that store information regarding HLA type from millions of volunteer donors (14--16). Another source of stem cells for allogeneic candidates without an HLA-matched sibling is a mismatched family member (17,18). However, persons who receive allogeneic grafts from donors who are not HLA-matched siblings are at a substantially greater risk for graft-versus-host disease (GVHD) (19). These persons are also at increased risk for suboptimal graft function and delayed immune system recovery (19). To reduce GVHD among allogeneic HSCTs, techniques have been developed to remove T-lymphocytes, the principal effectors of GVHD, from the donor graft. Although the recipients of T-lymphocyte--depleted marrow grafts generally have lower rates of GVHD, they also have greater rates of graft rejection, cytomegalovirus (CMV) infection, invasive fungal infection, and Epstein-Barr virus (EBV)-associated posttransplant lymphoproliferative disease (20). The patient's own cells are used in an autologous HSCT. Similar to autologous transplants are syngeneic transplants, among whom the HLA-identical twin serves as the donor. Autologous HSCTs are preferred for patients who require high-level or marrow-ablative chemotherapy to eradicate an underlying malignancy but have healthy, undiseased bone marrows. Autologous HSCTs are also preferred when the immunologic antitumor effect of an allograft is not beneficial. Autologous HSCTs are used most frequently to treat breast cancer, non-Hodgkin's lymphoma, and Hodgkin's disease (21). Neither autologous nor syngeneic HSCTs confer a risk for chronic GVHD. Recently, medical centers have begun to harvest hematopoietic stem cells from placental or umbilical cord blood (UCB) immediately after birth. These harvested cells are used primarily for allogeneic transplants among children. Early results demonstrate that greater degrees of histoincompatibility between donor and recipient might be tolerated without graft rejection or GVHD when UCB hematopoietic cells are used (22--24). However, immune system function after UCB transplants has not been well-studied. HSCT is also evolving rapidly in other areas. For example, hematopoietic stem cells harvested from the patient's peripheral blood after treatment with hematopoietic colony-stimulating factors (e.g., granulocyte colony-stimulating factor [G-CSF or filgastrim] or granulocyte-macrophage colony-stimulating factor [GM-CSF or sargramostim]) are being used increasingly among autologous recipients (25) and are under investigation for use among allogeneic HSCT. Peripheral blood has largely replaced bone marrow as a source of stem cells for autologous recipients. A benefit of harvesting such cells from the donor's peripheral blood instead of bone marrow is that it eliminates the need for general anesthesia associated with bone marrow aspiration. GVHD is a condition in which the donated cells recognize the recipient's cells as nonself and attack them. Although the use of intravenous immunoglobulin (IVIG) in the routine management of allogeneic patients was common in the past as a means of producing immune modulation among patients with GVHD, this practice has declined because of cost factors (26) and because of the development of other strategies for GVHD prophylaxis (27). For example, use of cyclosporine GVHD prophylaxis has become commonplace since its introduction during the early 1980s. Most frequently, cyclosporine or tacrolimus (FK506) is administered in combination with other immunosuppressive agents (e.g., methotrexate or corticosteroids) (27). Although cyclosporine is effective in preventing GVHD, its use entails greater hazards for infectious complications and relapse of the underlying neoplastic disease for which the transplant was performed. Although survival rates for certain autologous recipients have improved (28,29), infection remains a leading cause of death among allogeneic transplants and is a major cause of morbidity among autologous HSCTs (29). Researchers from the National Marrow Donor Program reported that, of 462 persons receiving unrelated allogeneic HSCTs during December 1987--November 1990, a total of 66% had died by 1991 (15). Among primary and secondary causes of death, the most common cause was infection, which occurred among 37% of 307 patients (15).** Despite high morbidity and mortality after HSCT, recipients who survive long-term are likely to enjoy good health. A survey of 798 persons who had received an HSCT before 1985 and who had survived for >5 years after HSCT, determined that 93% were in good health and that 89% had returned to work or school full time (30). In another survey of 125 adults who had survived a mean of 10 years after HSCT, 88% responded that the benefits of transplantation outweighed the side effects (31). Immune System Recovery After HSCTDuring the first year after an HSCT, recipients typically follow a predictable pattern of immune system deficiency and recovery, which begins with the chemotherapy or radiation therapy (i.e., the conditioning regimen) administered just before the HSCT to treat the underlying disease. Unfortunately, this conditioning regimen also destroys normal hematopoiesis for neutrophils, monocytes, and macrophages and damages mucosal progenitor cells, causing a temporary loss of mucosal barrier integrity. The gastrointestinal tract, which normally contains bacteria, commensal fungi, and other bacteria-carrying sources (e.g., skin or mucosa) becomes a reservoir of potential pathogens. Virtually all HSCT recipients rapidly lose all T- and B-lymphocytes after conditioning, losing immune memory accumulated through a lifetime of exposure to infectious agents, environmental antigens, and vaccines. Because transfer of donor immunity to HSCT recipients is variable and influenced by the timing of antigen exposure among donor and recipient, passively acquired donor immunity cannot be relied upon to provide long-term immunity against infectious diseases among HSCT recipients. During the first month after HSCT, the major host-defense deficits include impaired phagocytosis and damaged mucocutaneous barriers. Additionally, indwelling intravenous catheters are frequently placed and left in situ for weeks to administer parenteral medications, blood products, and nutritional supplements. These catheters serve as another portal of entry for opportunistic pathogens from organisms colonizing the skin (e.g., . coagulase-negative Staphylococci, Staphylococcus aureus, Candida species, and Enterococci) (32,33). Engraftment for adults and children is defined as the point at which a patient can maintain a sustained absolute neutrophil count (ANC) of >500/mm3 and sustained platelet count of >20,000, lasting >3 consecutive days without transfusions. Among unrelated allogeneic recipients, engraftment occurs at a median of 22 days after HSCT (range: 6--84 days) (15). In the absence of corticosteroid use, engraftment is associated with the restoration of effective phagocytic function, which results in a decreased risk for bacterial and fungal infections. However, all HSCT recipients and particularly allogeneic recipients, experience an immune system dysfunction for months after engraftment. For example, although allogeneic recipients might have normal total lymphocyte counts within >2 months after HSCT, they have abnormal CD4/CD8 T-cell ratios, reflecting their decreased CD4 and increased CD8 T-cell counts (27). They might also have immunoglobulin G (IgG)2, IgG4, and immunoglobulin A (IgA) deficiencies for months after HSCT and have difficulty switching from immunoglobulin M (IgM) to IgG production after antigen exposure (32). Immune system recovery might be delayed further by CMV infection (34). During the first >2 months after HSCT, recipients might experience acute GVHD that manifests as skin, gastrointestinal, and liver injury, and is graded on a scale of I--IV (32,35,36). Although autologous or syngeneic recipients might occasionally experience a mild, self-limited illness that is acute GVHD-like (19,37), GVHD occurs primarily among allogeneic recipients, particularly those receiving matched, unrelated donor transplants. GVHD is a substantial risk factor for infection among HSCT recipients because it is associated with a delayed immunologic recovery and prolonged immunodeficiency (19). Additionally, the immunosuppressive agents used for GVHD prophylaxis and treatment might make the HSCT recipient more vulnerable to opportunistic viral and fungal pathogens (38). Certain patients, particularly adult allogeneic recipients, might also experience chronic GVHD, which is graded as either limited or extensive chronic GVHD (19,39). Chronic GVHD appears similar to autoimmune, connective-tissue disorders (e.g., scleroderma or systemic lupus erythematosus) (40) and is associated with cellular and humoral immunodeficiencies, including macrophage deficiency, impaired neutrophil chemotaxis (41), poor response to vaccination (42--44), and severe mucositis (19). Risk factors for chronic GVHD include increasing age, allogeneic HSCT (particularly those among whom the donor is unrelated or a non-HLA identical family member) (40), and a history of acute GVHD (24,45). Chronic GVHD was first described as occurring >100 days after HSCT but can occur 40 days after HSCT (19). Although allogeneic recipients with chronic GVHD have normal or high total serum immunoglobulin levels (41), they experience long-lasting IgA, IgG, and IgG subclass deficiencies (41,46,47) and poor opsonization and impaired reticuloendothelial function. Consequently, they are at even greater risk for infections (32,39), particularly life-threatening bacterial infections from encapsulated organisms (e.g., Stre. pneumoniae, Ha. influenzae, or Ne. meningitidis). After chronic GVHD resolves, which might take years, cell-mediated and humoral immunity function are gradually restored. Opportunistic Pathogens After HSCTHSCT recipients experience certain infections at different times posttransplant, reflecting the predominant host-defense defect(s) (Figure). Immune system recovery for HSCT recipients takes place in three phases beginning at day 0, the day of transplant. Phase I is the preengraftment phase (<30 days after HSCT); phase II, the postengraftment phase (30--100 days after HSCT); and phase III, the late phase (>100 days after HSCT). Prevention strategies should be based on these three phases and the following information:
Preventing infections among HSCT recipients is preferable to treating infections. How ever, despite recent technologic advances, more research is needed to optimize health outcomes for HSCT recipients. Efforts to improve immune system reconstitution, particularly among allogeneic transplant recipients, and to prevent or resolve the immune dysregulation resulting from donor-recipient histoincompatibility and GVHD remain substantial challenges for preventing recurrent, persistent, or progressive infections among HSCT patients. BACTERIAL INFECTIONSGeneral RecommendationsPreventing Exposure Because bacteria are carried on the hands, health-care workers (HCWs) and others in contact with HSCT recipients should routinely follow appropriate hand-washing practices to avoid exposing recipients to bacterial pathogens (AIII). Preventing Disease Preventing Early Disease (0--100 Days After HSCT). Routine gut decontamination is not recommended for HSCT candidates (51--53) (DIII). Because of limited data, no recommendations can be made regarding the routine use of antibiotics for bacterial prophylaxis among afebrile, asymptomatic neutropenic recipients. Although studies have reported that using prophylactic antibiotics might reduce bacteremia rates after HSCT (51), infection-related fatality rates are not reduced (52). If physicians choose to use prophylactic antibiotics among asymptomatic, afebrile, neutropenic recipients, they should routinely review hospital and HSCT center antibiotic-susceptibility profiles, particularly when using a single antibiotic for antibacterial prophylaxis (BIII). The emergence of fluoquinolone-resistant coagulase-negative Staphylococci and Es. coli (51,52), vancomycin-intermediate Sta. aureus and vancomycin-resistant Enterococcus (VRE) are increasing concerns (54). Vancomycin should not be used as an agent for routine bacterial prophylaxis (DIII). Growth factors (e.g., GM-CSF and G-CSF) shorten the duration of neutropenia after HSCT (55); however, no data were found that indicate whether growth factors effectively reduce the attack rate of invasive bacterial disease. Physicians should not routinely administer IVIG products to HSCT recipients for bacterial infection prophylaxis (DII), although IVIG has been recommended for use in producing immune system modulation for GVHD prevention. Researchers have recommended routine IVIG*** use to prevent bacterial infections among the approximately 20%--25% of HSCT recipients with unrelated marrow grafts who experience severe hypogamma-globulinemia (e.g., IgG < 400 mg/dl) within the first 100 days after transplant (CIII). For example, recipients who are hypogammaglobulinemic might receive prophylactic IVIG to prevent bacterial sinopulmonary infections (e.g., from Stre. pneumoniae) (8) (CIII). For hypogammaglobulinemic allogeneic recipients, physicians can use a higher and more frequent dose of IVIG than is standard for non-HSCT recipients because the IVIG half-life among HSCT recipients (generally 1--10 days) is much shorter than the half-life among healthy adults (generally 18--23 days) (56--58). Additionally, infections might accelerate IgG catabolism; therefore, the IVIG dose for a hypogammaglobulinemic recipient should be individualized to maintain trough serum IgG concentrations >400--500 mg/dl (58) (BII). Consequently, physicians should monitor trough serum IgG concentrations among these patients approximately every 2 weeks and adjust IVIG doses as needed (BIII) (Appendix). Preventing Late Disease (>100 Days After HSCT). Antibiotic prophylaxis is recommended for preventing infection with encapsulated organisms (e.g., Stre. pneumoniae, Ha. influenzae, or Ne. meningitidis) among allogeneic recipients with chronic GVHD for as long as active chronic GVHD treatment is administered (59) (BIII). Antibiotic selection should be guided by local antibiotic resistance patterns. In the absence of severe demonstrable hypogammaglobulinemia (e.g., IgG levels < 400 mg/dl, which might be associated with recurrent sinopulmonary infections), routine monthly IVIG administration to HSCT recipients >90 days after HSCT is not recommended (60) (DI) as a means of preventing bacterial infections. Other Disease Prevention Recommendations. Routine use of IVIG among autologous recipients is not recommended (61) (DII). Recommendations for preventing bacterial infections are the same among pediatric or adult HSCT recipients. Recommendations Regarding Stre. pneumoniaePreventing Exposure Appropriate care precautions should be taken with hospitalized patients infected with Stre. pneumoniae (62,63) (BIII) to prevent exposure among HSCT recipients. Preventing Disease Information regarding the currently available 23-valent pneumococcal polysaccharide vaccine indicates limited immunogenicity among HSCT recipients. However, because of its potential benefit to certain patients, it should be administered to HSCT recipients at 12 and 24 months after HSCT (64--66) (BIII). No data were found regarding safety and immunogenicity of the 7-valent conjugate pneumococcal vaccine among HSCT recipients; therefore, no recommendation regarding use of this vaccine can be made. Antibiotic prophylaxis is recommended for preventing infection with encapsulated organisms (e.g., Stre. pneumoniae, Ha. influenzae, and Ne. meningitidis) among allogeneic recipients with chronic GVHD for as long as active chronic GVHD treatment is administered (59) (BIII). Trimethoprim-sulfamethasaxole (TMP-SMZ) administered for Pneumocystis carinii pneumonia (PCP) prophylaxis will also provide protection against pneumococcal infections. However, no data were found to support using TMP-SMZ prophylaxis among HSCT recipients solely for the purpose of preventing Stre. pneumoniae disease. Certain strains of Stre. pneumoniae are resistant to TMP-SMZ and penicillin. Recommendations for preventing pneumococcal infections are the same for allogeneic or autologous recipients. As with adults, pediatric HSCT recipients aged >2 years should be administered the current 23-valent pneumococcal polysaccharide vaccine because the vaccine can be effective (BIII). However, this vaccine should not be administered to children aged <2 years because it is not effective among that age population (DI). No data were found regarding safety and immunogenicity of the 7-valent conjugate pneumococcal vaccine among pediatric HSCT recipients; therefore, no recommendation regarding use of this vaccine can be made. Recommendations Regarding Streptococci viridansPreventing Exposure Because Streptococci viridans colonize the oropharynx and gut, no effective method of preventing exposure is known. Preventing Disease Chemotherapy-induced oral mucositis is a potential source of Streptococci viridans bacteremia. Consequently, before conditioning starts, dental consults should be obtained for all HSCT candidates to assess their state of oral health and to perform any needed dental procedures to decrease the risk for oral infections after transplant (67) (AIII). Generally, HSCT physicians should not use prophylactic antibiotics to prevent Streptococci viridans infections (DIII). No data were found that demonstrate efficacy of prophylactic antibiotics for this infection. Furthermore, such use might select antibiotic-resistant bacteria, and in fact, penicillin- and vancomycin-resistant strains of Streptococci viridans have been reported (68). However, when Streptococci viridans infections among HSCT recipients are virulent and associated with overwhelming sepsis and shock in an institution, prophylaxis might be evaluated (CIII). Decisions regarding the use of Streptococci viridans prophylaxis should be made only after consultation with the hospital epidemiologists or infection-control practitioners who monitor rates of nosocomial bacteremia and bacterial susceptibility (BIII). HSCT physicians should be familiar with current antibiotic susceptibilities for patient isolates from their HSCT centers, including Streptococci viridans (BIII). Physicians should maintain a high index of suspicion for this infection among HSCT recipients with symptomatic mucositis because early diagnosis and aggressive therapy are currently the only potential means of preventing shock when severely neutropenic HSCT recipients experience Streptococci viridans bacteremia (69). Recommendations Regarding Ha. influenzae type bPreventing Exposure Adults with Ha. influenzae type b (Hib) pneumonia require standard precautions (62) to prevent exposing the HSCT recipient to Hib. Adults and children who are in contact with the HSCT recipient and who have known or suspected invasive Hib disease, including meningitis, bacteremia, or epiglottitis, should be placed in droplet precautions until 24 hours after they begin appropriate antibiotic therapy, after which they can be switched to standard precautions. Household contacts exposed to persons with Hib disease and who also have contact with HSCT recipients should be administered rifampin prophylaxis according to published recommendations (70,71); prophylaxis for household contacts of a patient with Hib disease are necessary if all contacts aged <4 years are not fully vaccinated (BIII) (Appendix). This recommendation is critical because the risk for invasive Hib disease among unvaccinated household contacts aged <4 years is increased, and rifampin can be effective in eliminating Hib carriage and preventing invasive Hib disease (72--74). Pediatric household contacts should be up-to-date with Hib vaccinations to prevent possible Hib exposure to the HSCT recipient (AII). Preventing Disease Although no data regarding vaccine efficacy among HSCT recipients were found, Hib conjugate vaccine should be administered to HSCT recipients at 12, 14, and 24 months after HSCT (BII). This vaccine is recommended because the majority of HSCT recipients have low levels of Hib capsular polysaccharide antibodies >4 months after HSCT (75), and allogeneic recipients with chronic GVHD are at increased risk for infection from encapsulated organisms (e.g., Hib) (76,77). HSCT recipients who are exposed to persons with Hib disease should be offered rifampin prophylaxis according to published recommendations (70) (BIII) (Appendix). Antibiotic prophylaxis is recommended for preventing infection with encapsulated organisms (e.g., Stre. pneumoniae, Ha. influenzae, or Ne. meningitidis) among allogeneic recipients with chronic GVHD for as long as active chronic GVHD treatment is administered (59) (BIII). Antibiotic selection should be guided by local antibiotic-resistance patterns. Recommendations for preventing Hib infections are the same for allogeneic or autologous recipients. Recommendations for preventing Hib disease are the same for pediatric or adult HSCT recipients, except that any child infected with Hib pneumonia requires standard precautions with droplet precautions added for the first 24 hours after beginning appropriate antibiotic therapy (62,70) (BIII). Appropriate pediatric doses should be administered for Hib conjugate vaccine and for rifampin prophylaxis (71) (Appendix). VIRAL INFECTIONSRecommendations Regarding CytomegalovirusPreventing Exposure HSCT candidates should be tested for the presence of serum anti-CMV IgG antibodies before transplantation to determine their risk for primary CMV infection and reactivation after HSCT (AIII). Only Food and Drug Administration (FDA) licensed or approved tests should be used. HSCT recipients and candidates should avoid sharing cups, glasses, and eating utensils with others, including family members, to decrease the risk for CMV exposure (BIII). Sexually active patients who are not in long-term monogamous relationships should always use latex condoms during sexual contact to reduce their risk for exposure to CMV and other sexually transmitted pathogens (AII). However, even long-time monogamous pairs can be discordant for CMV infections. Therefore, during periods of immuno-compromise, sexually active HSCT recipients in monogamous relationships should ask partners to be tested for serum CMV IgG antibody, and discordant couples should use latex condoms during sexual contact to reduce the risk for exposure to this sexually transmitted OI (CIII). After handling or changing diapers or after wiping oral and nasal secretions, HSCT candidates and recipients should practice regular hand washing to reduce the risk for CMV exposure (AII). CMV-seronegative recipients of allogeneic stem cell transplants from CMV-seronegative donors (i.e., R-negative or D-negative) should receive only leukocyte-reduced or CMV-seronegative red cells or leukocyte-reduced platelets (<1 x 106 leukocytes/unit) to prevent transfusion-associated CMV infection (78) (AI). However, insufficient data were found to recommend use of leukocyte-reduced or CMV-seronega tive red cells and platelets among CMV-seronegative recipients who have CMV-seropositive donors (i.e., R-negative or D-positive). All HCWs should wear gloves when handling blood products or other potentially contaminated biologic materials (AII) to prevent transmission of CMV to HSCT recipients. HSCT patients who are known to excrete CMV should be placed under standard precautions (62) for the duration of CMV excretion to avoid possible transmission to CMV-seronegative HSCT recipients and candidates (AIII). Physicians are cautioned that CMV excretion can be episodic or prolonged. Preventing Disease and Disease Recurrence HSCT recipients at risk for CMV disease after HSCT (i.e., all CMV-seropositive HSCT recipients, and all CMV-seronegative recipients with a CMV-seropositive donor) should be placed on a CMV disease prevention program from the time of engraftment until 100 days after HSCT (i.e., phase II) (AI). Physicians should use either prophylaxis or preemptive treatment with ganciclovir for allogeneic recipients (AI). In selecting a CMV disease prevention strategy, physicians should assess the risks and benefits of each strategy, the needs and condition of the patient, and the hospital's virology laboratory support capability. Prophylaxis strategy against early CMV (i.e., <100 days after HSCT) for allogeneic recipients involves administering ganciclovir prophylaxis to all allogeneic recipients at risk throughout phase II (i.e., from engraftment to 100 days after HSCT). The induction course is usually started at engraftment (AI), although physicians can add a brief prophylactic course during HSCT preconditioning (CIII) (Appendix). Preemptive strategy against early CMV (i.e., <100 days after HSCT) for allogeneic recipients is preferred over prophylaxis for CMV-seronegative HSCT recipients of seropositive donor cells (i.e., D-positive or R-negative) because of the low attack rate of active CMV infection if screened or filtered blood product support is used (BII). Preemptive strategy restricts ganciclovir use for those patients who have evidence of CMV infection after HSCT. It requires the use of sensitive and specific laboratory tests to rapidly diagnose CMV infection after HSCT and to enable immediate administration of ganciclovir after CMV infection has been detected. Allogeneic recipients at risk should be screened >1 times/week from 10 days to 100 days after HSCT (i.e., phase II) for the presence of CMV viremia or antigenemia (AIII). HSCT physicians should select one of two diagnostic tests to determine the need for preemptive treatment. Currently, the detection of CMV pp65 antigen in leukocytes (antigenemia) (79,80) is preferred for screening for preemptive treatment because it is more rapid and sensitive than culture and has good positive predictive value (79--81). Direct detection of CMV-DNA (deoxyribonucleic acid) by polymerase chain reaction (PCR) (82) is very sensitive but has a low positive predictive value (79). Although CMV-DNA PCR is less sensitive than whole blood or leukocyte PCR, plasma CMV-DNA PCR is useful during neutropenia, when the number of leukocytes/slide is too low to allow CMV pp65 antigenemia testing. Virus culture of urine, saliva, blood, or bronchoalveolar washings by rapid shell-vial culture (83) or routine culture (84,85) can be used; however, viral culture techniques are less sensitive than CMV-DNA PCR or CMV pp65 antigenemia tests. Also, rapid shell-viral cultures require >48 hours and routine viral cultures can require weeks to obtain final results. Thus, viral culture techniques are less satisfactory than PCR or antigenemia tests. HSCT centers without access to PCR or antigenemia tests should use prophylaxis rather than preemptive therapy for CMV disease prevention (86) (BII). Physicians do use other diagnostic tests (e.g., hybrid capture CMV-DNA assay, Version 2.0 [87] or CMV pp67 viral RNA [ribonucleic acid] detection) (88); however, limited data were found regarding use among HSCT recipients, and therefore, no recommendation for use can be made. Allogeneic recipients <100 days after HSCT (i.e., during phase II) should begin preemptive treatment with ganciclovir if CMV viremia or any antigenemia is detected or if the recipient has >2 consecutively positive CMV-DNA PCR tests (BIII). After preemptive treatment has been started, maintenance ganciclovir is usually continued until 100 days after HSCT or for a minimum of 3 weeks, whichever is longer (AI) (Appendix). Antigen or PCR tests should be negative when ganciclovir is stopped. Studies report that a shorter course of ganciclovir (e.g., for 3 weeks or until negative PCR or antigenemia occurs) (89--91) might provide adequate CMV prevention with less toxicity, but routine weekly screening by pp65 antigen or PCR test is necessary after stopping ganciclovir because CMV reactivation can occur (BIII). Presently, only the intravenous formulation of ganciclovir has been approved for use in CMV prophylactic or preemptive strategies (BIII). No recommendation for oral ganciclovir use among HSCT recipients can be made because clinical trials evaluating its efficacy are still in progress. One group has used ganciclovir and foscarnet on alternate days for CMV prevention (92), but no recommendation can be made regarding this strategy because of limited data. Patients who are ganciclovir-intolerant should be administered foscarnet instead (93) (BII) (Appendix). HSCT recipients receiving ganciclovir should have ANCs checked >2 times/week (BIII). Researchers report managing ganciclovir-associated neutropenia by adding G-CSF (94) or temporarily stopping ganciclovir for >2 days if the patient's ANC is <1,000 (CIII). Ganciclovir can be restarted when the patient's ANC is >1,000 for 2 consecutive days. Alternatively, researchers report substituting foscarnet for ganciclovir if a) the HSCT recipient is still CMV viremic or antigenemic or b) the ANC remains <1,000 for >5 days after ganciclovir has been stopped (CIII) (Appendix). Because neutropenia accompanying ganciclovir administration is usually brief, such patients do not require antifungal or antibacterial prophylaxis (DIII). Currently, no benefit has been reported from routinely administering ganciclovir prophylaxis to all HSCT recipients at >100 days after HSCT (i.e., during phase III). However, persons with high risk for late CMV disease should be routinely screened biweekly for evidence of CMV reactivation as long as substantial immunocompromise persists (BIII). Risk factors for late CMV disease include allogeneic HSCT accompanied by chronic GVHD, steroid use, low CD4 counts, delay in high avidity anti-CMV antibody, and recipients of matched unrelated or T-cell--depleted HSCTs who are at high risk (95--99). If CMV is still detectable by routine screening >100 days after HSCT, ganciclovir should be continued until CMV is no longer detectable (AI). If low-grade CMV antigenemia (<5 positive cells/slide) is detected on routine screening, the antigenemia test should be repeated in 3 days (BIII). If CMV antigenemia indicates >5 cells/slide, PCR is positive, or the shell-vial culture detects CMV viremia, a 3-week course of preemptive ganciclovir treatment should be administered (BIII) (Appendix). Ganciclovir should also be started if the patient has had >2 consecutively positive viremia or PCR tests (e.g., in a person receiving steroids for GVHD or who received ganciclovir or foscarnet at <100 days after HSCT). Current investigational strategies for preventing late CMV disease include the use of targeted prophylaxis with antiviral drugs and cellular immunotherapy for those with deficient or absent CMV-specific immune system function. If viremia persists after 4 weeks of ganciclovir preemptive therapy or if the level of antigenemia continues to rise after 3 weeks of therapy, ganciclovir-resistant CMV should be suspected. If CMV viremia recurs during continuous treatment with ganciclovir, researchers report restarting ganciclovir induction (100) or stopping ganciclovir and starting foscarnet (CIII). Limited data were found regarding the use of foscarnet among HSCT recipients for either CMV prophylaxis or preemptive therapy (92,93). Infusion of donor-derived CMV-specific clones of CD8+ T-cells into the transplant recipient is being evaluated under FDA Investigational New Drug authorization; therefore, no recommendation can be made. Although, in a substantial cooperative study, high-dose acyclovir has had certain efficacy for preventing CMV disease (101), its utility is limited in a setting where more potent anti-CMV agents (e.g., ganciclovir) are used (102). Acyclovir is not effective in preventing CMV disease after autologous HSCT (103) and is, therefore, not recommended for CMV preemptive therapy (DII). Consequently, valacyclovir, although under study for use among HSCT recipients, is presumed to be less effective than ganciclovir against CMV and is currently not recommended for CMV disease prevention (DII). Although HSCT physicians continue to use IVIG for immune system modulation, IVIG is not recommended for CMV disease prophylaxis among HSCT recipients (DI). Cidofovir, a nucleoside analog, is approved by FDA for the treatment of AIDS-associated CMV retinitis. The drug's major disadvantage is nephrotoxicity. Cidofovir is currently in FDA phase 1 trial for use among HSCT recipients; therefore, recommendations for its use cannot be made. Use of CMV-negative or leukocyte-reduced blood products is not routinely required for all autologous recipients because most have a substantially lower risk for CMV disease. However, CMV-negative or leukocyte-reduced blood products can be used for CMV-seronegative autologous recipients (CIII). Researchers report that CMV-seropositive autologous recipients be evaluated for preemptive therapy if they have underlying hematologic malignancies (e.g., lymphoma or leukemia), are receiving intense conditioning regimens or graft manipulation, or have recently received fludarabine or 2-chlorodeoxyadenosine (CDA) (CIII). This subpopulation of autologous recipients should be monitored weekly from time of engraftment until 60 days after HSCT for CMV reactivation, preferably with quantitative CMV pp65 antigen (80) or quantitative PCR (BII). Autologous recipients at high risk who experience CMV antigenemia (i.e., blood levels of >5 positive cells/slide) should receive 3 weeks of preemptive treatment with ganciclovir or foscarnet (80), but CD34+-selected patients should be treated at any level of antigenemia (BII) (Appendix). Prophylactic approach to CMV disease prevention is not appropriate for CMV-seropositive autologous recipients. Indications for the use of CMV prophylaxis or preemptive treatment are the same for children or adults. Recommendations Regarding EBVPreventing Exposure All transplant candidates, particularly those who are EBV-seronegative, should be advised of behaviors that could decrease the likelihood of EBV exposure (AII). For example, HSCT recipients and candidates should follow safe hygiene practices (e.g., frequent hand washing [AIII] and avoiding the sharing of cups, glasses, and eating utensils with others) (104) (BIII), and they should avoid contact with potentially infected respiratory secretions and saliva (104) (AII). Preventing Disease Infusion of donor-derived, EBV-specific cytotoxic T-lymphocytes has demonstrated promise in the prophylaxis of EBV-lymphoma among recipients of T-cell--depleted unrelated or mismatched allogeneic recipients (105,106). However, insufficient data were found to recommend its use. Prophylaxis or preemptive therapy with acyclovir is not recommended because of lack of efficacy (107,108) (DII). Recommendations Regarding HSVPreventing Exposure HSCT candidates should be tested for serum anti-HSV IgG before transplant (AIII); however, type-specific anti-HSV IgG serology testing is not necessary. Only FDA-licensed or -approved tests should be used. All HSCT candidates, particularly those who are HSV-seronegative, should be informed of the importance of avoiding HSV infection while immunocompromised and should be advised of behaviors that will decrease the likelihood of HSV exposure (AII). HSCT recipients and candidates should avoid sharing cups, glasses, and eating utensils with others (BIII). Sexually active patients who are not in a long-term monogamous relationship should always use latex condoms during sexual contact to reduce the risk for exposure to HSV as well as other sexually transmitted pathogens (AII). However, even long-time monogamous pairs can be discordant for HSV infections. Therefore, during periods of immunocompromise, sexually active HSCT recipients in such relationships should ask partners to be tested for serum HSV IgG antibody. If the partners are discordant, they should consider using latex condoms during sexual contact to reduce the risk for exposure to this sexually transmitted OI (CIII). Any person with disseminated, primary, or severe mucocutaneous HSV disease should be placed under contact precautions for the duration of the illness (62) (AI) to prevent transmission of HSV to HSCT recipients. Preventing Disease and Disease Recurrence Acyclovir. Acyclovir prophylaxis should be offered to all HSV-seropositive allogeneic recipients to prevent HSV reactivation during the early posttransplant period (109--113) (AI). Standard approach is to begin acyclovir prophylaxis at the start of the conditioning therapy and continue until engraftment occurs or until mucositis resolves, whichever is longer, or approximately 30 days after HSCT (BIII) (Appendix). Without supportive data from controlled studies, routine use of antiviral prophylaxis for >30 days after HSCT to prevent HSV is not recommended (DIII). Routine acyclovir prophylaxis is not indicated for HSV-seronegative HSCT recipients, even if the donors are HSV-seropositive (DIII). Researchers have proposed administration of ganciclovir prophylaxis alone (86) to HSCT recipients who required simultaneous prophylaxis for CMV and HSV after HSCT (CIII) because ganciclovir has in vitro activity against CMV and HSV 1 and 2 (114), although ganciclovir has not been approved for use against HSV. Valacyclovir. Researchers have reported valacyclovir use for preventing HSV among HSCT recipients (CIII); however, preliminary data demonstrate that very high doses of valacyclovir (8 g/day) were associated with thrombotic thrombocytopenic purpura/hemolytic uremic syndrome among HSCT recipients (115). Controlled trial data among HSCT recipients are limited (115), and the FDA has not approved valacyclovir for use among recipients. Physicians wishing to use valacyclovir among recipients with renal impairment should exercise caution and decrease doses as needed (BIII) (Appendix). Foscarnet. Because of its substantial renal and infusion-related toxicity, foscarnet is not recommended for routine HSV prophylaxis among HSCT recipients (DIII). Famciclovir. Presently, data regarding safety and efficacy of famciclovir among HSCT recipients are limited; therefore, no recommendations for HSV prophylaxis with famciclovir can be made. Other Recommendations HSV prophylaxis lasting >30 days after HSCT might be considered for persons with frequent recurrent HSV (CIII) (Appendix). Acyclovir can be used during phase I for administration to HSV-seropositive autologous recipients who are likely to experience substantial mucositis from the conditioning regimen (CIII). Antiviral prophylaxis doses should be modified for use among children (Appendix), but no published data were found regarding valacyclovir safety and efficacy among children. Recommendations Regarding VZVPreventing Exposure HSCT candidates should be tested for the presence of serum anti-VZV IgG antibodies (AIII). However, these tests are not 100% reliable, particularly among severely immunosuppressed patients. Researchers recommend that a past history of varicella accompanied by a positive titer is more likely to indicate the presence of immunity to VZV than a low positive titer alone. All HSCT candidates and recipients, particularly those who are VZV-seronegative, should be informed of the potential seriousness of VZV disease among immunocompromised persons and advised of strategies to decrease their risk for VZV exposure (116--122) (AII). Although researchers report that the majority of VZV disease after HSCT is caused by reactivation of endogenous VZV, HSCT candidates and recipients who are VZV-seronegative, or VZV-seropositive and immunocompromised, should avoid exposure to persons with active VZV infections (123) (AII). HCWs, family members, household contacts, and visitors who are healthy and do not have a reported history of varicella infection or who are VZV-seronegative should receive VZV vaccination before being allowed to visit or have direct contact with an HSCT recipient (AIII). Ideally, VZV-susceptible family members, household contacts, and potential visitors of immunocompromised HSCT recipients should be vaccinated as soon as the decision is made to perform HSCT. The vaccination dose or doses should be completed >4 weeks before the conditioning regimen begins or >6 weeks (42 days) before the HSCT is performed (BIII). HSCT recipients and candidates undergoing conditioning therapy should avoid contact with any VZV vaccine recipient who experiences a rash after vaccination (BIII). When this rash occurs, it usually appears 14--21 days after VZV vaccination (median: 22 days; range: 5--35 days) (personal communication from Robert G. Sharrar, M.D., Merck & Co., Inc.). However, to date, no serious disease has been reported among immuno-compromised patients from transmission of VZV vaccine virus, and the VZV vaccine strain is susceptible to acyclovir. All HSCT recipients with VZV disease should be placed under airborne and contact precautions (62) (AII) to prevent transmission to other HSCT recipients. Contact precautions should be continued until all skin lesions are crusted. Airborne precautions should be instituted 10 days after exposure to VZV and continued until 21 days after last exposure or 28 days postexposure if the patient received varicella-zoster immunoglobulin (VZIG)**** (62) (AI) because a person infected with VZV can be infectious before the rash appears. Preventing Disease VZIG. VZV-seronegative HSCT recipients should be administered VZIG as soon as possible but ideally within 96 hours after close or household contact with a person having either chickenpox or shingles if the HSCT recipient is not immunocompetent (i.e., allogeneic patient <24 months after HSCT, >24 months after HSCT and on immunosuppressive therapy, or having chronic GVHD) (AII). Researchers report VZIG administration for VZV exposure as described for HSCT recipients who were VZV-seropositive before HSCT (CIII). Because of the high morbidity of VZV-associated disease among severely immunocompromised HSCT recipients and until further data are published, HSCT physicians should administer VZIG to all VZV-seronegative HSCT recipients or candidates undergoing conditioning therapy who are exposed to a VZV vaccinee having a varicella-like rash (BIII). Researchers also report VZIG administration for this situation for VZV-seropositive HSCT recipients and candidates undergoing conditioning therapy (CIII). These recommendations are made because the vaccinee might be unknowingly incubating wild-type varicella, particularly during the first 14 days after varicella vaccination, and because vaccine-strain VZV has been rarely transmitted by VZV vaccinees with vesicular rashes postvaccination (121). If VZV-seronegative HSCT recipients or candidates undergoing conditioning therapy are closely exposed to varicella >3 weeks after receiving VZIG, they should be administered another dose of VZIG (120) (BIII). Researchers also recommend VZIG administration for this condition for VZV-seropositive HSCT recipients and candidates undergoing conditioning therapy (CIII). Antiviral Drugs. Any HSCT recipient or candidate undergoing conditioning therapy who experiences a VZV-like rash (particularly after exposure to a person with wild-type varicella or shingles) should receive preemptive intravenous acyclovir until >2 days after all lesions have crusted (BIII) (Appendix). Any HSCT recipient or candidate undergoing conditioning therapy who experiences a VZV-like rash after exposure to a VZV vaccinee with a rash should be administered intravenous acyclovir preemptively to prevent severe, disseminated VZV disease (BII). Acyclovir should be administered until 2 days after all lesions have crusted. Long-term acyclovir prophylaxis to prevent recurrent VZV infection (e.g., during the first 6 months after HSCT) is not routinely recommended (124--126) (DIII); however, this therapy could be considered for use among HSCT recipients with severe, long-term immunodeficiency (CIII). When acyclovir resistance occurs among patients, HSCT physicians should use foscarnet for preemptive treatment of VZV disease (127) (BIII). Researchers report valacyclovir use for preventing HSV among HSCT recipients (CIII). However, preliminary data demonstrate that very high doses of valacyclovir (8 g/day) were associated with thrombotic thrombocytopenic purpura/hemolytic uremic syndrome among HSCT recipients (115). Controlled trial data regarding HSCT recipients are limited (115), and the FDA has not approved valacyclovir for use among HSCT recipients. Physicians wishing to use valacyclovir among HSCT recipients with renal impairment should exercise caution and decrease doses as needed (BIII) (Appendix). No data were found demonstrating safety and efficacy of preemptive treatment of famciclovir against herpes zoster among HSCT recipients. Consequently, no recommendation for its use can be made. Live-Attenuated VZV Vaccine. VZV vaccine use is contraindicated among HSCT recipients <24 months after HSCT (128) (EIII). Use of VZV vaccine among HSCT recipients is restricted to research protocols for recipients >24 months after HSCT who are presumed immunocompetent. Further research is needed to determine the safety, immunogenicity, and efficacy of VZV vaccine among HSCT recipients. Other Recommendations An inactivated VZV vaccine has been used investigationally among HSCT recipients (129); however, more studies are needed before a recommendation regarding its use can be made. Recommendations for VZV prevention are the same for allogeneic or autologous recipients. Recommendations for preventing VZV disease among pediatric or adult HSCT recipients are the same, except that appropriate dose adjustments for VZIG should be made for pediatric HSCT recipients (AIII) (Appendix). Recommendations Regarding CRV Infections: Influenza, Respiratory Syncytial Virus, Parainfluenza Virus, and AdenovirusPreventing Exposure Preventing CRV exposure is critical in preventing CRV disease (130,131). To prevent nosocomial CRV transmission, HSCT recipients and their HCWs should always follow HSCT infection control guidelines (AIII). To minimize the risk for CRV transmission, HCWs and visitors with upper respiratory infection (URI) symptoms should be restricted from contact with HSCT recipients and HSCT candidates undergoing conditioning therapy (AIII). At a minimum, active clinical surveillance for CRV disease should be conducted on all hospitalized HSCT recipients and candidates undergoing conditioning therapy; this clinical surveillance should include daily screening for signs and symptoms of CRV (e.g., URI or lower respiratory infection [LRI]) (AIII). Viral cultures of asymptomatic HSCT candidates are unlikely to be useful. HSCT recipients with URI or LRI symptoms should be placed under contact precautions to avoid transmitting infection to other HSCT candi dates and recipients, HCWs, and visitors until the etiology of illness is identified (62) (BIII). Optimal isolation precautions should be modified as needed after the etiology is identified (AIII). HSCT recipients and candidates, their family members and visitors, and all HCWs should be informed regarding CRV infection control measures and the potential severity of CRV infections among HSCT recipients (130--140) (BIII). Physicians have routinely conducted culture-based CRV surveillance among HSCT recipients; however, the cost effectiveness of this approach has not been evaluated. Influenza vaccination of family members and close or household contacts is strongly recommended during each influenza season (i.e., October--May) starting the season before HSCT and continuing >24 months after HSCT (141) (AI) to prevent influenza exposure among the recipients or candidates. All family members and close or household contacts of HSCT recipients who remain immunocompromised >24 months after HSCT should continue to be vaccinated annually as long as the HSCT recipient's immuno-compromise persists (141) (AI). Seasonal influenza vaccination is strongly recommended for all HCWs of HSCT recipients (142,143) (AI). If HCWs, family members, or other close contacts of HSCT recipients receive influenza vaccination during an influenza A outbreak, they should receive amantadine or rimantadine chemoprophylaxis for 2 weeks after influenza vaccination (BI) while the vaccinee experiences an immunologic response to the vaccine. Such a strategy is likely to prevent transmission of influenza A to HCWs and other close contacts of HSCT recipients, which could prevent influenza A transmission to HSCT recipients themselves. However, if a nosocomial outbreak occurs with an influenza A strain that is not contained in the available influenza vaccine, all healthy family members, close and household contacts, and HCWs of HSCT recipients and candidates should be administered influenza A chemoprophylaxis with amantadine or rimantadine until the end of the outbreak (141) (BIII). In 1999, two neuroaminidase inhibitors (zanamivir and oseltamivir) were approved for treatment of influenza, but are not currently approved for prophylaxis. To date, experience is limited regarding use of zanamivir or oseltamivir in the treatment or prophylaxis of influenza among HSCT settings. However, HCWs, family members, or other close contacts can be offered a neuroaminidase inhibitor (e.g., zanamivir or oseltamivir) using the same strategies outlined previously, if a) rimantadine or amantadine cannot be tolerated, b) the outbreak strain of influenza A is amantadine or rimantadine-resistant, or c) the outbreak strain is influenza B (144--147) (BI). Zanamivir can be administered to persons aged >12 years, and oseltamivir can be administered to persons aged >18 years. Patients with influenza should be placed under droplet and standard precautions (AIII) to prevent transmission of influenza to HSCT recipients. HCWs with influenza should be excused from patient care until they are no longer infectious (AIII). Preventing Disease HSCT physicians should determine the etiology of a URI in an HSCT recipient or candidate undergoing conditioning therapy, if possible, because respiratory syncytial virus (RSV), influenza, parainfluenza, and adenovirus URIs can progress to more serious LRI, and certain CRVs can be treated (BIII). Appropriate diagnostic samples include nasopharyngeal washes, swabs or aspirates, throat swabs, and bronchoalveolar lavage (BAL) fluid. HSCT candidates with URI symptoms at the time conditioning therapy is scheduled to start should postpone their conditioning regimen until the URIs resolve, if possible, because certain URIs might progress to LRI during immunosuppression (131,133,137,138) (BIII). Recommendations Regarding Influenza. Life-long seasonal influenza vaccination is recommended for all HSCT candidates and recipients, beginning during the influenza season before HSCT and resuming >6 months after HSCT (142) (BIII). Influenza vaccinations administered to HSCT recipients <6 months after HSCT are unlikely to be beneficial and are not recommended (142) (DII). HSCT recipients <6 months after HSCT should receive chemoprophylaxis with amantadine or rimantadine during community or nosocomial influenza A outbreaks (BIII). These drugs are not effective against influenza B. Additionally, antiviral-resistant strains of influenza can emerge during treatment with amantadine or rimantadine and transmission of resistant strains can occur (148,149). During such outbreaks, HSCT recipients 6--24 months after HSCT, or >24 months after HSCT and still substantially immunocompromised (i.e., receiving immunosuppressive therapy, have had a relapse of their underlying disease, or have GVHD) and who have not yet received a current influenza vaccination, should be vaccinated against influenza immediately (BIII). Additionally, to allow sufficient time for the patient to experience an immunologic response to influenza vaccine, chemoprophylaxis with amantadine or rimantadine can be used for these HSCT recipients for 2 weeks after vaccination during a nosocomial or community influenza A outbreak (CIII). Influenza A chemoprophylaxis with amantadine or rimantadine has been recommended for all influenza A-exposed HSCT recipients <24 months after HSCT or >24 months after HSCT and substantially immunocompromised regardless of vaccination history, because of their likely suboptimal immunologic response to influenza vaccine (142,143). However, no recommendation regarding such chemoprophylaxis can be made because of lack of data. To prevent severe disease, early preemptive therapy with amantadine or rimantadine has been reported for HSCT recipients with unexplained acute URI or LRI symptoms during a community or nosocomial outbreak of influenza A (141). However, the effectiveness in preventing influenza-related complications and the safety of this strategy have not been evaluated among HSCT recipients. Therefore, data are insufficient to make a recommendation. Neuroaminidase inhibitors (zanimivir and oseltamivir), intravenous and aerosol ribavirin, and combination drug therapy (e.g., rimantadine or amantadine with ribavirin or interferon) (143,150--153) have been proposed for investigational, preemptive treatment to prevent severe influenza disease among HSCT recipients. However, because of lack of data, no recommendation for use of these strategies among HSCT recipients can be made. Recommendations Regarding RSV. Respiratory secretions of any hospitalized HSCT candidate or recipient who experiences signs or symptoms of CRV infection should be tested promptly by viral culture and rapid diagnostic tests for RSV (BIII). If two diagnostic samples taken >2 days apart do not identify a respiratory pathogen despite persistence of respiratory symptoms, BAL and further testing are advised (BIII). This testing is critical because of the high morbidity and case fatality of RSV disease among HSCT recipients (154,155). HSCT recipients, particularly those who are preengraftment and at highest risk for severe RSV pneumonia, should have their illness diagnosed early (i.e., during RSV URI), and their illness should be treated aggressively to prevent fatal RSV disease (BIII). Although a definitive, uniformly effective preemptive therapy for RSV infection among HSCT recipients has not been identified, certain strategies have been proposed, including use of aerosolized ribavirin (155,156), RSV antibodies (i.e., passive immunization with high RSV-titered IVIG or RSV immunoglobulin) in combination with aerosolized ribavirin (137,157), and RSV monoclonal antibody (158). Clinical trials are currently underway to evaluate the efficacy of these strategies. No recommendation regarding the optimal method for RSV prevention and preemptive therapy can be made because of limited data. Further, current data do not support use of intravenous ribavirin for preemptive therapy for RSV pneumonia among HSCT recipients (60) (DIII), and no commercially licensed vaccines against RSV are currently available. Recommendations Regarding Parainfluenza Virus and Adenovirus. Immuno-prophylaxis, chemoprophylaxis, and preemptive treatment for parainfluenza virus and adenovirus infections among HSCT recipients have been proposed (159,160). However, no recommendation can be made in these guidelines because of insufficient data. No commercially licensed vaccines against parainfluenza or adenovirus are currently available. Other Disease Prevention Recommendations The recommendations for preventing CRV infections and their recurrence are the same for allogeneic or autologous recipients. Generally, these recommendations apply to children or adults (161--164), but with appropriate adjustments in antiviral drug and influenza vaccine doses for children (Appendix). For pediatric HSCT recipients and candidates aged >6 months, annual seasonal influenza vaccination is recommended HSCT (BIII). Children aged <9 years who are receiving influenza vaccination for the first time require two doses administered >1 months apart (AI). Healthy children who receive influenza vaccination for the first time might not generate protective antibodies until 2 weeks after receipt of the second dose of influenza vaccine. Therefore, during an influenza A outbreak, pediatric recipients aged <9 years, >6 months after HSCT, and receiving their first influenza vaccination, should be administered >6 weeks of influenza A chemoprophylaxis after the first dose of influenza vaccine (141) (BIII) (Appendix). Amantadine and rimantadine are not FDA-approved for children aged <1 year (141,161) (DIII). To prevent RSV disease, researchers report substituting RSV-IVIG for IVIG during RSV season (i.e., November--April) for pediatric recipients (i.e., children aged <18 years) who receive routine IVIG therapy (164) (i.e., those with hypogammaglobulinemia) (CIII) (Appendix). Other researchers report that pediatric recipients with RSV can be considered for preemptive therapy (e.g., during URI or early LRI) with aerosolized ribavirin (CIII), although this therapy remains controversial (164) (Appendix). Droplet and contact precautions for the duration of illness are required for pediatric recipients for the duration of adenovirus (62) (AIII). FUNGAL INFECTIONSGeneral RecommendationsPreventing Exposure Limited data were found that demonstrate to what extent preventing fungal exposures is effective in preventing infection and disease. However, HSCT recipients and candidates undergoing conditioning therapy have been advised to avoid contact with certain areas and substances, including foods, that might increase a patient's risk for fungal exposures (CII). Specific precautions have included avoiding areas of high dust exposure (e.g., excavation sites, areas of building construction or renovation, chicken coops, and caves), occupations involving soil, and foods that contain molds (e.g., blue cheese). Preventing Disease Growth factors (e.g., GM-CSF and G-CSF) shorten the duration of neutropenia after HSCT (165); however, no data were found that indicate which growth factors effectively reduce the attack rate of invasive fungal disease. Therefore, no recommendation for use of growth factors solely for prophylaxis against invasive fungal disease can be made. Topical antifungal drugs, which are applied to the skin or mucosa (e.g., nystatin or clotrimazole), might reduce fungal colonization in the area of application. However, these agents have not been proven to prevent generation of locally invasive or disseminated yeast infections (e.g., candidiasis) or mold infections (e.g., aspergillosis) and are not recommended for their prophylaxis (DII). Performing fungal surveillance cultures is not indicated for asymptomatic HSCT recipients (166,167) (DII), but cultures should be obtained from symptomatic HSCT recipients (BIII). Recommendations Regarding Yeast InfectionsPreventing Exposure Invasive candidiasis is usually caused by dissemination of endogenous Candida species that have colonized a patient's gastrointestinal tract (168). Consequently, methods of preventing exogenous yeast exposure usually do not prevent invasive yeast infections after HSCT. However, because Candida species can be carried on the hands, HCWs and others in contact with HSCT recipients should follow appropriate hand-washing practices to safeguard patients from exposure (AIII). Preventing Disease Allogeneic recipients should be administered fluconazole prophylaxis to prevent invasive disease with fluconazole-susceptible Candida species during neutropenia, particularly among centers where Can. albicans is the predominant cause of invasive fungal disease preengraftment (AI) (Appendix). Because candidiasis occurs during phase I (169), fluconazole (400 mg/day by mouth or intravenously) should be administered (169,170) from the day of HSCT until engraftment (AII). However, fluconazole is not effective against certain Candida species, including Can. krusei (171) and Can. glabrata and is, therefore, not recommended for their prevention (DI). Further studies are needed to determine the optimal duration of fluconazole prophylaxis. Preliminary studies have reported that low-dose fluconazole prophylaxis (100--200 mg/day by mouth) among neutropenic patients has variable efficacy in preventing candidiasis (172). Therefore, this therapy is not recommended for HSCT recipients (DII). Oral, nonabsorbable antifungal drugs, including oral amphotericin B (500 mg suspension every 6 hours), nystatin, and clotrimazole troches, might reduce superficial colonization and control local mucosal candidiasis, but have not been demonstrated to reduce invasive candidiasis (CIII). Other Recommendations HSCT candidates with candidemia or invasive candidiasis can safely receive transplants (173) if a) their infection was diagnosed early and treated immediately and aggressively with amphotericin B or alternatively with appropriate doses of fluconazole if the organism is susceptible; and b) evidence of disease control is reported (e.g., by serial computed tomography scans) before the transplant (BIII). Such patients should continue receiving therapeutic doses of an appropriate antifungal drug throughout phase I (BII) and until a careful review of clinical, laboratory, and serial computed tomography scans verifies resolution of candidiasis (BII). Because autologous recipients generally have an overall lower risk for invasive fungal infection than allogeneic recipients, certain autologous recipients do not require routine antiyeast prophylaxis (DIII). However, researchers recommend administering antiyeast prophylaxis to a subpopulation of autologous recipients with underlying hematologic malignancies (e.g., lymphoma or leukemia) and who have or will have prolonged neutropenia and mucosal damage from intense conditioning regimens or graft manipulation, or have received fludarabine or 2-CDA recently (BIII). Recommendations regarding preventing invasive yeast infections among pediatric or adult HSCT recipients are the same, except that appropriate dose adjustments for prophylactic drugs should be made for pediatric recipients (Appendix). Recommendations Regarding Mold InfectionsPreventing Exposure Nosocomial mold infections among HSCT recipients result primarily from respiratory exposure to and direct contact with fungal spores (174). Ongoing hospital construction and renovation have been associated with an increased risk for nosocomial mold infection, particularly aspergillosis, among severely immunocompromised patients (175--177). Therefore, whenever possible, HSCT recipients who remain immunocompromised should avoid hospital construction or renovation areas (AIII). When constructing new HSCT centers or renovating old ones, hospital planners should ensure that rooms for HSCT patients have an adequate capacity to minimize fungal spore counts through use of
Preventing Disease No regimen has been reported to be clearly effective or superior in preventing aspergillosis, and therefore, no recommendation can be made. Further studies are needed to determine the optimal strategy for aspergillosis prevention. Moderate-dose (0.5 mg/kg/day) amphotericin B (181--184), low-dose (0.1--0.25 mg/kg/day) amphotericin B (185--187), intranasal amphotericin B spray (188), lipid formulations of amphotericin B (182,189), and aerosolized amphotericin B (190) have been administered for aspergillosis prophylaxis, but data are limited regarding the safety and efficacy of these formulations among HSCT recipients. Additionally, itraconazole capsules are not recommended for fungal prophylaxis among HSCT recipients (191) (DII) for three reasons. First, itraconazole capsules are poorly absorbed gastrointestinally, particularly among patients who are fasting (192) or receiving cytotoxic agents (193). Second, persons taking itraconazole capsules do not achieve steady-state serum levels for 2 weeks (188,194), and when achieved, these levels are lower than the average Aspergillus species minimum inhibitory concentration (MIC) among HSCT recipients (195). Third, itraconazole has adverse interactions with other drugs (e.g., antiepileptics, rifampin, oral hypoglycemics, protease inhibitors, vinca alkaloids, cyclosporine, methylprednisolone, and warfarin-like anticoagulants) (196). Trials assessing the efficacy of the recently licensed cyclodextrin oral solution and intravenous formulations of itraconazole in preventing invasive fungal disease among HSCT recipients are in progress; however, no recommendations regarding its use for Aspergillus species infection prophylaxis can be made. For HSCT recipients whose respiratory specimens are culture positive for Aspergillus species, acute invasive aspergillosis should be diagnosed presumptively (197) and treated preemptively and aggressively (e.g., with intravenous amphotericin) (AIII). The risk for aspergillosis recurrence has been high among allogeneic recipients with preexisting invasive aspergillosis. Previously, allogeneic HSCTs were avoided among persons with uncontrolled, proven aspergillosis. However, HSCT center personnel have recently reported successful allogeneic or autologous HSCT among a limited number of persons who have had successfully treated, prior invasive pulmonary aspergillosis (198--200). Because of limited data, no recommendations regarding strategies for preventing aspergillosis recurrence can be made. PROTOZOAL AND HELMINTHIC INFECTIONSRecommendations Regarding PCPPreventing Exposure Although a possible cause of PCP is reactivation of latent infection among immunocompromised persons, cases of person-to-person transmission of PCP have been reported (201--206). Generally, standard precautions should be used for patients with PCP (62) (BIII), but researchers have reported patients with PCP being isolated (201,204) and contact precautions being used if evidence existed of person-to-person transmission in the institution (CIII). This subject remains controversial, and until further data are published, HSCT recipients should avoid exposure to persons with PCP (62) (CIII). Preventing Disease and Disease Recurrence Physicians should prescribe PCP prophylaxis for allogeneic recipients throughout all periods of immunocompromise (207) after engraftment. Prophylaxis should be administered from engraftment until 6 months after HSCT (AII) for all patients, and >6 months after HSCT for the duration of immunosuppression for those who a) are receiving immunosuppressive therapy (e.g. prednisone or cyclosporine) (AI), or b) have chronic GVHD (BII). However, PCP prophylaxis can be initiated before engraftment if engraftment is delayed (CIII). Researchers report an additional 1- to 2-week course of PCP prophylaxis before HSCT (i.e., day --14 to day --2) (CIII). Preferred PCP prophylaxis is TMP-SMZ (AII); however, if TMP-SMZ is administered before engraftment, the associated myelosuppression could delay engraftment, and patients might experience sensitivity to the drug. Every effort should be made to keep such patients on the drug, including assessment of desensitization therapy, although data regarding this technique among HSCT recipients are limited. For patients who cannot tolerate TMP-SMZ, physicians can choose to use alternative PCP prophylaxis regimens (e.g., dapsone) (208) (BIII). Use of aerosolized pentamidine (209) is associated with the lowest PCP prevention rates and should only be used if other agents cannot be tolerated. Atovaquone is a possible alternative drug for PCP prophylaxis among dapsone-intolerant persons with HIV infection (210); however, no recommendation regarding use of atovaquone among HSCT recipients can be made because of lack of data. Although data are limited, concomitant use of leucovorin (folinic acid) and TMP-SMZ is not recommended (211,212) (DIII). A patient's history of PCP should not be regarded as a contraindication to HSCT (213) (DIII). Recurrent PCP among HSCT recipients is rare; however, patients with continued immunosuppression should remain on PCP prophylaxis until their immunosuppression is resolved (AI). The regimen recommended for preventing toxoplasmosis recurrence among HSCT recipients (i.e., TMP-SMZ) will also prevent PCP recurrence. Other Recommendations PCP prophylaxis should be considered for autologous recipients who have underlying hematologic malignancies (i.e., lymphoma or leukemia), are receiving intense conditioning regimens or graft manipulation, or have recently received fludarabine or 2-CDA (207,214) (BIII). PCP prophylaxis should be administered >6 months after HSCT if substantial immunosuppression or immunosuppressive therapy (e.g., steroids) persists (CIII). Use of PCP prophylaxis among other autologous recipients is controversial (CIII). Generally, indications for PCP prophylaxis are the same among children or adults, but pediatric doses should be used (Appendix). Recommendations Regarding Toxoplasma gondiiPreventing Exposure All HSCT recipients should be provided information regarding strategies to reduce their risk for Toxoplasma species exposure. Researchers report that potential donors for allogeneic HSCT be tested for To. gondii antibodies (215,216) by using FDA-licensed or -approved screening tests that include IgG antibody testing because To. gondii has been reported to be transmitted by leukocyte transfusion (217) and HSCT (218,219) (CIII). Preventing Disease and Disease Recurrence Because most toxoplasmosis among HSCT recipients is caused by disease reactivation, researchers report that candidates for allogeneic HSCT can be tested for IgG antibody to determine whether they are at risk for disease reactivation after HSCT (215,216,218) (CIII). However, the value of such testing is controversial because a limited number of patients who were seronegative for To. gondii pretransplant experienced the infection posttransplant (220). If testing is performed, only FDA-licensed or -approved screening tests should be used. Researchers recommend toxoplasmosis prophylaxis for seropositive allogeneic recipients with active GVHD or a prior history of toxoplasmic chorioretinitis (221,222), but data demonstrating efficacy are limited (CIII). The optimal prophylactic regimen for toxoplasmosis among HSCT recipients has not been determined, but a proposed drug is TMP-SMZ (BII), although allogeneic recipients have experienced break-through clinical disease despite TMP-SMZ prophylaxis (218). For patients who are TMP-SMZ--intolerant, a combination of clindamycin, pyramethamine, and leucovorin can be substituted for To. gondii prophylaxis (Appendix). After therapy for toxoplasmosis, HSCT recipients should continue receiving suppressive doses of TMP-SMZ or an alternate regimen for the duration of their immunosuppression (BIII) (Appendix). Other Recommendations Recipients of autologous transplants are at negligible risk for toxoplasmosis reactivation (218). No prophylaxis or screening for toxoplasmosis infection is recommended for such patients (DIII). Indications for toxoplasmosis prophylaxis are the same among children or adults, but pediatric doses should be used among children (Appendix). Recommendations Regarding Strongyloides stercoralisPreventing Exposure Allogeneic recipients should avoid contact with outhouses and cutaneous exposure to soil or other surfaces that might be contaminated with human feces (223) (AIII). Allogeneic recipients who work in settings (e.g., hospitals or institutions) where they could be exposed to fecal matter should wear gloves when working with patients or in areas with potential fecal contamination (AIII). Preventing Disease and Disease Recurrence Travel and residence histories should be obtained for all patients before HSCT to determine any exposures to high-risk areas (e.g., such moist temperate areas as the tropics, subtropics, or the southeastern United States and Europe) (223) (BIII). HSCT candidates who have unexplained peripheral eosinophilia or who have resided in or traveled to areas endemic for strongyloidiasis, even during the distant past, should be screened for asymptomatic strongyloidiasis before HSCT (BIII). Serologic testing with an enzyme-linked immunosorbent assay is the preferred screening method and has a sensitivity and specificity of >90% (223,224) (BIII). FDA-licensed or -approved screening tests should be used. Although stool examinations for strongyloidiasis are specific, the sensitivity obtained from >3 stool examinations is 60%--70%; the sensitivity obtained from concentrated stool exams is, at best, 80% (223). A total of >3 stool examinations should be performed if serologic tests are unavailable or if strongyloidiasis is clinically suspected in a seronegative patient (BIII). HSCT candidates whose screening tests before HSCT are positive for Strongyloides species, and those with an unexplained eosinophilia and a travel or residence history indicative of exposure to Strongyloides stercoralis should be empirically treated before transplantation (225,226), preferably with ivermectin (BIII), even if seronegative or stool-negative (Appendix). To prevent recurrence among HSCT candidates with parasitologically confirmed strongyloidiasis, cure after therapy should be verified with >3 consecutive negative stool examinations before proceeding with HSCT (AIII). Data are insufficient to recommend a drug prophylaxis regimen after HSCT to prevent recurrence of strongyloidiasis. HSCT recipients who had strongyloidiasis before or after HSCT should be monitored carefully for signs and symptoms of recurrent infection for 6 months after treatment (BIII). Other Recommendations Hyperinfection strongyloidiasis has not been reported after autologous HSCT; however, the same screening precautions should be used among autologous recipients (BIII). Indications for empiric treatment for strongyloidiasis before HSCT are the same among children or adults except for children weighing <15 kg, for whom the preferred drug is thiabendazole (BIII) (Appendix). Recommendations Regarding Trypanosoma cruziPreventing Exposure HSCT physicians should be aware that Trypanosoma cruzi, the etiologic agent of Chagas' disease, can be transmitted congenitally, through blood transfusion (227), and possibly through HSCT. Additionally, treatment for persons infected with Tr. cruzi is not always effective, even during the acute stage of infection (227). Therefore, potential donors who were born, received a blood transfusion, or ever lived for >6 months in a Chagas' disease endemic area (e.g., parts of South and Central America and Mexico) should be screened serologically for anti-Tr. cruzi serum IgG antibody (228) (BIII). Persons who lived <6 months in a Chagas'-endemic area but who had high-risk living conditions (e.g., having had extensive exposure to the Chagas' disease vector --- the reduviid bug --- or having lived in dwellings with mud walls, unmilled logs and sticks, or a thatched roof) should also be screened for evidence of Tr. cruzi infection (BIII). Because Chagas' disease can be transmitted congenitally, researchers report that any person with extensive multigenerational maternal family histories of cardiac disease (e.g., cardiomegaly and arrhythmias) should be screened serologically for serum IgG anti-Tr. cruzi antibodies (227) (CIII). To decrease the risk for misdiagnosis by false-positive or false-negative serologic tests, Tr. cruzi screening should consist of >2 conventional serologic tests (e.g., enzyme immunoassay, indirect hemagglutination, indirect fluorescent antibody) or >1 conventional serologic tests, followed by a confirmatory serologic test (e.g., radioimmunoprecipitation assay) (229) (BIII). Persons with active Chagas' disease should not serve as HSCT donors (DIII). Researchers also recommend deferral of HSCT donation for a past history of Chagas' disease (CIII). Preventing Disease HSCT candidates who are at risk for being infected with Tr. cruzi should be screened for serum IgG anti-Tr. cruzi antibody (228) (BIII). Tr. cruzi seropositivity is not a contraindication to HSCT (228,230). However, if an acute illness occurs in a Tr. cruzi-seropositive HSCT recipient, particularly during neutropenia, Tr. cruzi reactivation should be included in the differential diagnosis (230) (BIII). Researchers have proposed use of beznidazole or nifurtimox for preemptive therapy or prophylaxis of recurrent Tr. cruzi among seropositive HSCT recipients (230,231), but insufficient data were found to make a recommendation.***** Other Recommendations Recommendations are the same for autologous or allogeneic recipients. However, recurrence of Chagas' disease is probably less likely to occur among autologous recipients because of the shorter duration of immunosuppression. Recommendations are the same among children or adults. HOSPITAL INFECTION CONTROLRoom VentilationHSCT center personnel should follow published guidelines for hospital room design and ventilation (140,180) (BIII). HSCT centers should also prevent birds from gaining access to hospital air-intake ducts (140,174) (AII). All allogeneic recipients should be placed in rooms with >12 air exchanges/hour (232,233) and point-of-use HEPA filters that are capable of removing particles >0.3 µm in diameter (140,178,180,233) (AIII). Correct filtration is critical in HSCT centers with ongoing construction and renovation (179). When portable HEPA filters are used as adjuncts to the primary ventilation system, they must be placed centrally in patient rooms so that space is available around all surfaces to allow free air circulation (BIII). The need for environmental HEPA filtration for autologous recipients has not been established. However, HEPA-filtered rooms should be evaluated for autologous recipients if they experience prolonged neutropenia, a substantial risk factor for nosocomial aspergillosis (CIII). A laminar air flow (LAF) room contains filtered air that moves in parallel, unidirectional flow --- the air enters the room from one wall and exits the room on the opposite wall (232). Although LAF has been demonstrated to protect patients from infection during aspergillosis outbreaks related to hospital construction (234,235), the value of routine LAF room use for all HSCT recipients is doubtful because substantial overall survival benefit has not been reported (236). During 1983, LAF rooms were preferred for allogeneic recipients with aplastic anemia and HLA-identical sibling donors because use of regular rooms was associated with a mortality rate that was approximately four times higher than for those recipients treated in LAF rooms (237). However, the survival of aplastic anemia HSCT recipients during the late 1990s exceeds that reported during the early 1980s, and no studies have been done to determine whether HSCT recipients with aplastic anemia still have an improved survival rate when treated in an LAF room. Therefore, HSCT centers need not construct LAF rooms for each HSCT recipient. Use of LAF rooms, if available, is optional (CII). Hospital rooms should have directed airflow so that air intake occurs at one side of the room and air exhaust occurs at the opposite side (140) (BIII). Each hospital room should also be well-sealed (e.g, around windows and electrical outlets) (140) (BIII). To provide consistent positive pressure in the recipient's room, HSCT centers should maintain consistent pressure differentials between the patient's room and the hallway or anteroom at >2.5 Pa (i.e., 0.01 inches by water gauge) (232,233) (BIII). Generally, hospital rooms for HSCT recipients should have positive room air pressure when compared with any adjoining hallways, toilets, and anterooms, if present. Anterooms should have positive air pressure compared with hallways (180). An exception is the HSCT recipient with an active disease that has airborne transmission (e.g., pulmonary or laryngeal Mycobacteria tuberculosis [TB] or measles). These HSCT patients should be placed in negative isolation rooms (62) (BIII), and a room with an anteroom is recommended for such patients (180) (BIII). Whenever possible, HSCT centers should have self-closing doors to maintain constant pressure differentials among the HSCT recipients' room and anterooms, if available, and hallways (233) (BIII). To enable the nursing staff to observe the HSCT recipient even when the doors are closed, windows can be installed in either the door or the wall of the HSCT recipient's room (233) (CIII). HSCT centers should provide backup emergency power and redundant air-handling and pressurization systems to maintain a constant number of air exchanges and room pressurization in the center when the central ventilation system is shut off for maintenance and repair (238) (BIII). Additionally, infection control personnel should work with maintenance personnel to develop protocols to protect HSCT centers at all times from bursts of mold spores that might occur when air-handling systems are restarted after routine maintenance shut-downs (BIII). Construction, Renovation, and Building CleaningConstruction and Renovation Hospital construction and renovation have been associated with an increased risk for nosocomial fungal infection, particularly aspergillosis, among severely immuno-compromised patients (175,176). Therefore, persons responsible for HSCT center construction or renovation should consult published recommendations regarding environmental controls during construction (239,240) (AIII). Whenever possible, HSCT recipients, HCWs, and visitors should avoid construction or renovation areas (240) (AIII). Also, equipment and supplies used by HSCT recipients or their HCWs should not be exposed to construction or renovation areas (240). When planning for construction or renovation, the HSCT center should include plans for intensified aspergillosis-control measures (AIII). Construction and renovation infection control planning committees should include engineers, architects, housekeeping staff, infection control personnel, the director of the HSCT center, the administration, and safety officers (241) (BIII). When constructing new HSCT centers, planners should ensure that patient rooms will have adequate capacity to minimize fungal spore counts by following room ventilation recommendations. During outdoor construction and demolition, the intake air should be sealed (BIII), if possible; if not, filters should be checked frequently. Additionally, to protect HSCT patient care areas during fire drills and emergencies, weather stripping should be placed around stairwell doors, or alternatively, the stairwell air should be filtered to the level of safety of the adjacent hospital air (BIII). False ceilings should be avoided whenever possible (174) (BII). If use of false ceilings cannot be avoided, the area above false ceilings should be vacuumed routinely to minimize dust and, therefore, fungal exposure to patients (174) (BIII). During hospital construction or renovation, hospitals should construct rigid, dust-proof barriers with airtight seals (242) between patient care and construction or renovation areas to prevent dust from entering patient care areas; these barriers (i.e., sealed drywall) should be impermeable to Aspergillus species (140,175,176,179,240) (BIII). If impervious barriers cannot be created around the construction or renovation area, patients should be moved from the area until renovation or construction is complete and the area has been cleaned appropriately (176) (BIII). HSCT centers should direct pedestrian traffic occurring near construction or renovation areas away from patient care areas to limit the opening and closing of doors or other barriers that might cause dust dispersion, entry of contaminated air, or tracking of dust into patient areas (140), particularly those in the HSCT center (176) (BIII). If possible, specific corridors, entrances, and exits should be dedicated to construction use only (240). An elevator to which patients do not have access also should be dedicated to construction use only (240). Construction workers, whose clothing might be contaminated with Aspergillus species spores, should use the construction elevator and avoid contact with patients, patient care areas, other elevators, and nonconstruction areas (BIII). Hospital construction or renovation areas should have negative air pressure relative to that in adjacent patient care areas, if no contraindications exist for such pressure differential (140,176,179,240,242) (BIII). Ideally, air from the construction or renovation areas should be exhausted to the outside of the hospital (176) (BIII) or if recirculated, it should be HEPA-filtered first (BIII). Researchers have proposed that HSCT recipients wear the N95 respirator to prevent mold exposure during transportation near hospital construction or renovation areas (CIII) because the N95 respirators are regarded as effective against any aerosol. However, to be maximally effective, N95 respirators must be fit-tested and all users must be trained. With correct personnel fit-testing and training, N95 respirators reliably reduce aerosol exposure by 90%. Without fit-testing and training, aerosol exposure would be reduced but not necessarily by 90% (243). For patients who cannot use or tolerate an N95 respirator, researchers have proposed using the powered air purifying respirator (244,245), which can be used by patients in wheelchairs. Limitations of the powered air purifying respirator include its cost and that it is not appropriate for young children and infants. General limitations of using respirators are that no commercially available respi rator, including N95, has been tested specifically for its efficacy in reducing exposure to Aspergillus species in hospital construction or renovation areas, and no studies have been done that assess the usefulness and acceptability of using respirators among HSCT recipients. Standard surgical masks provide negligible protection against mold spores and are not recommended for this indication (DIII). Newly constructed or renovated areas should be cleaned before patients are allowed to enter them (140,176) (AIII). Decontamination of fungal-contaminated areas that cannot be extracted and replaced should be done using copper-8-quinolate (179) (BIII). Also, areas above false ceilings located under or adjacent to construction areas should be vacuumed (174) (BIII). Additionally, the ventilation, direction of airflow, and room pressurization should be tested and correctly adjusted before patients are allowed to enter (BIII). Cleaning HSCT centers should be cleaned >1 times/day with special attention to dust control (BIII). Exhaust vents, window sills, and all horizontal surfaces should be cleaned with cloths and mop heads that have been premoistened with an FDA- or Environmental Protection Agency (EPA)-registered hospital disinfectant (BIII). Thorough cleaning during and after any construction activity, including minor renovation projects, is critical (BIII). HSCT center personnel should prohibit exposures of patients to such activities as vacuuming or other floor or carpet vacuuming that could cause aerosolization of fungal spores (e.g., Aspergillus species) (140) (AIII). Accordingly, doors to patient rooms should be closed when vacuuming HSCT center corridors. All vacuum cleaners used in the HSCT center should be fitted with HEPA filters. An FDA- or EPA-registered disinfectant (246,247) should be used daily for environmental disinfection and when wet vacuuming is performed in the HSCT center (BIII). If an HSCT center provides care for infants, phenolic disinfectants can be used to clean the floors only if the compound is diluted according to the product label; but phenolic compounds should not be used to clean basinets or incubators (246) (DIII). Water leaks should be cleaned up and repaired as soon as possible but within 72 hours to prevent mold proliferation in floor and wall coverings, ceiling tiles, and cabinetry in and around all HSCT patients care areas (BIII). If cleanup and repair are delayed >72 hours after the water leak, the involved materials should be assumed to contain fungi and handled accordingly. Use of a moisture meter to detect water penetration of walls should be used whenever possible to guide decision-making (238) (BIII). For example, if the wall does not have <20% moisture content >72 hours after water penetration, it should be removed (BIII). Design and selection of furnishings should focus on creating and maintaining a dust-free environment. Flooring and finishes (i.e., wall coverings, window shades, and countertops) used in HSCT centers should be scrubbable, nonporous, easily disinfected, and they should collect minimal dust (BIII). Isolation and Barrier PrecautionsHSCT center personnel should follow published guidelines for hospital isolation practices, including CDC guidelines for preventing nosocomial infections (62,140,248) (AIII). However, the efficacy of specific isolation and barrier precautions in preventing noso-comial infections among HSCT recipients has not been evaluated. HSCT recipients should be placed in private (i.e., single-patient) rooms (BIII). If contact with body fluids is anticipated, standard precautions should be followed (AIII). These precautions include hand washing and wearing appropriate gloves, surgical masks or eye and face protection, and gowns during procedures and activities that are likely to generate splashes or sprays of blood, body fluids, secretions or excretions, or cause soiling of clothing (62). When indicated, HSCT recipients should also be placed on airborne, droplet, or contact precautions in addition to standard precautions (62) (AIII). Careful observation of isolation precautions is critical in preventing transmission of infectious agents among HSCT recipients, HCWs, visitors, and other HSCT recipients. Physicians are cautioned that HSCT recipients might have a prolonged or episodic excretion of organisms (e.g., CMV). Researchers have proposed that HSCT recipients wear surgical mask and gloves when exiting their hospital rooms before engraftment (CIII). All HSCT recipients who are immunocompromised (phases I--III of immune system recovery) and candidates undergoing conditioning therapy should minimize the time spent in crowded areas of the hospital (e.g., waiting areas and elevators) (BIII) to minimize potential exposure to persons with CRV infections. Hand HygieneHand washing is the single-most critical and effective procedure for preventing nosocomial infection (62). All persons, but particularly HCWs, should wash their hands before entering and after leaving the rooms of HSCT recipients and candidates undergoing conditioning therapy (62,249) or before and after any direct contact with patients regardless of whether they were soiled from the patient, environment, or objects (AI). HSCT recipients should be encouraged to practice safe hand hygiene (e.g., washing hands before eating, after using the toilet, and before and after touching a wound) (BIII). Hand washing should be done with an antimicrobial soap and water (AIII); alternatively, use of hygienic hand rubs is another acceptable means of maintaining hand hygiene (250,251). If gloves are worn, HCWs should put them on in the patient's room after hand washing and then discard them in the same patient's room before washing hands again after exiting the room. When worn, gloves should always be changed between patients or when soiled before touching a clean area (e.g., change gloves after touching the perineum and before going to a "clean" area) (AIII). Appropriate gloves should be used by all persons when handling potentially contaminated biological materials (AII). Items worn on the hands and fingers (e.g., rings or artificial nails [248,252]) and adhesive bandage strips, can create a nidus for pathogenic organisms that is difficult to clean. Thus, HCWs should avoid wearing such items whenever possible (BII). EquipmentAll HSCT center personnel should sterilize or disinfect and maintain equipment and devices using only EPA-registered compounds as directed by established guidelines (140,180,246,247,253--256) (AIII). HSCT center personnel should monitor opened and unopened wound-dressing supplies (e.g., adhesive bandages [257,258] and surgical and elastic adhesive tape [259]) to detect mold contamination and prevent subsequent cutaneous transmission to patients (BII). Monitoring should consist of discarding all bandages and wound dressings that are out of date, have damaged packaging, or are visually contaminated by construction debris or moisture (BIII). When arm boards are used to provide support for intravenous lines, only sterile dressing materials should be used (260), and arm boards should be changed frequently (e.g., daily) (BIII). Additionally, unsterile tongue depressors inserted into a piece of foam tubing should not be used as splints for intravenous and arterial catheter sites because these have been associated with an outbreak of fatal invasive nosocomial Rhizopus microsporus among preterm (i.e., very low-birth--weight) infants (261) (DII). HSCT centers should not install carpeting in hallways outside (DII) or in patient rooms (DIII) because contaminated carpeting has been associated with outbreaks of aspergillosis among HSCT recipients (262,263). Plants, Play Areas, and ToysAlthough to date, exposure to plants and flowers has not been conclusively reported to cause fungal infections among HSCT recipients, most researchers strongly recommend that plants and dried or fresh flowers should not be allowed in the rooms of hospitalized HSCT candidates undergoing conditioning therapy and HSCT recipients (phases I--III of immune system recovery) because Aspergillus species have been isolated from the soil of potted ornamental plants (e.g., cacti), the surface of dried flower arrangements, and fresh flowers (140,174,178,264) (BIII). Play areas for pediatric HSCT recipients and candidates undergoing conditioning therapy should be cleaned and disinfected >1 times/week and as needed (BIII). Only toys, games, and videos that can be kept clean and disinfected should be allowed in the HSCT center (BIII). HSCT centers should follow published recommendations for washing and disinfecting toys (265) (BIII). All HSCT center toys, games, and videos should be routinely and thoroughly washed or wiped down when brought into the HSCT center and thereafter >1 times/week and as needed by using a nontoxic FDA- or EPA-registered disinfectant (246,247,265) followed by a water rinse (BIII). Cloth or plush toys should be washed in a hot cycle of a washing machine or dry-cleaned >1 times/week and as needed (BIII). Alternatively, machine washing in a cold cycle is acceptable if laundry chemicals for cold water washing are used in proper concentration (265). Hard plastic toys should be scrubbed with warm soapy water using a brush to clean crevices, rinsed in clean water, immersed in a mild bleach solution, which should be made fresh daily, for 10--20 minutes, rinsed again, and allowed to air dry (246). Alternatively, hard plastic toys can be washed in a dishwasher or hot cycle of a washing machine (BIII). Broviac dolls****** should be disassembled upon completion of play and washed with a nontoxic FDA- or EPA-registered disinfectant (246,247), rinsed with tap water, and allowed to air dry before other children are allowed to play with them (BIII). Toys that cannot be washed, disinfected, or dry-cleaned after use should be avoided (BIII). Infants, toddlers, and children who put toys in their mouths should not share toys (265) (DIII). For children in isolation, researchers recommend the following:
HCWsHSCT center personnel should have a written comprehensive policy regarding their immunizations and vaccinations, and that policy should meet current CDC, Advisory Committee on Immunization Practices, and Healthcare Infection Control Practices Advisory Committee recommendations (267) (BIII). Immunizations are needed to prevent transmission of vaccine-preventable diseases to HSCT recipients and candidates undergoing conditioning therapy. All HCWs with diseases transmissible by air, droplet, and direct contact (e.g., VZV, infectious gastroenteritis, HSV lesions of lips or fingers, and URIs) should be restricted from patient contact and temporarily reassigned to other duties (AI). HSCT center personnel should follow published recommendations regarding the duration of work restrictions for HCWs with infectious diseases (268,269) (BIII). HSCT center HCWs with bloodborne viruses (e.g., HIV or hepatitis B or C viruses) should not be restricted from patient contact (DIII) as long as they do not perform procedures that pose a high risk for injury that could result in patient exposure to the HCW's blood or body fluids. Work exclusion policies should be designed to encourage HCWs to report their illnesses or exposures (AII). HSCT Center VisitorsHospitals should have written policies for screening HSCT center visitors, particularly children, for potentially infectious conditions. Such screening should be performed by clinically trained HCWs (BII). Visitors who might have communicable infectious diseases (e.g., URIs, flu-like illnesses, recent exposure to communicable diseases, an active shingles rash whether covered or not, a VZV-like rash within 6 weeks of receiving a live-attenuated VZV vaccine, or a history of receiving an oral polio vaccine within the previous 3--6 weeks) should not be allowed in the HSCT center or allowed to have direct contact with HSCT recipients or candidates undergoing conditioning therapy (AII). No absolute minimum age requirement for HSCT center visitors exists; however, all visitors must be able to understand and follow appropriate hand washing and isolation precautions (AIII). The number of HSCT center visitors at any one time should be restricted to a number that permits the nursing staff to perform appropriate screening for contagious diseases and adequate instruction and supervision of hand washing, glove and mask use, and biosafety precautions (BIII). Patient Skin and Oral CareTo optimize skin care, HSCT recipients should take daily showers or baths during and after transplantation (BIII), using a mild soap (BIII). Skin care during neutropenia should also include daily inspection of skin sites likely to be portals of infection (e.g., the perineum and intravascular access sites) (BIII). HSCT recipients and candidates undergoing conditioning therapy should maintain good perineal hygiene to minimize loss of skin integrity and risk for infection (BIII). To facilitate this precaution, HSCT center personnel should develop protocols for patient perineal care, including recommendations for gentle but thorough perineal cleaning after each bowel movement and thorough drying of the perineum after each urination (BIII). Females should always wipe the perineum from front to back after using the toilet to prevent fecal contamination of the urethra and urinary tract infections (AIII). Moreover, to prevent vaginal irritation, menstruating immunocompromised HSCT recipients should not use tampons (DIII) to avoid the risk for cervical and vaginal abrasions. Additionally, the use of rectal thermometers, enemas, suppositories, and rectal exams are contraindicated among HSCT recipients to avoid skin or mucosal breakdown (DIII). All HSCT candidates and their caregivers should be educated regarding the importance of maintaining good oral and dental hygiene for at least the first year after HSCT to reduce the risk for oral and dental infections (AIII). For example, HSCT candidates should be informed that establishment of the best possible periodontal health before HSCT is a substantial step in avoiding short- and long-term oral infections and that maintenance of safe oral hygiene after HSCT can minimize the severity of infections and facilitate healing of mucositis, particularly before engraftment (BIII). All HSCT candidates should receive a dental evaluation and relevant treatment before conditioning therapy begins (270,271) (AIII). Likely sources of dental infection should be vigorously eliminated (271) (AIII). For example, teeth with moderate to severe caries should be restored; ill-fitting dental prostheses should be repaired; and teeth compromised by moderate to severe periodontal disease should be extracted (271). Ideally, 10--14 days should elapse between the completion of tissue-invasive oral procedures and onset of conditioning therapy to allow for adequate healing and monitoring for postsurgical complications (AIII). HSCT recipients with mucositis and HSCT candidates undergoing conditioning therapy should maintain safe oral hygiene by performing oral rinses 4--6 times/day with sterile water, normal saline, or sodium bicarbonate solutions (270) (AIII). HSCT recipients and candidates should brush their teeth >2 times/day with a soft regular toothbrush (270) (BIII). If the recipient cannot tolerate these brushings, use of an ultrasoft toothbrush or toothette (i.e., foam swab on a stick), can be used (CIII), but physicians should be aware that using the latter products are less desirable than using soft regular or ultrasoft toothbrushes because the toothettes remove less dental debris (270). Using toothpaste is optional, depending on the recipient's tolerance (270) (CIII). HSCT recipients and candidates undergoing conditioning therapy who are skilled at dental flossing should floss daily if this can be done without trauma (BIII). Routine dental supervision is advised to monitor and guide the patient's maintenance of oral and dental hygiene (BIII). To decrease the risk for mechanical trauma and infection of oral mucosa, fixed orthodontic appliances and space maintainers should not be worn from the start of conditioning therapy until preengraftment mucositis resolves, and these devices should not be worn during any subsequent periods of mucositis (270) (DIII). Dental and transplant teams and the patient's community dentist should coordinate removal of these appliances and long-term rehabilitation of any oral lesions (BIII). However, patients who normally wear removable dental prostheses might be able to wear them during conditioning therapy before HSCT and during mucositis after HSCT, depending on the degree of tissue integrity at the denture-bearing sites and the ability of the patient to maintain denture hygiene on a daily basis (CIII). Preventing Bacterial Intravascular Catheter-Related InfectionsHSCT center personnel are advised to implement published guidelines for preventing intravascular device-related infections (33) (AIII). Contact with tap water at the central venous catheter site should be avoided (BIII). For long-term central venous access among children, HSCT physicians can use a totally implantable device among children aged <4 years if the anticipated duration of vascular access is >30 days (CII). However, such a device among children aged <4 years is not generally used as the actual HSCT infusion site because a) problems with skin fragility contraindicate repeated punctures over the port site and b) the port device might have an insufficient number of lumens for optimal patient management immediately after HSCT. To prevent bloodstream infections associated with needleless intravenous access devices, HSCT recipients should a) cover and protect the catheter tip or end cap during bathing or showering to protect it from tap water contamination, b) change the device in accordance with manufacturers' recommendations, if available, and c) have a caregiver perform intravenous infusions whenever possible (272,273) (BII). Also, HSCT recipients and their caregivers should be educated regarding proper care of needleless intravenous access devices (272) (BII). No recommendation regarding the use of antibiotic-impregnated central venous catheters among HSCT recipients can be made because of lack of data. Control of Specific Nosocomial InfectionsRecommendations Regarding Legionella Species HSCT physicians should always include Legionnaires' disease (LD) in the differential diagnosis of pneumonia among HSCT recipients (140) (AIII). Appropriate tests to confirm LD include a) culturing sputum, BAL, and tissue specimens; b) testing BAL specimens for Legionellae by direct fluorescent antibody; and c) testing for Legionella pneumophila serogroup 1 antigen in urine. The incubation period for LD is usually 2--10 days; thus, laboratory-confirmed legionellosis that occurs in a patient who has been hospitalized continuously for >10 days before the onset of illness is regarded as a definite case of nosocomial LD, and a laboratory-confirmed infection that occurs 2--9 days after hospital admission is a possible case of nosocomial LD (140). When a case of laboratory-confirmed nosocomial LD (274,275) is identified in a person who was in the inpatient HSCT center during all or part of the 2--10 days before illness onset, or if two or more cases of laboratory-confirmed LD occur among patients who had visited an outpatient HSCT center, hospital personnel should
The source of Legionella infection should be identified and decontaminated or removed (AIII). Extensive hospital investigations of an isolated case of possible nosocomial LD might not be indicated if the patient has had limited contact with the inpatient center during most of the incubation period (CIII). Because HSCT recipients are at much higher risk for disease and death from legionellosis compared with other hospitalized persons (274), periodic routine culturing for Legionellae in water samples from the center's potable water supply could be regarded as part of an overall strategy for preventing LD in HSCT centers (CIII). However, the optimal methodology (i.e., frequency or number of sites) for environmental surveillance cultures in HSCT centers has not been determined, and the cost-effectiveness of this strategy has not been evaluated. Because HSCT recipients are at high risk for LD and no data were found to determine a safe concentration of Legionellae organisms in potable water, the goal, if environmental surveillance for Legionellae is undertaken, should be to maintain water systems with no detectable organisms (AIII). Physicians should suspect legionellosis among HSCT recipients with nosocomial pneumonia even when environmental surveillance cultures do not yield Legionellae (AIII). If Legionella species are detected in the water supplying an HSCT center, the following should be done until Legionella species are no longer detected by culture:
When a new hospital with an HSCT center is constructed, the cooling towers should be placed so that the tower drift is directed away from the hospital's air-intake system, and the cooling towers should be designed so that the volume of aerosol drift is minimized (140) (BII). For operational hospital cooling towers, hospitals should
Recommendations Regarding Methicillin-Resistant Sta. aureus HSCT center HCWs should follow basic infection control practices (e.g., hand washing between patients and use of barrier precautions, including wearing gloves whenever entering the methicillin-resistant Sta. aureus [MRSA] infected or colonized patient's room); these practices are essential for MRSA control (62) (AII). If MRSA is a substantial problem in the HSCT center and evidence exists of ongoing MRSA transmission, MRSA infected or colonized patients should be treated as a cohort (e.g., cared for exclusively by a limited number of HCWs) (BIII). HSCT transplant recipients with recurrent Sta. aureus infections should undergo extensive evaluation for persistent colonization, including cultures of nares, groin, axilla, and ostomy sites (e.g., tracheostomy or gastrointestinal tube) (BIII). For patients with recurrent MRSA infection, elimination of the carrier state should be attempted by applying a 2% mupirocin calcium ointment to the nares (BIII), although this strategy has been only marginally effective in certain institutions (278) (Appendix). High-level mupirocin-resistant MRSA has been reported in Europe, the Middle East, and South America (279--283) but is uncommon in the United States. As with any antibiotic, incorrect or overuse of mupirocin can result in mupirocin-resistant Staphylococci; therefore, mupirocin use should be reserved for infection control strategies only (279,280). For patients who fail mupirocin, physicians have used bacitracin, TMP-SMZ, or rifampin administered with another antibiotic, but no standardized protocol using these drugs for this indication has been evaluated and no recommendations can be made because of lack of data. Selection of a systemic antibiotic should be guided by susceptibility patterns. Intravascular cannulas or other implantable devices that are infected or colonized with MRSA should be removed (AIII). Patients with MRSA should be placed under contact precautions until all antibiotics are discontinued and until three consecutive cultures, taken >1 weeks apart, are negative (62) (BIII). Screening cultures for MRSA include the anterior nares, any body site previously positive for MRSA, and any wounds or surgical sites. Recommendations Regarding Staphylococcus Species with Reduced Susceptibility to Vancomycin All HSCT centers should have sufficient laboratory capability to identify all Staphylococci isolates and their susceptibility patterns to antibiotics, including vancomycin (284,285) (AIII). Additionally, all HSCT center personnel should conduct routine surveillance for the emergence of Staphylococcus species strains with reduced susceptibility to vancomycin (285,286) (AIII). Reduced susceptibility should be considered for all Sta. aureus strains that have a vancomycin MIC of >4 µg/mL and all coagulase-negative Staphylococci that have a vancomycin MIC of >8 µg/mL. If repeat testing of the organism in pure culture confirms the genus, species, and elevated vancomycin MICs, the following steps should be taken (287):
Recommendations Regarding VRE Use of intravenous vancomycin is associated with VRE emergence. Vancomycin and all other antibiotics, particularly antianaerobic agents (e.g., metronidazole and third-generation cephalosporins) must be used judiciously (284,290--292) (AII). Oral van-comycin use can be limited by treating recurrences of Cl. difficile diarrhea with oral metronidazole instead of vancomycin (BIII). Physicians have placed patients with a history of VRE or VRE colonization into continuous isolation during clinic visits and hospitalizations; however, this practice is controversial because certain non-HSCT recipients might clear VRE from their stools. No recommendation regarding use of continuous isolation among HSCT recipients can be made because of lack of data. To control VRE exposure, strict adherence to the following standard infection control measures is necessary (292) (AI):
Recommendations Regarding Cl. difficile HSCT physicians should follow published recommendations for preventing and controlling Cl. difficile disease, including minimizing the duration of antibiotic therapy and number of antibiotics used for any indication (295,296) (AIII). All patients with Cl. difficile disease should be placed under contact precautions for the duration of illness (62) (AII). All HCWs who anticipate contact with a Cl. difficile-infected patient or the patient's environment or possessions should put on gloves before entering the patient's room (62,295--298) and before handling the patient's secretions and excretions (AI). During Cl. difficile outbreaks, HSCT center personnel should restrict use of antibiotics (e.g., clindamycin) (299) (BII). To prevent transmission of Cl. difficile to patients during nosocomial Cl. difficile outbreaks, HSCT center HCWs should a) use disposable rectal thermometers or tympanic thermometers; b) disinfect gastrointestinal endoscopes with 2% glutaraldehyde immersion for 10 minutes or use an equivalent disinfectant strategy (255,256); and c) perform surface sterilization of the hospital ward environment (e.g., floors, walls, bed frames, doors, bathroom surfaces) with an FDA- or EPA-registered sterilant (e.g., phosphate-buffered sodium hypochlorite solution [1,660 ppm available chloride]; unbuffered hypochlorite solution [500 ppm available chloride]; 0.04% formaldehyde and 0.03% glutaraldehyde [255,295,300]; or ethylene oxide [247,296]) (BII). Additionally, physicians should treat patients with Cl. difficile disease with antibiotics as recommended in published reports (62,295) (BII). Certain researchers also recommend antibiotic treatment of Cl. difficile carriers (301). However, other researchers have reported that treatment of asymptomatic Cl. difficile carriers with metronidazole is not effective and that treatment with vancomycin is only effective temporarily (i.e., <2 months after treatment) (302). Consequently, no recommendation regarding treatment of asymptomatic Cl. difficile carriers can be made. Similarly, although symptomatic Cl. difficile disease recurrence or relapse occurs among 7%--20% of patients (295), data are insufficient to make a recommendation for preventing multiple Cl. difficile relapses. The following practices are not recommended for Cl. difficile control:
Recommendations Regarding CRV Infections Physicians should institute appropriate precautions and infection control measures for preventing nosocomial pneumonia among hospitalized HSCT recipients and candidates undergoing conditioning therapy, particularly during community or nosocomial CRV outbreaks (140) (AIII). Patients with URI or LRI symptoms should be placed under a) contact precautions for most viral respiratory infections including varicella; b) droplet precautions for influenza or adenovirus; or c) airborne precautions for measles or varicella to avoid transmitting infection to other HSCT candidates and recipients as well as to HCWs and visitors (BIII). Identifying HSCT recipients with RSV infection and placing them under contact precautions immediately (AIII) to prevent nosocomial transmission is critical. When suctioning the respiratory tract of patients with URI or LRI symptoms, HCWs should wear gowns, surgical masks, and eye protection to avoid contamination from the patient's respiratory secretions. All protective clothing (e.g., gown, gloves, surgical mask, and eye protection) should be put on when entering a patient's room and discarded in the same room before exiting; protective clothing should always be changed between patient rooms (140) (AIII). When caring for an HSCT recipient or candidate undergoing conditioning therapy with URI or LRI, HCWs and visitors should change gloves and wash hands a) after contact with a patient; b) after handling respiratory secretions or objects contaminated with secretions from one patient and before contact with another patient, object, or environmental surface; and c) between contacts with a contaminated body site and the respiratory tract of or respiratory device used on the same patient (140) (AII). This practice is critical because most respiratory infections are usually transmitted by contact, particularly by hand to nose and eye. Therefore just wearing a mask, without appropriate hand washing, glove-wearing, or use of eye protection is insufficient to prevent transmission of CRV infections. Researchers have proposed that HSCT recipients or candidates undergoing conditioning therapy be placed under contact precautions during nosocomial outbreaks (131) (CIII). Even when no nosocomial or community outbreak of CRV infections exists, all persons who enter the HSCT center should be screened daily for URI symptoms, including visitors and HCWs (BIII). Researchers also describe systems where HCWs provide daily verification (e.g., using sign-in sheets) that they are free of URI symptoms before being allowed to provide HSCT patient care. HCWs and visitors with URI symptoms should be restricted from contact with HSCT recipients and candidates undergoing conditioning therapy to minimize the risk for CRV transmission (131) (AIII). All HCWs with URI symptoms should be restricted from patient contact and reassigned to nonpatient care duties until the HCW's symptoms resolve (BIII). Visitors with URI symptoms should be asked to defer their visit to the HSCT center (131) until their URI symptoms resolve (BIII). Respiratory secretions of any hospitalized HSCT candidate or recipient with signs or symptoms of CRV infection should be tested promptly by viral culture and rapid diagnostic tests for CRV (BIII). Appropriate samples include nasopharyngeal washes, swabs, aspirates, throat swabs, and BAL fluid. This practice is critical because preemptive treatment of certain CRVs (e.g., influenza and RSV) (133) might prevent severe disease and death among HSCT recipients. Viral shedding among HSCT recipients with CRV infection has been reported to last <4 months for influenza (143), <2 years for adenovirus (305,306), and <22 days for RSV (136); however, RSV viral shedding has been reported to last 112 days in a child with severe combined immunodeficiency (307). Therefore, to prevent nosocomial transmission of CRV (136), HSCT center HCWs should recognize that prolonged CRV shedding can occur when determining the duration of appropriate precautions for CRV-infected HSCT recipients or candidates undergoing conditioning therapy (CIII). HSCT centers should use serial testing by using cultures from nasopharyngeal swabs, throat swabs or aspirates, or rapid antigen tests to help determine whether patients have stopped shedding influenza virus (BIII). Researchers have proposed that HSCT physicians conduct routine CRV surveillance among HSCT recipients to detect outbreaks and implement infection control measures as early as possible (CIII). During RSV season, HSCT recipients and candidates with signs or symptoms should be tested for RSV infection (i.e., the presence of RSV antigen in respiratory secretions tested by enzyme-linked immunosorbent assay and viral culture) starting with admission to the HSCT center. All patients who are RSV-antigen positive should be treated as a cohort during nosocomial RSV outbreaks because this practice reduces nosocomial RSV transmission (130,131) (BII). Symptomatic HCWs should be excluded from patient contact until symptoms resolve. HCWs and visitors with infectious conjunctivitis should be restricted from direct patient contact until the drainage resolves (i.e., usually, 5--7 days for adenovirus) and the ophthalmology consultant concurs that the infection and inflammation have resolved (268) (AII) to avoid possible transmission of adenovirus to HSCT recipients. Preventing CRV exposure among HSCT recipients after hospital discharge is more challenging because of high CRV prevalence. Preventive measures should be individualized in accordance with the immunologic status and tolerance of the patient. In outpatient waiting rooms, patients with CRV infections should be separated to the extent possible from other patients (BIII). Recommendations Regarding TB HSCT candidates should be screened for TB by careful medical history and chart review to ascertain any history of prior TB exposure (AIII) because immunocompromised persons have higher risk for progression from latent TB infection to active disease (244). Also, physicians can administer a tuberculin skin test (TST) using the Mantoux method with five tuberculin units of purified protein derivative (CIII); but because of a patient's immunocompromise, this test might not be reliable. If a TST is administered, either the Tubersol® or Aplisol® formulation of purified protein derivative can be used (244,308). Persons with a recently positive TST or a history of a positive TST and no prior preventive therapy should be administered a chest radiograph and evaluated for active TB (309) (AI). For immunocompromised persons, a positive TST is defined as >5 mm of induration (309,310) because of their decreased ability to mount a delayed hypersensitivity response (CIII). Because immunosuppressive therapy decreases the sensitivity of the TST, HSCT physicians should not rely solely on the TST to determine whether latent TB infection is present and whether preventive therapy should be administered to HSCT recipients or candidates (DIII). Instead, a full 9-month course of isonicotinic acid hydrazide preventive therapy should be administered to immunocompromised HSCT recipients or candidates who have been substantially exposed to someone with active, infectious (i.e., sputum-smear positive) pulmonary or laryngeal TB, regardless of the HSCT recipient's or candidate's TST status (309) (BIII). A full 9-month course of isonicotinic acid hydrazide preventive therapy should also be administered to HSCT recipients or candidates with a positive TST who were not previously treated and have no evidence of active TB disease (309) (AIII) (Appendix). Routine anergy screening might not be reliable among HSCT recipients and candidates undergoing conditioning therapy and, therefore, is not recommended (DIII). An HSCT should not be canceled or delayed because of a positive TST (DIII). Use of a 2-month course of a daily pyrazinamide/rifampin (PZA/RIF) regimen has been recommended as an alternate preventive therapy for persons with TB (309). However, limited data were found regarding safety and efficacy of this regimen among non-HIV--infected persons. Furthermore, rifampin has substantial drug interactions with certain medications, including cyclosporine, tacrolimus (FK506), corticosteroids, fluconazole, and pain medications. Therefore, routine use of the 2-month PZA/RIF prophylactic regimen among HSCT recipients is not recommended (DIII). However, this regimen can be used for HSCT candidates who are not at risk for serious rifampin drug interactions and whose HSCT is not scheduled until >2 weeks after completion of the 2-month PZA/RIF course (CIII). This delay will diminish the possibility of adverse effects of rifampin on drugs used for routine HSCT OI prophylaxis (e.g., fluconazole) (311). An HSCT candidate or recipient who has been exposed to an active case of extrapulmonary, and therefore, noninfectious TB does not require preventive therapy (DIII). HSCT center personnel should follow guidelines regarding the control of TB in health-care facilities (244,245), including instituting airborne precautions and negative-pressure rooms for patients with suspected or confirmed pulmonary or laryngeal TB (62,244) (AII). HCWs should wear N95 respirators, even in isolation rooms, to protect themselves from possible TB transmission from patients with active pulmonary or laryngeal TB, particularly during cough-inducing procedures (62,244,245,312) (AIII). To be maximally effective, respirators (e.g., N95) must be fit-tested, and all respirator users must be trained to use them correctly (243) (AIII). Unless they become soiled or damaged, changing N95 respirators between patient rooms is not necessary (DIII). Bacillus of Calmette and Guérin vaccination is contraindicated among HSCT candidates and recipients because it might cause disseminated or fatal disease among immunocompromised persons (313,314) (EII). No role has been identified for chronic suppressive therapy or follow-up surveillance cultures among HSCT recipients who have a history of successfully treated TB (DIII). Infection Control SurveillanceHSCT center personnel are advised to follow standard guidelines for surveillance of antimicrobial use and nosocomial pathogens and their susceptibility patterns (315) (BIII). HSCT center personnel should not perform routine fungal or bacterial cultures of asymptomatic HSCT recipients (166,167) (DII). In the absence of epidemiologic clusters of infections, HSCT center personnel should not perform routine periodic bacterial surveillance cultures of the HSCT center environment or of equipment or devices used for respiratory therapy, pulmonary-function testing, or delivery of inhalation anesthesia (140) (DIII). Researchers recommend that hospitals perform routine sampling of air, ceiling tiles, ventilation ducts, and filters to test for molds, particularly when construction or renovation occurs near or around the rooms of immunocompromised patients (167,174) or when clinical surveillance demonstrates a possible increase in mold (i.e., aspergillosis) cases (CIII). Strategies that might decrease fungal spores in the ventilation system include eliminating access of birds (i.e., primarily pigeons) to air-intake systems, removing bird droppings from the air-intake ducts, and eliminating moss from the hospital roof (174). Furthermore, in the absence of a nosocomial fungal outbreak, HSCT centers need not perform routine fungal cultures of devices and dust in the rooms of HSCT recipients and candidates undergoing conditioning therapy (DIII). HSCT center personnel should routinely perform surveillance for the number of aspergillosis cases occurring among HSCT recipients, particularly during hospital construction or renovation (BIII). A two-fold or greater increase in the attack rate of aspergillosis during any 6-month period indicates that the HSCT center environment should be evaluated for breaks in infection control techniques and procedures and that the ventilation system should be investigated carefully (174) (BIII). STRATEGIES FOR SAFE LIVING AFTER HSCT -- PREVENTING EXPOSURE AND DISEASEAvoiding Environmental ExposuresHSCT recipients and candidates undergoing conditioning therapy, particularly allogeneic recipients, and parents of pediatric HSCT recipients and candidates should be educated regarding strategies to avoid environmental exposures to opportunistic pathogens (AIII). Preventing Infections Transmitted by Direct Contact HSCT recipients and candidates should wash their hands thoroughly (i.e., with soap and water) and often. For example, hands should be washed
Preventing Respiratory Infections To prevent respiratory infections after hospital discharge, HSCT recipients should observe the following precautions:
Researchers report that allogeneic recipients should avoid construction or excavation sites or other dust-laden environments for the first 6 months after HSCT and during other periods of substantial immunosuppression (e.g., GVHD, systemic steroid use, or relapse of the underlying disease for which the transplant was performed) to avoid exposures to molds (CIII). Researchers also report that outpatient HSCT recipients should be advised of travel routes to the HSCT center that will avoid or minimize exposure to construction sites (CIII). Coccidioidomycosis is uncommon after allogeneic HSCT; however, researchers report that HSCT recipients traveling to or residing in coccidioidomycosis-endemic areas (e.g., the American southwest, Mexico, and Central and South America) should avoid or minimize exposure to disturbed soil, including construction or excavation sites, areas with recent earthquakes, farms, or other rural areas (CIII). Histoplasmosis (Histoplasma capsulatum) after allogeneic HSCT is also rare; however, researchers report that HSCT recipients in histoplasmosis-endemic areas should avoid exposure to chicken coops and other bird-roosting sites and caves for the first 6 months after HSCT and during periods of substantial immunosuppression (e.g., GVHD, systemic steroid use, or relapse of the underlying disease for which the transplant was performed) (CIII). Smoking tobacco and exposure to environmental tobacco smoke are risk factors for bacterial and CRV infections among healthy adults and children (320--325); consequently, logic dictates that physicians advise HSCT recipients not to smoke and to avoid exposure to environmental tobacco smoke (CIII). However, no data were found that specifically assess whether smoking or environmental smoke exposure are risk factors for OIs among HSCT recipients. Researchers have reported that marijuana smoking might be associated with generation of invasive pulmonary aspergillosis among immunocompromised persons, including HSCT recipients (326--329). Therefore, HSCT recipients should refrain from smoking marijuana to avoid Aspergillusspecies exposure (326,330--334) (BIII). Preventing Infections Transmitted Through Direct Contact and Respiratory Transmission Researchers have proposed that immunocompromised HSCT recipients and candidates who are undergoing conditioning therapy avoid gardening or direct contact with soil, plants, or their aerosols to reduce exposure to potential pathogens (e.g., To. gondii, Hi. capsulatum, Cryptococcus neoformans, Nocardia species, and Aspergillus species) (CIII). HSCT recipients, particularly allogeneic recipients, could wear gloves while gardening or touching plants or soil (335) (CIII), and they should avoid creating plant or soil aerosols (BIII). Additionally, they should always wash their hands afterwards (335) and care for skin abrasions or cuts sustained during soil or plant contact (AIII). Persons whose occupations involve animal contact (e.g., veterinarians, pet store employees, farmers, or slaughterhouse workers) could be at increased risk for toxoplasmosis and other zoonotic diseases. Although data are insufficient to justify a general recommendation against HSCT recipients working in such settings, these exposures should be avoided during the first 6 months after HSCT and during other periods of substantial immunosuppression (e.g., GVHD, systemic steroid use, or relapse of the underlying disease for which the transplant was performed) (BIII). Safe SexSexually active HSCT recipients should avoid sexual practices that could result in oral exposure to feces (5,316) (AIII). Sexually active patients who are not in long-term monogamous relationships should always use latex condoms during sexual contact to reduce their risk for exposure to CMV, HSV, HIV, hepatitis B and C, and other sexually transmitted pathogens (AII). However, even long-time monogamous partners can be discordant for these infections. Therefore, during periods of immunocompromise, sexually active HSCT recipients in such relationships should consider using latex condoms during sexual contact to reduce the risk for exposure to these sexually transmitted infections (CIII). Pet SafetyPreventing Pet-Transmitted Zoonotic Infections HSCT physicians should advise recipients and candidates undergoing conditioning therapy of the potential infection risks posed by pet ownership; however, they should not routinely advise HSCT recipients to part with their pets, with limited exceptions. Generally, immunocompromised HSCT recipients and candidates undergoing conditioning therapy should minimize direct contact with animals (336,337), particularly those animals that are ill (e.g., with diarrhea) (335) (BIII). Immunocompromised persons who choose to own pets should be more vigilant regarding maintenance of their pet's health than immunocompetent pet owners (BIII). This recommendation means seeking veterinary care for their pet early in the pet's illness to minimize the possible transmission of the pet's illness to the owner (335) (BIII). Feeding pets only high-quality commercial pet foods reduces the possibility of illness caused by spoiled or contaminated foods, thus reducing the possibility of transmitting illness from the pet to the HSCT recipient. If eggs, poultry, or meat products are given to the pet as supplements, they should be well-cooked. Any dairy products given to pets should be pasteurized (335) (BIII). Pets should be prevented from drinking toilet bowl water and from having access to garbage; pets should not scavenge, hunt, or eat other animals' feces (335) (BIII). If HSCT recipients have contact with pets or animals, they should wash their hands after handling them (particularly before eating) and after cleaning cages; HSCT recipients should avoid contact with animal feces to reduce the risk for toxoplasmosis, cryptosporidiosis, salmonellosis, and campylobacteriosis (335) (BIII). Adults should supervise hand washing of pediatric HSCT recipients (BIII). Immunocompromised HSCT recipients and candidates should not clean pet litter boxes or cages or dispose of animal waste (DIII). If this cannot be avoided, patients should wear disposable gloves during such activities and wash their hands thoroughly afterwards (BIII). Immunocompromised HSCT recipients and candidates should avoid adopting ill or juvenile pets (e.g., aged <6 months for cats) (335) and any stray animals (5,316) (BIII). Any pet that experiences diarrhea should be checked by a veterinarian for infection with Cryptosporidium (5,316), Giardia species (335), Salmonella, and Campylobacter (5,335,337) (BIII). Immunocompromised HSCT recipients and candidates should not have contact with reptiles (e.g., snakes, lizards, turtles, or iguanas) (DII) to reduce their risk for acquiring salmonellosis (335,338--341). Additionally, patients should be informed that salmonellosis can occur from fomite contact alone (342). Therefore, HSCT recipients and candidates should avoid contact with a reptile, its food, or anything that it has touched, and if such contact occurs, recipients and candidates should wash their hands thoroughly afterwards (AIII). Immunocompromised HSCT recipients and candidates should avoid contact with ducklings and chicks because of the risk for acquiring Salmonella or Campylobacter species infections (338,343) (BIII). Immunocompromised HSCT recipients and candidates should avoid contact with exotic pets (e.g., nonhuman primates) (BIII). Bird cage linings should be cleaned regularly (e.g., daily) (337). All persons, but particularly immunocompromised HSCT candidates and recipients, should wear gloves whenever handling items contaminated with bird droppings (337) (BIII) because droppings can be a source of Cryptococcus neoformans, Mycobacterium avium, or Hi. capsulatum. However, routine screening of healthy birds for these diseases is not recommended (335) (DIII). To minimize potential exposure to Mycobacterium marinum, immunocompromised HSCT recipients and candidates should not clean fish tanks (DIII). If this task cannot be avoided, patients should wear disposable gloves during such activities and wash their hands thoroughly afterwards (335,337) (BIII). Preventing Toxoplasmosis The majority of toxoplasmosis cases in the United States is acquired through eating undercooked meat (335,337). However, all HSCT recipients and candidates, particularly those who are To. gondii seronegative, should be informed of the risks for contracting toxoplasmosis from cat feces (BIII), but need not be advised to give away their cats (DII). For households with cats, litter boxes should not be placed in kitchens, dining rooms, or other areas where food preparation and eating occur (335). Additionally, litter boxes should be cleaned daily by someone other than the HSCT recipient during the first 6 months after HSCT and during periods of substantial immunosuppression (e.g., GVHD, steroid use, or relapse of the underlying disease for which the transplant was performed) to reduce the risk for transmitting toxoplasmosis to the HSCT recipient (BIII). Daily litter box changes will minimize the risk for fecal transmission of To. gondii oocysts, because fecal oocysts require >2 days of incubation to become infectious. If HSCT recipients perform this task during the first 6 months after HSCT and during subsequent periods of substantial immunocompromise (e.g., during GVHD, systemic steroid use, or relapse of the underlying neoplastic disease for which the transplant was performed), they should wear disposable gloves (335). Gloves should be discarded after a single use (BIII). Soiled, dried litter should be disposed of carefully to prevent aerosolizing the To. gondii oocysts (BIII). Cat feces (but not litter) can be flushed down the toilet (BIII). Also, persons who clean cat litter, particularly HSCT recipients, should wash their hands thoroughly with soap and water afterwards to reduce their risk for acquiring toxoplasmosis (BIII). HSCT recipients and candidates with cats should keep their cats inside (BIII) and should not adopt or handle stray cats (DIII). Cats should be fed only canned or dried commercial food or well-cooked table food, not raw or undercooked meats, to eliminate the possibility of causing an illness that could be transmitted from the cat to the HSCT recipient (BIII). Pet cats of HSCT recipients do not need to be tested for toxo-plasmosis (EII). Playground sandboxes should be kept covered when not in use to prevent cats from soiling them (BIII). HSCT recipients and candidates undergoing conditioning therapy should avoid drinking raw goat's milk to decrease the risk for acquiring toxoplasmosis (BIII). Water and Other Beverage SafetyAlthough limited data were found regarding the risks for and epidemiology of Cryptosporidium disease among HSCT recipients, HSCT recipients are prudent to avoid possible exposures to Cryptosporidium (BIII) because it has been reported to cause severe, chronic diarrhea, malnutrition, and death among other immunocompromised persons (5,318,319). HSCT recipients should avoid walking, wading, swimming, or playing in recreational water (e.g., ponds or lakes) that is likely to be contaminated with Cryptosporidium, Es. coli O157:H7 (344--346), sewage, or animal or human waste (BII). HSCT recipients should also avoid swallowing such water (e.g., while swimming) (5,344,346) as well as any water taken directly from rivers and lakes (5,316) (AIII). HSCT recipients should not use well water from private wells or from public wells in communities with limited populations (DIII) because tests for microbial contamination are performed too infrequently (e.g., in certain locations, tests are performed <1 times/month) to detect sporadic bacterial contamination. However, drinking well water from municipal wells serving highly populated areas is regarded as safe from bacterial contamination because the water is tested >2 times/day for bacterial contamination. If HSCT recipients consume tap water, they should routinely monitor mass media (e.g., radio, television, or newspapers) in their area to immediately implement any boil-water advisories that might be issued for immunocompromised persons by state or local governments (BIII). A boil-water advisory means that all tap water should be boiled for >1 minutes before it is consumed. Tap water might not be completely free of Cryptosporidium. To eliminate the risk for Cryptosporidium exposure from tap water, HSCT recipients can boil tap water for >1 minutes before consuming it (e.g., drinking or brushing teeth) (5) (CIII). Alternately, they can use certain types of water filters (316) or a home distiller (317) to reduce their risk for Cryptosporidium (5) and other waterborne pathogens (CIII). If a home water filter******* is used, it should be capable of removing particles >1 µm in diameter, or filter by reverse osmosis. However, the majority of these filters are not capable of removing smaller microbes (e.g., bacteria or viruses), and therefore, should only be used on properly treated municipal water. Further, the majority of these devices would not be appropriate for use on an unchlorinated private well to control viral or bacterial pathogens. Bottled water can be consumed if it has been processed to remove Cryptosporidium by one of three processes --- reverse osmosis, distillation, or 1-µm particulate absolute filtration. To confirm that a specific bottled water has undergone one of these processes, HSCT recipients should contact the bottler directly.******** Patients can take other precautions in the absence of boil-water advisories to further reduce their risk for cryptosporidiosis. These extra precautions include avoiding fountain beverages and ice made from tap water at restaurants, bars, and theaters (5), fruit drinks made from frozen concentrate mixed with tap water, and iced tea or coffee made with tap water (317). Drinks that are likely to be Cryptosporidium safe for HSCT recipients include nationally distributed brands of bottled or canned carbonated soft drinks and beers (5); commercially packaged noncarbonated drinks that contain fruit juice; fruit juices that do not require refrigeration until after opening (e.g., those that are stored unrefrigerated on grocery shelves) (5); canned or bottled soda, seltzer or fruit drinks; steaming hot (>175 F) tea or coffee (317); juices labeled as pasteurized; and nationally distributed brands of frozen fruit juice concentrate that are reconstituted with water from a safe source (5). HSCT recipients should not drink unpasteurized milk or fruit or vegetable juices (e.g., apple cider or orange juice) to avoid infection with Brucella species, Es. coli O157:H7, Salmonella species, Cryptosporidium, and others (319,347--351) (DII). Food SafetyHSCT candidates and household or family members who prepare food for them after HSCT should review food safety practices that are appropriate for all persons (352) (AIII), and food preparers should be educated regarding additional food safety practices appropriate for HSCT recipients. This review and education should be done before the conditioning regimen (i.e., chemotherapy and radiation) begins (BIII). Adherence to these guidelines will decrease the risk for foodborne disease among HSCT recipients. Food Safety Practices Appropriate for All Persons Raw poultry, meats, fish, and seafood should be handled on separate surfaces (e.g., cutting board or counter top) from other food items. Food preparers should always use separate cutting boards (i.e., one for poultry and other meats and one for vegetables and remaining cutting or carving tasks) (AIII), or the board(s) should be washed with warm water and soap between cutting different food items (AIII). To prevent foodborne illnesses caused by Campylobacter jejuni and Salmonella enteritidis, which can cause severe and invasive infections among immunocompromised persons (353,354), uncooked meats should not come in contact with other foods (BIII). After preparing raw poultry, meats, fish, and seafood and before preparing other foods, food handlers should wash their hands thoroughly in warm, soapy water. Any cutting boards, counters, knives, and other utensils used should be washed thoroughly in warm, soapy water also (AIII). Food preparers should keep shelves, counter tops, refrigerators, freezers, utensils, sponges, towels, and other kitchen items clean (AIII). All fresh produce should be washed thoroughly under running water before serving (355) (AIII). Persons preparing food should follow published U.S. Department of Agriculture recommendations regarding safe food thawing (356) (BIII). Persons cooking food for HSCT recipients should follow established guidelines for monitoring internal cooking temperatures for meats (357) (AII). The only method for determining whether the meat has been adequately cooked is to measure its internal temperature with a thermometer because the color of the meat after cooking does not reliably reflect the internal temperature. Different kinds of meat should be cooked to varying internal temperatures, all >150 F (AII). Specifically, the U.S. Department of Agriculture recommends that poultry be cooked to an internal temperature of 180 F; other meats and egg-containing casseroles and souffles should be cooked to an internal tem perature of >160 F. Cold foods should be stored at <40 F; hot foods should be kept at >140 F (BIII). Food preparers should
Additional Food Safety Practices Appropriate for HSCT Recipients HSCT recipients' diets should be restricted to decrease the risk for exposure to foodborne infections from bacteria, yeasts, molds, viruses, and parasites (BIII). Currently, a low microbial diet is recommended for HSCT recipients (358,359) (BIII). This diet should be continued for 3 months after HSCT for autologous recipients. Allogeneic recipients should remain on the diet until all immunosuppressive drugs (e.g., cyclosporine, steroids, and tacrolimus) are discontinued. However, the HSCT physician should have final responsibility for determining when the diet can be discontinued safely. Only one study has reported that dietary changes (e.g., consuming yogurt) have decreased the risk for mycotic infections (e.g., candidal vaginitis) (360) (Table 3). HSCT recipients should not eat any raw or undercooked meat, including beef, poultry, pork, lamb, venison or other wild game, or combination dishes containing raw or undercooked meats or sweetbreads from these animals (e.g., sausages or casseroles) (AII). Also, HSCT recipients should not consume raw or undercooked eggs or foods that might contain them (e.g., certain preparations of hollandaise sauce, Caesar and other salad dressings, homemade mayonnaise, and homemade eggnog) because of the risk for infection with Salmonella enteritidis (354) (AII). HSCT recipients should not consume raw or undercooked seafood (e.g., oysters or clams) to prevent exposure to Vibrio species, viral gastroenteritis, and Cryptosporidium parvum (361--364) (AII). HSCT recipients and candidates should only consume meat that is welldone when they or their caretakers do not have direct control over food preparation (e.g., when eating in a restaurant) (AI). To date, no evidence exists in the United States that eating food at a fast food restaurant is riskier than eating at a conventional sit-down restaurant. Generally, HSCT candidates undergoing conditioning therapy and HSCT recipients with neutropenia (i.e., ANC < 1,000/ml3), GVHD, or immunosuppression should avoid exposures to naturopathic medicines that might contain molds (365) (DIII). HSCT recipients wishing to take naturopathic medications are advised to use them only as prescribed by a licensed naturopathic physician working in consultation with the recipient's transplant and infectious disease physicians (CIII). Travel SafetyTravel to developing countries can pose substantial risks for exposure to opportunistic pathogens for HSCT recipients, particularly allogeneic recipients chronically immunosuppressed. HSCT recipients should not plan travel to developing countries without consulting their physicians (AIII), and travel should not occur until the period of severe immunosuppression has resolved. Generally, allogeneic recipients should not plan travel to developing countries for 6--12 months after HSCT, particularly if GVHD has occurred. Autologous recipients can travel to developing countries 3--6 months after HSCT if their physicians agree. HSCT recipients should be informed regarding strategies to minimize the risk for acquiring foodborne and waterborne infections while traveling. They should obtain updated, detailed health information for international travelers from health organizations (366,367) (AIII). Generally, while traveling in developing countries, HSCT recipients should avoid consuming the following (BIII):
Antimicrobial prophylaxis for traveler's diarrhea is not recommended routinely for HSCT recipients traveling to developing countries (DIII) because traveler's diarrhea is not known to be more frequent or more severe among immunocompromised hosts. However, HSCT physicians who wish to provide prophylaxis to HSCT recipients who are traveling can prescribe a fluoroquinolone (e.g., ciprofloxacin hydrochloride) or TMP-SMZ (CIII), although resistance to TMP-SMZ is now common and resistance to fluoroquinolones is increasing in tropical areas (Appendix). Researchers recommend using bismuth subsalicylate to prevent traveler's diarrhea among adults (366). However, no data were found regarding safety and efficacy among HSCT recipients, and salicylates are not recommended for use among persons aged <18 years because salicylates are associated with Reye's syndrome (369). HSCT recipients' immunization status should be assessed and their vaccinations updated as needed before travel (366). Influenza chemoprophylaxis with rimantadine or amantadine can be used for immunocompromised HSCT recipients who are traveling outside the continental United States and who could be exposed to influenza A (CIII). HSCT RECIPIENT VACCINATIONSAntibody titers to vaccine-preventable diseases (e.g., tetanus, polio, measles, mumps, rubella, and encapsulated organisms) decline during the 1--4 years after allogeneic or autologous HSCT (66,370--373) if the recipient is not revaccinated. Clinical relevance of decreased antibodies to vaccine-preventable diseases among HSCT recipients is not immediately apparent because a limited number of cases of vaccine-preventable diseases are reported among U.S. recipients. However, vaccine-preventable diseases still pose risks to the U.S. population. Additionally, evidence exists that certain vaccine-preventable diseases (e.g., encapsulated organisms) can pose increased risk for HSCT recipients (66); therefore, HSCT recipients should be routinely revaccinated after HSCT so that they can experience immunity to the same vaccine-preventable diseases as others (Table 4). HSCT center personnel have developed vaccination schedules for HSCT recipients (374). One study determined that HSCT center personnel used 3--11 different vaccination schedules per vaccine (374); consequently, the study authors requested national guidelines for doses and timing of vaccines after HSCT to eliminate confusion among HSCT center personnel regarding how to vaccinate their patients. To address this need, an interim vaccination schedule for HSCT recipients was drafted in collaboration with partner organizations, including CDC's Advisory Committee on Immunization Practices. The purpose of the vaccination schedule in these guidelines is to provide guidance for HSCT centers (Table 4). Although limited data were found regarding safety and immunogenicity (e.g., serologic studies of antibody titers after vaccination) among HSCT recipients, no data were found regarding vaccine efficacy among HSCT recipients (e.g., which determine whether vaccinated HSCT recipients have decreased attack rates of disease compared with unvaccinated HSCT recipients). Because certain HSCT recipients have faster immune system recovery after HSCT than others, researchers have proposed that different vaccination schedules be recommended for recipients of different types of HSCT. However, to date, data are too limited to do so. Therefore, the same vaccination schedule is recommended for all HSCT recipients (e.g., allogeneic, autologous, and bone marrow, peripheral, or UCB grafts) until additional data are published. In the tables, vaccines have only been recommended for use among HSCT recipients if evidence exists of safety and immunogenicity for those recipients. Vaccination of family members, household contacts, and HCWs are also recommended to minimize exposure of vaccine-preventable diseases among HSCT recipients (Tables 5--8). HEMATOPOIETIC STEM CELL SAFETYWith allogeneic HSCT, the life of the recipient might depend on the timely selection of an acceptable HLA-matched donor. Only a limited number of HLA-matched donors might be identified; hence, the transplant physician often has to accept a higher risk for transmission of an infectious agent through HSCT than would be permitted for routine blood transfusion. This section provides strategies for the HSCT physician to minimize transmission of infectious diseases, whenever possible, from donors to recipients.********* ********** Whether to select a donor who is at risk for or who has an infectious disease transmissible by HSCT, should be determined on a case-by-case basis (AIII) and is the final responsibility of the HSCT physician (AIII). If the only possible donor is at risk for or known to be infected with a bloodborne pathogen and the patient is likely to succumb rapidly from his or her disease if an HSCT is not received, the physician must carefully weigh the risks and benefits of using potentially infected donor cells. No person should be denied a potentially life-saving HSCT procedure solely on the basis of the risk for an infectious disease. However, HSCT physicians should avoid transplanting any infected or infectious donor hematopoietic stem cell product unless no other stem cell product can be obtained and the risk for death from not undergoing transplantation is deemed to be greater than the risk for morbidity or death from the infection that could potentially be transmitted (DII). If such a product is selected for use, it should be done on a case-by-case basis (375) and the following should be noted in the recipient's chart:
Preventing Transmission of Infections from HSCT Donors to RecipientsAll prospective HSCT donors should be evaluated through a physical history and examination to determine their general state of health and whether they pose a risk for transmitting infectious diseases to the recipient (376). To detect transmissible infections, all HSCT donor collection site personnel should follow up-to-date published guidelines and standards for donor screening (e.g., medical history), physical exam, and serologic testing (377--383) (AIII). Initial donor screening and physical exam should be performed <8 weeks before the planned donation (BIII). Donor serologic testing should be done <30 days before donation to detect potentially transmissible infections (BII); additionally, researchers recommend that donors be retested <7 days before collection. If testing is done >7 days before donation, donor screening should be repeated to ensure that no new risk behaviors have occurred during the interval between the original screening and the time of donation (BIII). This practice is critical because if new behavioral risk factors have occurred, the potential donor might need to be deferred. Screening and testing should be done on all allogeneic or syngeneic donors (AIII). Screening and testing of autologous donors is recommended to ensure the safety of laboratory personnel and to prevent cross contamination (BIII). If autologous donors are not tested, their autologous units should be specially labeled and handled as if potentially infected (BIII). For donors screened in the United States, FDA-licensed or -approved tests should be used in accordance with the manufacturers' instructions (AIII), and the donor samples should be tested in laboratories certified by the Clinical Laboratory Improvement Amendments of 1988 (AIII). All HSCT donors should be in good general health (376) (BIII). Acute or chronic illness in the prospective donor should be investigated to determine the etiology. Generally, persons who are ill should not be HSCT donors (DIII). A flu-like illness in a prospective donor at the time of evaluation or between the time of evaluation and donation should prompt evaluation of and serologic testing for infections that might pose a risk to the recipient (e.g., EBV, CMV, To. gondii) (BIII). Persons with a positive serum EBV-viral capsid antigen IgM but negative serum EBV-viral capsid antigen IgG should not serve as donors for allogeneic T-cell--depleted HSCT, particularly for unrelated or mismatched transplants, until their serum EBV-viral capsid antigen IgG becomes positive (DIII). Persons with acute toxoplasmosis should not donate until the acute illness has resolved (DII); however, physicians should be aware that persons who are asymptomatically seropositive for To. gondii might transmit this infection through HSCT (218). Prospective donors with symptoms of active TB should be evaluated for that disease (383) (BIII). Prospective donors with active TB should not donate (EIII) until the TB is well-controlled (e.g., no longer contagious as determined by the donor's primary physician) after appropriate medical therapy. However, no known risk exists from transplanting marrow from an untreated, tuberculin-positive donor who has no evidence of active disease. Screening potential donors for TB with Mantoux skin tests (DIII) is not necessary. Prospective HSCT donors who reside in or have traveled to areas endemic for rickettsia or other tickborne pathogens and who are suspected of having an acute tickborne infection should be temporarily deferred as donors until infection with these pathogens is excluded (DIII). Relevant pathogens include Rickettsia rickettsii, Babesia microti and other Babesia species, Coxiella burnetii, and the Colorado tick fever virus, which are the etiologic agents of Rocky Mountain spotted fever, babesiosis, Q fever, and Colorado tick fever, respectively; these pathogens have been reported to be transmitted by blood transfusion (384--388). Researchers recommend deferral for a past history of Q fever or babesiosis because these infections can be chronic and the babesiosis parasite might persist despite appropriate therapy (389) (CIII). Additionally, researchers have recommended deferring persons with acute human ehrlichiosis (e.g., human active human granulocytic ehrlichiosis [390], human monocytic ehrlichiosis, as well as any infections from Ehrlichia ewingii) from HSCT donation (CIII). The medical history of the prospective HSCT donor should include the following:
The following serologic tests should be performed for each prospective donor:
HSCT transplant center personnel should keep accurate records of all HSCT received and the disposition of each sample obtained (381). These tracking records must be separate from patients' medical records (e.g., in a log book) so that this information is easily obtainable. Recorded information should include the donor identification number, name of procurement of distribution center supplying the HSCT, recipient-identifying information, name of recipient's physician, and dates of a) receipt by the HSCT center and b) either transplantation to the recipient or further distribution (381) (AIII). All centers for donation, transplantation, or collection of hematopoietic stem cells should keep records of donor screening and testing, and HSCT harvesting, processing, testing, cryopreservation, storage, and infusion or disposal of each aliquot of donated hematopoietic progenitor cells for >10 years after the date of implantation, transplantation, infusion, or transfer of the product (378) (AIII). However, if that date is not known, records should be retained >10 years after the product's distribution, disposition, or expiration, whichever is latest. Pediatric DonorsChildren aged >18 months who are born to mothers with or at risk for HIV infection, who have not been breast-fed during the past 12 months, and whose HIV antibody tests, physical examination, and medical records do not indicate evidence of HIV infection can be accepted as donors (381) (BIII). Children aged <18 months who are born to mothers with or at risk for HIV infection and who have not been breast-fed by an HIV-infected woman during the past 12 months can be accepted as donors only if HIV infection has been excluded according to established criteria (402) (BIII). Children who have been breast-fed by an HIV-infected woman during the past 12 months should be excluded as stem cell donors regardless of HIV infection status (AIII). The mother and, if possible, the father of all pediatric stem-cell donors who are at risk for perinatal transmission of HIV and other bloodborne infections, should be interviewed by a health-care professional competent to elicit information regarding risk factors for possible bloodborne infection in the potential pediatric donor (AIII). Children who meet any of the adult donor exclusion criteria should not become HSCT donors (381) (EIII). Preventing Infection from Extraneous Contamination of Donated UnitsPersonnel of donation, collection, or transplantation centers, cell-processing laboratories, and courier services should follow current standards for detecting and preventing extrinsic bacterial and fungal contamination of collected stem cell units at the collection site, during processing and transportation, and at the transplant center (376) (AIII). Quality improvement programs and procedure manuals of collection centers, cell-processing laboratories, and transplant programs should include strategies for preventing transplant-associated infections. For example, collection centers should use aseptic techniques when collecting marrow, peripheral blood, and UCB hematopoietic stem cells (376,378) (AIII). Whenever possible, closed systems should be used for pooling hematopoietic stem cells during a collection procedure (BIII) because higher rates of microbial contamination seen in marrow harvests versus blood stem cell collections can be caused by use of open collecting systems (375,403,404). The highest risk for extraneous microbial contamination of hematopoietic stem cells occurs during extensive manipulation and processing in the laboratory (404,405). Potential sources include unprotected hands and laboratory equipment and freezers (406), particularly the liquid phases of liquid nitrogen freezers (407). Therefore, stem cell processing should be performed according to current standards (378) using approved manufacturing practices (AIII). Hematopoietic stem cell units thawed in a water bath should be enclosed in a second bag (i.e., double-bagged technique) to prevent contamination of the ports or caps from unsterile bath water (407) (BIII). Additionally, water baths should be cleaned routinely (BIII) and certain researchers have proposed that the bath contain sterile water (407) (CIII). Researchers also report sterilizing liquid nitrogen freezers before initial use for hematopoietic stem cell storage (407) until fungal and bacterial cultures are negative (CIII). Cell-processing laboratory personnel should implement programs to detect extrinsic bacterial or fungal contamination of collected stem cell units, ideally before transplantation (AIII). Although repeated cultures are costly (408), donated hematopoietic stem cells should be cultured for aerobic bacteria and fungi >1 times during initial processing and freezing (BIII). Researchers also have proposed adding anaerobic bacterial cultures and culturing twice, once at the end of processing, and once after thawing just before use (407) (CIII). If bacterial culture results are positive, antibiotic-susceptibility tests should be performed (BIII). Results of cultures and antibiotic-susceptibility tests should be provided to the transplant physician before release of a cryopreserved marrow or blood stem cell unit, and as soon as feasible for transplants infused before completion of culture incubation (BIII). Collection center, cell-processing laboratory, and transplant program personnel should maintain active surveillance of infections among persons who have received hematopoietic stem cells from those facilities to collect data regarding the number of infections after HSCT that might have been caused by exogenous contamination of donor stem cells (BIII) because this type of infection has been reported (405). In Utero or Fetal HSCTNo national standards exist for in utero or fetal HSCT, and the overall risks for transmitting infections to a fetus through HSCT (409,410) have not been determined. However, in addition to precautions appropriate for adult recipients, physicians performing in utero or fetal HSCT are advised to evaluate potential donors for evidence of active infectious diseases that could cause serious congenital infections (e.g., rubella, varicella, CMV, syphilis, or To. gondii) in the fetus (CIII). AcknowledgmentsThe authors gratefully acknowledge the assistance of the following persons in the preparation of this report: Jon S. Abramson, M.D.; Saundra N. Aker, R.D.; George J. Alangaden, M.D.; Ann Arvin, M.D.; Carol Baker, M.D.; Michael Boeckh, M.D.; Brian J. Bolwell, M.D.; John M. Boyce, M.D.; C. Dean Buckner, M.D.; Pranatharthi H. Chandrasekar, M.D.; D.W. Chen, M.D., M.P.H.; Joan Chesney, M.D.; Raymond Chinn, M.D.; Christina Cicogna, M.D.; Dennis Confer, M.D.; Stella M. Davies, M.D., Ph.D.; Alfred DeMaria, Jr., M.D.; David W. Denning, M.B.B.S.; Joseph Fay, M.D.; Stephen Forman, M.D.; Michael Gerber, M.D.; Anne A. Gershon, M.D.; Stuart L. Goldberg, M.D.; Marie Gourdeau, M.D.; Christine J. Hager, Ph.D.; Rebecca Haley, M.D.; Liana Harvath, Ph.D.; Kelly Henning, M.D.; Steve Heyse; Elizabeth Higgs; Kevin High, M.D.; Mary M. Horowitz, M.D.; Craig W.S. Howe, M.D., Ph.D.; David D. Hurd, M.D.; Hakan Kuyu, M.D.; Amelia A. Langston, M.D.; Catherine Laughlin, Ph.D.; Hillard M. Lazarus, M.D.; Joseph H. Laver, M.D.; Helen Leather, Phar.D.; Paul R. McCurdy, M.D.; Carole Miller, M.D.; I. George Miller, M.D.; Per Ljungman, M.D., Ph.D.; Paul R. McCurdy, M.D.; Richard J. O'Reilly, M.D.; Gary Overturf, M.D.; Jan E. Patterson, M.D.; Lauren Patton, D.D.S.; Doug Peterson, D.D.S., Ph.D.; Donna Przepiorka, M.D., Ph.D.; Philip A. Pizzo, M.D.; Charles G. Prober, M.D.; Issam Raad, M.D.; Elizabeth C. Reed, M.D.; Frank Rhame, M.D.; Olle Ringdén, M.D.; Stephen M. Rose, Ph.D.; Scott D. Rowley, M.D.; Pablo Rubinstein, M.D.; Martin Ruta; Joel Ruskin, M.D.; Thomas N. Saari, M.D.; Stephen Schoenbaum, M.D.; Mark Schubert, D.D.S., M.S.D.; Jane D. Siegel, M.D.; Jacqueline Sheridan; Alicia Siston, M.D.; Trudy N. Small, M.D.; Frank O. Smith, M.D.; Ruth Solomon, M.D.; Cladd Stevens, M.D.; Patrick J. Stiff, M.D.; Andrew J. Streifel, M.P.H.; Donna A. Wall, M.D.; Thomas Walsh, M.D.; Phyllis Warkentin, M.D.; Robert A. Weinstein, M.D.; Estella Whimbey, M.D.; Richard Whitley, M.D.; Catherine Wilfert, M.D.; Drew J. Winston, M.D.; Jeffrey Wolf, M.D.; Andrew Yeager, M.D.; John A. Zaia, M.D.; and Carol Zukerman. Contributions from the following CDC staff are also gratefully acknowledged: Larry J. Anderson, M.D.; Fred Angulo, D.V.M.; Richard Besser, M.D.; Jay C. Butler, M.D.; Donald Campbell; Mary E. Chamberland, M.D.; James Childs, Sc.D.; Nancy J. Cox, Ph.D.; Robert B. Craven, M.D.; Jackie Curlew; Vance J. Dietz, M.D., M.P.H.T.M.; Richard Facklam, Ph.D.; Cindy R. Friedman, M.D.; Keiji Fukuda, M.D., M.P.H.; Rana A. Hajjeh, M.D.; Barbara Herwaldt, M.D., M.P.H.; AnnMarie Jenkins; Dennis D. Juranek, D.V.M., M.Sc.; Paul Kilgore, M.D.; William Kohn, D.D.S.; Jacobus Kool, M.D., D.T.M.H.; John R. Livengood, M.D., M.Phil.; Eric Mast, M.D., M.P.H.; Michael McNeil, M.D., M.P.H.; Robin R. Moseley, M.A.T.; Thomas R. Navin, M.D.; Adelisa Panlilio, M.D., M.P.H.; Monica Parise, M.D.; Michele Pearson, M.D.; Bradford A. Perkins, M.D.; Renee Ridzon, M.D.; Martha Rogers, M.D.; Nancy Rosenstein, M.D.; Charles Rupprecht, Ph.D., V.M.D.; Peter Schantz, Ph.D.; Lawrence B. Schonberger, M.D., M.P.H.; Jane Seward, M.B.B.S., M.P.H.; John A. Stewart, M.D.; Raymond Strikas, M.D.; Robert V. Tauxe, M.D., M.P.H.; Theodore Tsai, M.D.; Rodrigo Villar, M.D.; David Wallace; and Sherilyn Wainwright, D.V.M., M.D. The contributions of staff from other federal and nongovernmental agencies are also gratefully acknowledged: Agency for Healthcare Research and Quality; Food and Drug Administration; Health Resources and Services Administration; National Institutes of Health; National Cancer Institute; National Heart, Lung, and Blood Institute; National Institute for Allergy and Infectious Diseases; CDC's Advisory Committee on Immunization Practices; American Academy of Pediatrics' Committee on Infectious Diseases; American Association of Blood Banks; Hospital Infection Control Advisory Committee; International Society of Hematotherapy and Graft Engineering; National Marrow Donor Program; Southwest Oncology Group; Foundation for the Accreditation of Hematopoietic Cell Therapy; and Society for Healthcare Epidemiology of America. ReferencesIntroduction
Background
Bacterial Infections
Viral Infections
Fungal Infections
Protozoal and Helminthic Infections
Hospital Infection Control
Strategies for Safe Living After HSCT -- Prevention of Exposure and Disease
HSCT Recipient Vaccinations
Hematopoietic Stem Cell Safety
* For this report, HSCT is defined as any transplantation of blood- or marrow-derived hematopoietic stem cells, regardless of transplant type (e.g., allogeneic or autologous) or cell source (e.g., bone marrow, peripheral blood, or placental/umbilical cord blood). In addition, HSCT recipients are presumed immunocompetent at >24 months after HSCT if they are not on immunosuppressive therapy and do not have graft-versus-host disease (GVHD), a condition that occurs when the transplanted cells recognize that the recipient's cells are not the same cells and attack them. ** Presently, no updated data have been published. *** Since November 1997, the United States has had a shortage of intravenous immunoglobulin (IVIG) (Source: CDC. Availability of immune globulin intravenous for treatment of immune deficient patients---United States, 1997--1998. MMWR 1999:48[8];159--162). Physicians who have difficulty obtaining IVIG should contact the following sources:
Physicians who are unable to obtain IVIG for a licensed indication from one of these sources should contact the Product Shortage Officer at the Food and Drug Administration's Center for Biologics Evaluation and Research, Office of Compliance, (301) 827-6220, for assistance. **** VZIG is distributed by FFF Enterprises, Inc., under contract with the American Red Cross, except in Massachusetts where it is distributed by the Massachusetts Public Health Biologic Laboratories (now a unit of the University of Massachusetts) (19) . FFF Enterprises, Inc., can be contacted at
***** For additional information regarding the epidemiology of Chagas' disease, contact CDC/National Center for Infectious Diseases/Division of Parasitic Diseases, (770) 488-7760. ****** Broviac dolls are used to demonstrate medical procedures (e.g., insertion of catheters) to children to lessen their fears. ******* For a list of filters certified under NSF Standard 053 for cyst (i.e., Cryptosporidium removal, contact the NSF International consumer line at (800) 673-8010 or <http://www.nsf.org/notice/crypto.html>. ******** The International Bottled Water Association can be contacted at (703) 683-5213 from 9 a.m. to 5 p.m. EST or anytime at their Internet site (<http://www.bottledwater.org>) to obtain contact information regarding water bottlers. ********* The U.S. Public Health Service is reexamining the current donor deferral recommendations regarding risk behaviors for donors of organs, cells, tissues, xenotransplantation, and reproductive cells and tissue, including semen, and revisions to these guidelines could become necessary as the research evolves. ********** Guidelines for screening UCB donors and their mothers are evolving and will not be addressed in this document. Table 1 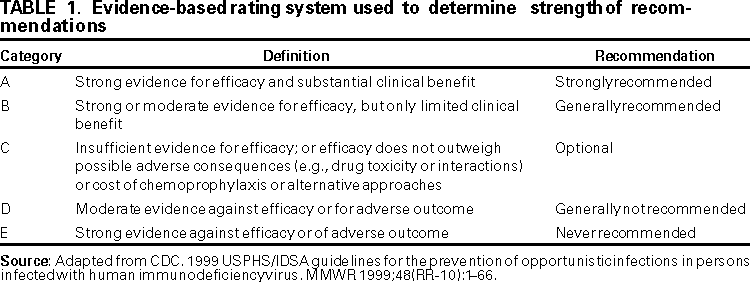 Return to top. Figure 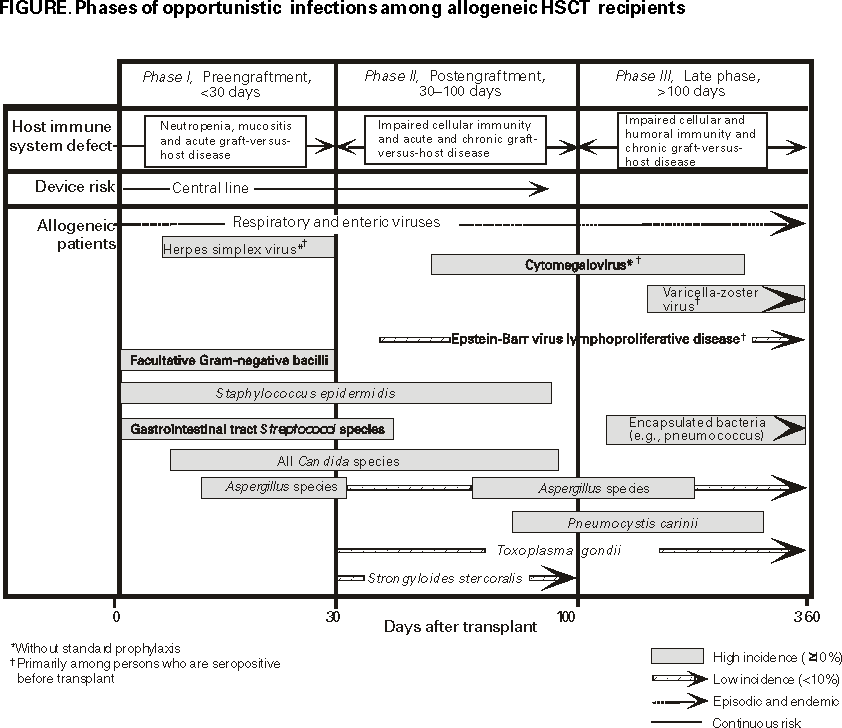 Return to top. Table 2 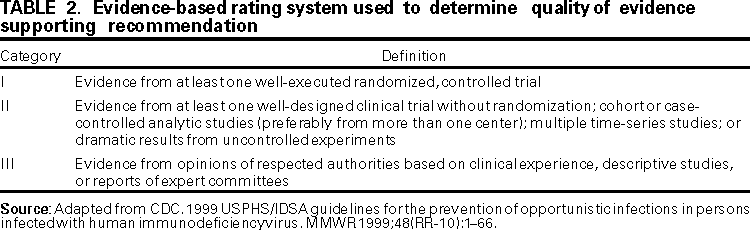 Return to top. Table 3  Return to top. Table 4 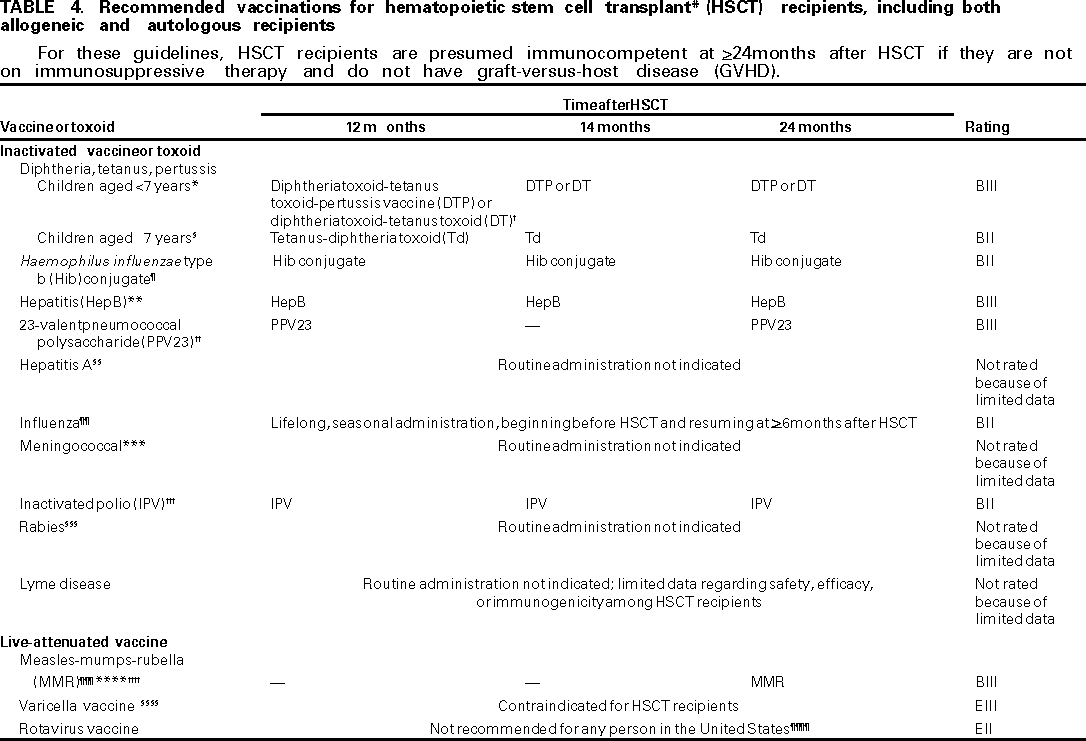   Return to top. Table 5 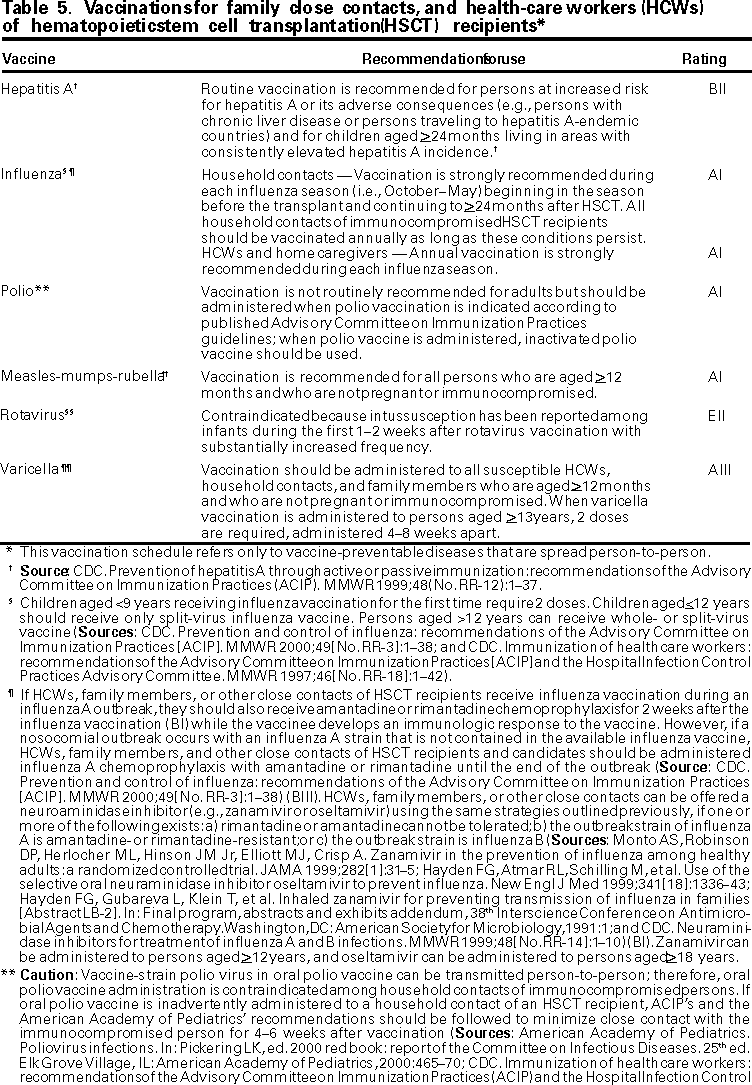  Return to top. Table 6 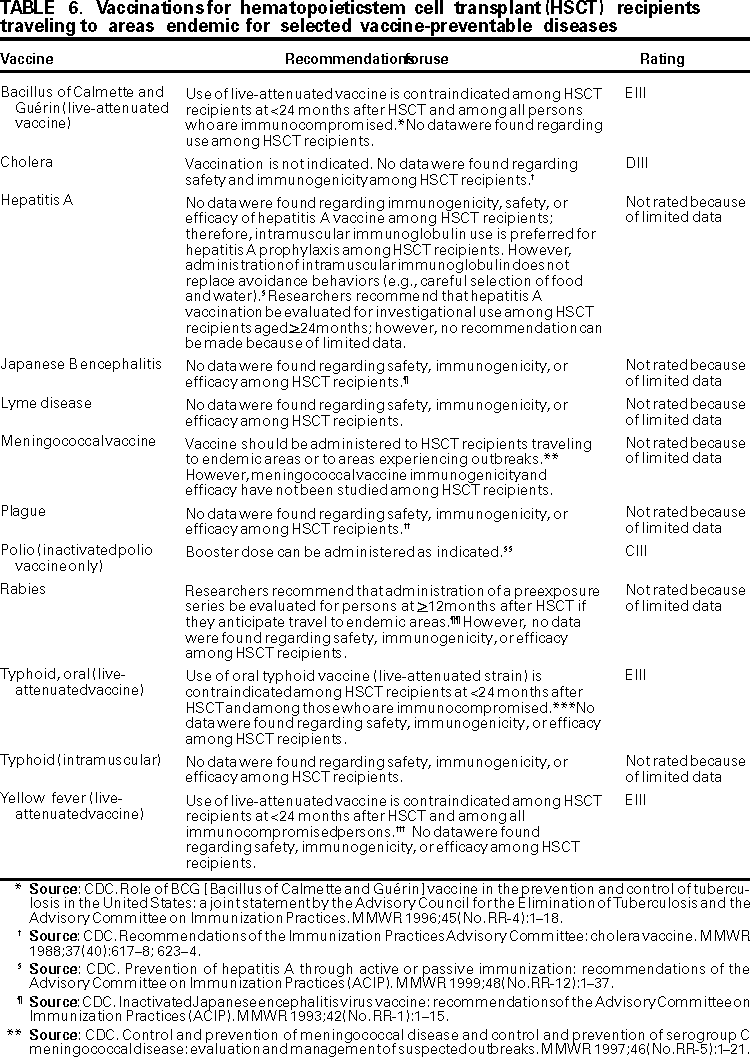  Return to top. Table 7 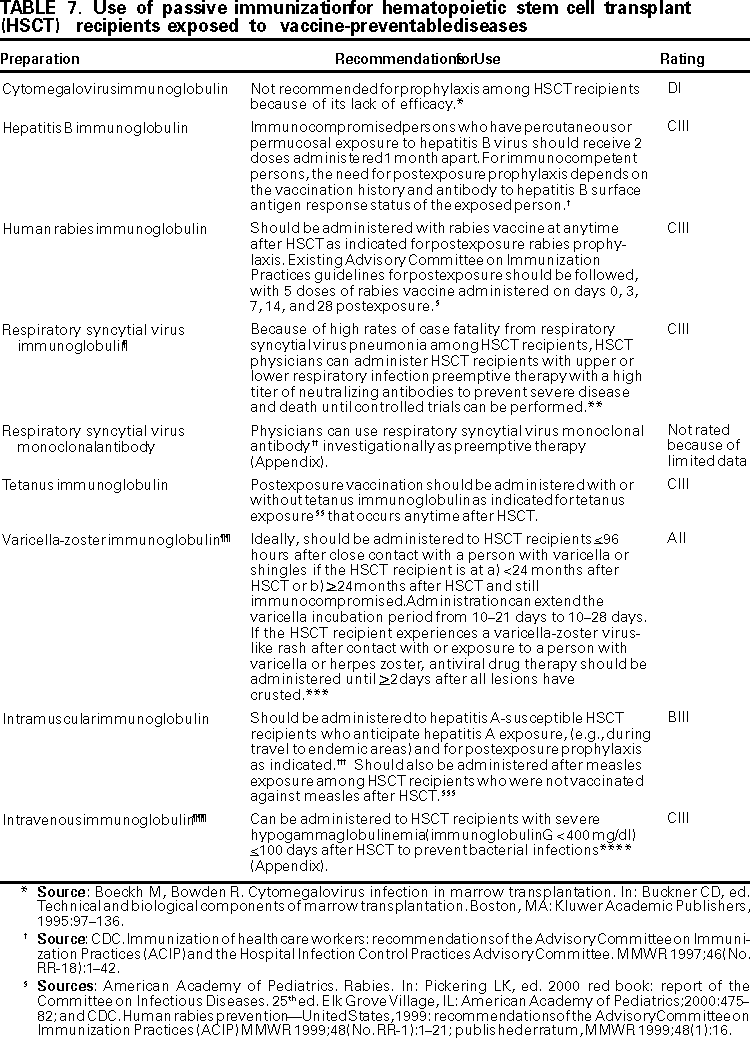  Return to top. Table 8   Return to top. Appendix AppendixDosing Charts for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant Recipients
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/11/2000 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This page last reviewed 5/2/01
|