 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention and Control of Meningococcal DiseaseRecommendations of the Advisory Committee on Immunization Practices (ACIP) An update has been published for this report. To view the update, please click here. Prepared by The material in this report originated in the National Center for Infectious Diseases, Ann Schuchat, MD, Acting Director, Division of Bacterial and Mycotic Diseases, Judith Aguilar, Acting Director; and the National Immunization Program, Stephen Cochi, MD, Acting Director, Epidemiology and Surveillance Division, Gina Mootrey, DO, Acting Director, and Immunization Services Division, Lance Rodewald, MD, Director.
Corresponding preparer: Oleg Bilukha, MD, PhD, National Center for Infectious Diseases, CDC, 1600 Clifton Road NE, MS C-09, Atlanta, GA, 30333. Telephone: 404-639-1367; Fax: 404-639-3059; e-mail: OBB0@cdc.gov. Summary In January 2005, a tetravalent meningococcal polysaccharide-protein conjugate vaccine ([MCV4] Menactra,™ manufactured by Sanofi Pasteur, Inc., Swiftwater, Pennsylvania) was licensed for use among persons aged 11--55 years. CDC's Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of young adolescents (defined in this report as persons aged 11--12 years) with MCV4 at the preadolescent health-care visit (at age 11--12 years). Introducing a recommendation for MCV4 vaccination among young adolescents might strengthen the role of the preadolescent visit and have a positive effect on vaccine coverage among adolescents. For those persons who have not previously received MCV4, ACIP recommends vaccination before high-school entry (at approximately age 15 years) as an effective strategy to reduce meningococcal disease incidence among adolescents and young adults. By 2008, the goal will be routine vaccination with MCV4 of all adolescents beginning at age 11 years. Routine vaccination with meningococcal vaccine also is recommended for college freshmen living in dormitories and for other populations at increased risk (i.e., military recruits, travelers to areas in which meningococcal disease is hyperendemic or epidemic, microbiologists who are routinely exposed to isolates of Neisseria meningitidis, patients with anatomic or functional asplenia, and patients with terminal complement deficiency). Other adolescents, college students, and persons infected with human immunodeficiency virus who wish to decrease their risk for meningococcal disease may elect to receive vaccine. This report updates previous reports from ACIP concerning prevention and control of meningococcal disease. It also provides updated recommendations regarding use of the tetravalent meningococcal polysaccharide vaccine (MPSV4) and on antimicrobial chemoprophylaxis. IntroductionNeisseria meningitidis has become a leading cause of bacterial meningitis in the United States after dramatic reductions in the incidence of Streptococcus pneumoniae (1) and Haemophilus influenzae type b (Hib) (2) infections have been achieved as a result of using conjugate vaccines. CDC's Advisory Committee on Immunization Practices (ACIP) previously recommended a tetravalent polysaccharide vaccine (Menomune®-A,C,Y,W-135, manufactured by Sanofi Pasteur, Inc., Swiftwater, Pennsylvania) for use among certain populations at increased risk, including travelers to countries with epidemic or hyperendemic meningococcal disease, persons who have certain medical conditions (i.e., terminal complement component deficiencies and anatomic or functional asplenia), and laboratory personnel who are routinely exposed to N. meningitdis in solutions that might be aerosolized (3). Use of this vaccine also was recommended for control of meningococcal disease outbreaks (4). Recommendations permitting use of MPSV4 among college freshmen have been published previously (5). The new tetravalent A, C, Y, W-135 conjugate vaccine (Menactra™, manufactured by Sanofi Pasteur, Inc.) licensed for persons aged 11--55 years should become a key addition to existing meningococcal disease prevention measures. This report provides ACIP's recommendations on prevention and control of meningococcal disease, including recommendations on use of the new tetravalent conjugate vaccine (MCV4) as well as updated recommendations on use of the polysaccharide vaccine (MPSV4) and on antimicrobial chemoprophylaxis. BackgroundEpidemiology of Meningococcal DiseaseEach year, an estimated 1,400--2,800 cases of meningococcal disease occur in the United States, a rate of 0.5--1.1/100,000 population (CDC, unpublished data, 2004). N. meningitidis colonizes mucosal surfaces of nasopharynx and is transmitted through direct contact with large droplet respiratory secretions from the patients or asymptomatic carriers. Humans are the only host. Despite the continued sensitivity of meningococcus to multiple widely available antibiotics, including penicillin (6,7), the case-fatality ratio for meningococcal disease is 10%--14% (CDC, unpublished data, 2004). Meningococcal disease also causes substantial morbidity; 11%--19% of survivors have sequelae (e.g., neurologic disability, limb loss, and hearing loss) (8,9). During 1991--2002, the highest rate of meningococcal disease (9.2/100,000) occurred among infants aged <1 year; the rate for persons aged 11--19 years (1.2/100,000) also was higher than that for the general population (Figure 1). Although rates of disease are highest among children aged <2 years, 62% of meningococcal disease in the United States occurs among persons aged >11 years (CDC, unpublished data, 2004). In the United States, >98% of cases of meningococcal disease are sporadic; however, since 1991, the frequency of localized outbreaks has increased (10,11). The proportion of meningococcal cases caused by serogroup Y increased from 2% during 1989--1991 (12) to 37% during 1997--2002 (CDC, unpublished data, 2004). Serogroups B, C, and Y are the major causes of meningococcal disease in the United States, each being responsible for approximately one third of cases. The proportion of cases caused by each serogroup varies by age group. Among infants aged <1 year, >50% of cases are caused by serogroup B, for which no vaccine is licensed or available in the United States (13,14). Of all cases of meningococcal disease among persons aged >11 years, 75% are caused by serogroups (C, Y, or W-135), which are included in vaccines available in the United States (CDC, unpublished data, 2004). Persons who have deficiencies in the terminal common complement pathway (C3, C5--9) (15,16) and those with anatomic or functional asplenia (17) are at increased risk for acquiring meningococcal disease. Antecedent viral infection, household crowding, chronic underlying illness, and both active and passive smoking also are associated with increased risk for meningococcal disease (18--25). During outbreaks, bar or nightclub patronage and alcohol use also have been associated with higher risk for meningococcal disease (26--28). In the United States, blacks and persons of low socioeconomic status (SES) have been consistently at higher risk for meningococcal disease (12,13). However, race and low SES are likely risk markers rather than risk factors for this disease. A multistate case-control study in which controls were matched to case-patients by age group indicated that in a multivariable analysis (controlling for sex and education), active and passive smoking, recent respiratory illness, corticosteroid use, new residence, new school, Medicaid insurance, and household crowding all were associated with increased risk for meningococcal disease, whereas income and race were not (18). Additional research is needed to identify groups at risk that might benefit from prevention efforts. Meningococcal Disease and College StudentsMultiple studies have been conducted in the United States (29--31) and the United Kingdom (32,33) concerning the risk for meningococcal disease among college students. The risk for meningococcal disease among U.S. college students was higher for those who resided in dormitories than for those residing in other types of accommodations. Overall incidence among college students usually is similar to or somewhat lower than that observed among persons in the general population of similar age. The earliest of these studies (conducted during the 1990--91 and 1991--92 academic years) had a poor response rate (38%) and indicated a low overall incidence of meningococcal disease among U.S. college students (1.0/100,000 population/year) (31). Cases of meningococcal disease occurred 9--23 times more frequently among students living in dormitories than among those living in other types of accommodations. A retrospective cohort study conducted in Maryland during 1992--1997 (30) indicated that the overall incidence of meningococcal disease among college students was similar to that among the U.S. population of persons the same age (1.7/100,000 and 1.4/100,000, respectively); however, rates of disease among students living in dormitories were higher than rates among students living off campus (3.2/100,000 and 1.0/100,000, respectively; p = 0.05). U.S. surveillance data from the 1998--99 school year (29) indicated that the overall rate of meningococcal disease among undergraduate college students was lower than the rate among persons aged 18--23 years who were not enrolled in college (0.7 and 1.4/100,000, respectively) (Table 1). Rates were somewhat higher among freshmen (1.9/100,000). Among the approximately 600,000 freshmen living in dormitories, rates were higher (5.1/100,000) than among any age group in the population other than children aged <2 years but lower than the threshold (10/100,000) recommended for initiating meningococcal vaccination campaigns (4). In a case-control study involving 50 cases detected among college students (29), multivariate analysis indicated that freshmen living in dormitories were at higher risk for meningococcal disease than other students (matched odds ratio [OR]: 3.6; 95% confidence interval [CI] = 1.6--8.5). In the United Kingdom, rates of meningococcal disease were higher among university students than among nonstudents of similar age (32). Regression analysis indicated that the main risk factor was catered hall accommodations (the U.K. equivalent of U.S. dormitories). A recent study conducted in the United Kingdom demonstrated a rapid increase in carriage rates of meningococci among university students in the first week of the fall semester, although rates of disease peaked later in the academic year (33). The increased rate of disease among university students has prompted the United Kingdom to initiate routine vaccination of incoming university students with a bivalent A/C polysaccharide vaccine as part of a new vaccination program (34). In 2000, ACIP and the Committee on Infectious Diseases of the American Academy of Pediatrics (AAP) concluded that college students, especially those living in dormitories, are at moderately increased risk for meningococcal disease compared with other persons their age (5). ACIP and AAP recommended that 1) college students and their parents be informed by health-care providers of the risks of meningococcal disease and of the potential benefits of vaccination with MPSV4; 2) college and university health services facilitate implementation of educational programs about meningococcal disease and the availability of vaccination services; and 3) MPSV4 be made available to those persons requesting vaccination. As of November 2004, a total of 31 states had adopted legislation requiring colleges to provide information on risks of meningococcal disease either to matriculating students or to students residing on campus, and 10 states had mandated vaccination for certain students, unless a vaccination waiver is provided (Figure 2) (35). In 2004, the American College Health Association conducted an Internet-based survey of college policies and practices related to meningococcal vaccination (36). Of the 72 (10%) contacted colleges and universities that responded, 60% reported having a written policy on meningococcal vaccination, and 80% reported conducting some type of outreach awareness program among college students or their parents. Median vaccination rates reported for the 2002--03 and 2003--04 academic years were 20% and 35%, respectively; 67% reported an increase in vaccination rates during the previous 3 years. On the basis of the number of vaccine doses sold, during the 2004--05 academic year, approximately 1.1 million college students received MPSV4 before arrival on campus, and an estimated 50,000--100,000 students received vaccine after arrival on campus (Sanofi Pasteur, Inc., unpublished data, 2004). Evaluation and Management of Suspected Outbreaks of Meningococcal DiseaseSince the early 1990s, outbreaks of meningococcal disease have occurred with increasing frequency in the United States. During July 1994--June 2002, a total of 76 outbreaks were identified (annual median: 10; range: 4--16) (11), including 48 (63%) outbreaks caused by serogroup C, 19 (25%) by serogroup B, and nine (12%) by serogroup Y. These outbreaks occurred in 32 states and involved 247 patients (accounting for <2% of total cases of meningococcal disease in the United States during this period). Of the 76 outbreaks, 26 (34%) were community-based and accounted for 53% of all outbreak-related cases. Of the 50 (65%) outbreaks that were organization-based, 13 (26%) occurred in colleges; 19 (38%) in primary and secondary schools; and nine (18%) in nursing homes. Vaccination campaigns (using an average of 2,500 doses of MPSV4 per outbreak) were conducted in 34 outbreaks (30 of which were caused by serogroup C and four by serogroup Y) (11). The decision to implement a mass vaccination campaign to prevent meningococcal disease depends on whether the occurrence of more than one case represents an outbreak or an unusual clustering of endemic disease. Because the number of cases in outbreaks is usually not substantial, this determination often requires evaluation and analysis of the patterns of disease occurrence. Mass vaccination campaigns are expensive, require a massive public health effort, and can create unwarranted concern among the public. Detailed information on evaluation and management of suspected outbreaks has been published previously (4) and is presented in this report. Case DefinitionsThe following case definitions are used in this report:
Organization- and Community-Based OutbreaksAn outbreak usually is classified as organization-based if it involves the occurrence of three or more confirmed or probable cases of meningococcal disease of the same serogroup in <3 months among persons who have a common affiliation but no close contact with each other, resulting in primary disease attack rate of >10 cases/100,000 persons. Calculation of attack rates for organization-based outbreaks is most useful for large organizations (e.g., universities). However, in the majority of organization-based outbreaks with three or even two cases of disease, the rate will be >10 cases/100,000 population. In such situations, public health officials also might consider vaccination after only two primary cases are identified. An outbreak is classified as community-based if it involves the occurrence of three or more confirmed or probable cases of meningococcal disease in <3 months among persons residing in the same area who are not close contacts of each other and who do not share a common affiliation, with a primary disease attack rate of >10 cases/100,000 persons. Distinguishing whether an outbreak should be classified as organization- or community-based is complicated by the fact that, in certain instances, these types of outbreaks occur simultaneously. Population at RiskIn addition to close contacts, persons considered to be at increased risk for meningococcal disease compared with historical rates of disease in the same population in the general U.S. population are classified as being at risk. The population at risk is used as the denominator in calculations of the disease attack rate. The population at risk is usually defined on the basis of organizational affiliation or community of residence. In organization-based outbreaks, cases are linked by a common affiliation other than a shared, geographically delineated community; the population at risk is thus usually the group of persons who best represent that affiliation. For example, if the only association between patients is attending the same school or university, the population at risk is all persons attending the school or university. In community-based outbreaks, patients have no common affiliation other than a shared, geographically defined community. The population at risk can be defined as the smallest geographically contiguous population that includes all (or nearly all) patients. This population is usually a neighborhood, town, city, or county, whose size is obtained from census data. Attack Rate and Decision To VaccinateFor a primary attack rate to be calculated, all confirmed cases of the same serogroup should be summed; secondary cases should be excluded and each set of co-primary cases counted as one case. Because attack rates are calculated both to characterize the risk for disease among the general population and to determine whether overall rates have increased, related cases (secondary and co-primary) should not be included. From an epidemiologic perspective, secondary and co-primary cases can be considered as representing single episodes of disease with direct spread to one or more close contact(s), which is consistent with endemic disease. If three or more cases have occurred in either an organization- or a community-based outbreak during <3 months (starting at the time of the first confirmed or probable case), a primary attack rate should be calculated. Because of the limited number of cases typically involved and the seasonal patterns of meningococcal disease (more cases occur during fall than other times of the year), rate calculations should not be annualized. The following formula is used to calculate attack rates: Attack rate per 100,000 = [(number of primary confirmed or probable cases during a 3-month period) / (number of population at risk)] x 100,000 Vaccination of the population at risk should be considered if the attack rate is >10 cases/100,000 persons. The actual attack rate at which the decision to vaccinate is made varies. Public health personnel should consider the following factors: 1) completeness of case reporting and number of possible cases of meningococcal disease for which bacteriologic confirmation or serogroup data are not available; 2) occurrence of additional cases of meningococcal disease after recognition of a suspected outbreak (e.g., if the outbreak occurred 2 months previously and if no additional cases have occurred, in which case vaccination might be unlikely to prevent additional cases of meningococcal disease); and 3) logistic and financial considerations. Because available vaccines are not effective against N. meningitdis serogroup B, vaccination should not be considered during serogroup B outbreaks. Vaccination GroupThose persons designated to be administered vaccine during a vaccination campaign comprise a vaccination group. The vaccination group usually includes either the whole or a subset of the population of risk. Because meningococcal disease outbreak cases occur predominantly among persons aged <30 years (10,11), and available vaccines are not recommended among children aged <2 years, the vaccination group usually is that portion of the population at risk aged 2--29 years. In the majority of organization-based outbreaks, the vaccination group includes the whole population at risk, provided that all persons are aged >2 years. If a substantial proportion of patients are aged <2 years and thus are not eligible to receive vaccine, patients aged <2 years should be excluded, and, if at least three patients remain, the attack rate should be recalculated. If the recalculated attack rate remains >10 cases/100,000 persons, vaccination should be considered for part or all of the population at risk aged >2 years. In certain organization-based outbreaks, a vaccination group larger than the population at risk might be designated. For example, in a high school in which all outbreak-associated cases occurred among students, authorities might decide to offer vaccine to staff. In community-based outbreaks, the vaccination group usually can be defined as a subset of the population at risk (e.g., persons aged 2--29 years). If a substantial proportion of patients are aged <2 years, these patients might be excluded from calculation of an attack rate. In rare situations (e.g., in a town with a limited population) in which multiple cases have occurred among adults aged >29 years, the entire population aged >2 years might be considered for vaccination. For more substantial populations, this decision would be costly in terms of finances and human resources, and restricting the vaccination group to the persons in age groups with the highest attack rates might be more appropriate. Age-specific attack rates can be calculated by using the formula previously provided and by restricting the numerator and denominator to persons within specific age groups (e.g., persons aged 2--29 years). Genotyping of N. meningitdis IsolatesGenotyping of N. meningitdis isolates by using such methods as pulsed-field gel electrophoresis or ribotyping might provide useful information for determining whether a group of cases represents an outbreak (38). Outbreaks of meningococcal disease usually are caused by closely related strains. Genotyping data can allow identification of an outbreak strain and help to better define the extent of the outbreak. If strains from a group of patients are unrelated by genotyping, the group of cases most likely does not represent an outbreak. Because molecular subtyping testing might not be readily available or accessible, initiation of outbreak-control efforts should not be delayed until genotyping results are available. Other Control MeasuresMass chemoprophylaxis (i.e., administration of antibiotics to substantial populations) is not recommended to control large outbreaks of disease. Disadvantages of mass chemoprophylaxis include cost of the drug and administration, difficulty of ensuring simultaneous administration of drugs to substantial populations, drug side effects, and emergence of resistant organisms. In addition, multiple sources and prolonged risk for exposure make this approach impractical and unlikely to succeed. In the majority of outbreak settings, these disadvantages outweigh the possible benefit in disease prevention. However, in outbreaks involving limited populations (e.g., an outbreak in a single school), administration of chemoprophylaxis might be considered (39), especially in serogroup B outbreaks, for which available vaccines are not effective (40). When making a decision about initiating mass chemoprophylaxis in these settings, public health officials should consider not only the potential for prevention of new cases but also the logistics, cost, and potential for developing antimicrobial resistance (39,41). If mass chemoprophylaxis is undertaken, it should be administered to all targeted persons at the same time. In the United States, measures that have not been recommended for control of meningococcal disease outbreaks include restricting travel to areas with an outbreak, closing schools or universities, or canceling sporting or social events. Educating communities, physicians, and other health-care workers about meningococcal disease to promote an early case recognition and early care-seeking behaviors is an important part of managing suspected meningococcal disease outbreaks. Education efforts should be initiated as soon as an outbreak of meningococcal disease is suspected (4). Information about the signs and symptoms of meningococcal disease is avail-able at http://www.cdc.gov/ncidod/dbmd/diseaseinfo/meningococcal_g.htm. Meningococcal Tetravalent Polysaccharide VaccineVaccine CompositionMPSV4 is a tetravalent meningococcal polysaccharide vaccine (Menomune-A,C,Y,W-135, manufactured by Sanofi Pasteur, Inc., Swiftwater, Pennsylvania) available in the United States (42). Each dose consists of the four (A, C, Y, W-135) purified bacterial capsular polysaccharides (50 µg each). MPSV4 (Menomune) is available in single-dose (0.5-mL) and 10-dose (5-mL) vials; 50-dose vials are no longer available. Vaccine Immunogenicity and EfficacyThe immunogenicity and clinical efficacy of the serogroups A and C meningococcal vaccines have been well established. The serogroup A polysaccharide induces antibody response among certain children as young as age 3 months, although a response comparable with that occurring in adults is not achieved until age 4--5 years; the serogroup C component is poorly immunogenic among recipients aged <18--24 months (43,44). The serogroups A and C vaccines have demonstrated estimated clinical efficacies of >85% among school-aged children and adults and are useful in controlling outbreaks (45--49). Serogroups Y and W-135 polysaccharides are safe and immunogenic among adults and children aged >2 years (50--52); although clinical protection has not been documented, vaccination with these polysaccharides induces production of bactericidal antibodies. The antibody responses to each of the four polysaccharides in the tetravalent vaccine are serogroup specific and independent. Persons whose spleens have been removed because of trauma or nonlymphoid tumors and persons who have inherited complement deficiencies have acceptable antibody responses to polysaccharide meningococcal vaccine (53--55). A 2003 study indicated that tetravalent polysaccharide vaccine substantially reduced the incidence of invasive meningococcal disease among patients with terminal complement deficiency compared with similar patients who were unvaccinated (16). Reduced clinical efficacy has not been demonstrated among persons who have received multiple doses of vaccine. However, recent serologic studies have reported that multiple doses of serogroup A and C polysaccharide vaccine might cause immunologic hyporesponsiveness (i.e., a reduced antibody response after subsequent challenge with the same polysaccharide antigen) to group A (56,57) and C (58,59) polysaccharide. The clinical relevance of such hyporesponsiveness is unclear. Duration of ProtectionAmong infants and children aged <5 years, measurable levels of antibodies against group A and C polysaccharides decreased substantially during the first 3 years after a single dose of vaccine; among healthy adults, antibody levels also decreased, but antibodies were still detectable <10 years after vaccine administration (43,60--63). Similarly, although vaccine-induced clinical protection likely persists among school-aged children and adults for >3 years, the efficacy of the group A vaccine among children aged <5 years might decrease markedly within this period. In one study, efficacy among children aged <4 years at the time of vaccination declined from >90% to <10% within 3 years after vaccination; efficacy was 67% among children who were aged >4 years when vaccinated (64). Precautions and ContraindicationsMeningococcal polysaccharide vaccines have been used extensively in mass vaccination programs as well as in the military and among international travelers. Adverse reactions to polysaccharide meningococcal vaccines are usually mild; the most frequent reaction is pain and redness at the injection site, lasting for 1--2 days. Estimates of the incidence of such local reactions have varied (range: 4%--56%) (65,66). In certain studies, transient fever occurred among <5% of persons vaccinated, more commonly among infants (44,67). Severe reactions to polysaccharide meningococcal vaccine are uncommon (44,52,65--71). The majority of studies report the rate of systemic allergic reactions (e.g., urticaria, wheezing, and rash) as 0--0.1/100,000 vaccine doses (44,71). Anaphylaxis has been documented among <0.1/100,000 vaccine recipients (42,70). Neurologic reactions (e.g., seizures, anesthesias, and paresthesias) have also been observed infrequently (65,70). Meningococcal Conjugate VaccinesAdvantages of Meningococcal Conjugate VaccinesBacterial polysaccharides, including those comprising the capsule of N. meningitdis, are T-cell--independent antigens. T-cell--independent antigens do not elicit a memory response; they stimulate mature B-lymphocytes but not T-lymphocytes, thus inducing a response that is neither long-lasting nor characterized by an anamnestic response after subsequent challenge with the same polysaccharide antigen (72). Thus, meningococcal polysaccharide vaccines have inherent limitations. The serogroup C polysaccharide is poorly immunogenic among children aged <2 years (73--75). The A polysaccharide induces antibody response in infants, but vaccine efficacy declines rapidly (64). Meningococcal polysaccharide vaccines do not confer long-lasting immunity (61,64); they also do not cause a sustainable reduction of nasopharyngeal carriage of N. meningitdis (76,77) and therefore do not substantially interrupt transmission to elicit herd immunity. Finally, multiple doses of serogroup A and C polysaccharide vaccine might cause immunologic hyporesponsiveness to the group A (56,57) and C (58,59) polysaccharide, although clinical implications of this phenomenon are unknown. Conjugation (i.e., covalent coupling) of polysaccharide to a protein carrier that contains T-cell epitopes changes the nature of immune response to polysaccharide from T-cell--independent to T-cell--dependent, leading to a substantial primary response among infants and a strong anamnestic response at re-exposure (78). Both conjugate Hib and conjugate S. pneumoniae vaccines (introduced for mass infant immunization in the United States in 1990 and 2000, respectively) have reduced incidence of disease caused by vaccine-preventable serotypes (1,79). In addition, both vaccines reduce asymptomatic carriage of respective bacteria (80--82), thus protecting unvaccinated persons through a herd immunity effect (1). Meningococcal Serogroup C Conjugate Vaccine in the United KingdomIn November 1999, monovalent serogroup C conjugate vaccines were introduced in the United Kingdom. The national vaccination campaign introduced a routine 3-dose infant vaccination series and implemented a mass catch-up campaign during 1999--2000 targeting all persons aged 12 months--17 years (34). The three serogroup C conjugate vaccines used in the United Kingdom are Meningtec™ (Wyeth Lederle Vaccines and Pediatrics, Pearl River, New York); Menjugate™ (Chiron Vaccines, Siena, Italy); and NeisVac™ (Baxter Hyland Immuno, Beltsville, Maryland). Two vaccines (Meningtec and Menjugate) contain short-chain oligosaccharide (O-acetylated) derived from serogroup C capsular polysaccharide, conjugated to CRM197, a nontoxic mutant diphtheria toxin. The third vaccine (NeisVac) contains serogroup C polysaccharide (de-O-acetylated) conjugated to tetanus toxoid (83,84). The serogroup C conjugate meningococcal vaccines used in this campaign were licensed on the basis of data on safety and immunogenicity but without data on clinical efficacy (85). By 2001--2002, vaccine coverage in the United Kingdom was estimated as 80% among infants, 84% among toddlers, 76% among preschoolers, and 86%--87% among schoolchildren (86). Effectiveness of the vaccine within the first year of vaccination ranged from 88% to 98% among different age groups (87--89). Insufficient data are available to differentiate efficacy of the three meningococcal conjugate vaccines. Because the vaccine campaign was initiated only in 1999, long-term data on duration of protection are not yet available. However, among infants who received 3 doses of vaccine at ages 2, 3, and 4 months, efficacy declined to -81% (95% CI = -7,430--71) after only 1 year (88). Although the number of cases remains low, likely in part as a result of vaccine-induced herd immunity, this study raises questions about the meningococcal vaccine schedule and the need for a booster dose. During 1999--2000, carriage rates of group C meningococci in the United Kingdom declined 66% (90). In addition, incidence of meningococcal serogroup C disease declined 67% among unvaccinated persons aged 1--17 years and 35% among persons aged >25 years who were not targeted for vaccination, indicating the additional vaccine benefit of eliciting herd immunity (86). Meningococcal Tetravalent Conjugate VaccineVaccine CompositionMCV4 is a tetravalent meningococcal conjugate vaccine (Menactra, manufactured by Sanofi Pasteur, Inc., Swiftwater, Pennsylvania) that was licensed for use in the United States in January 2005. A 0.5-mL single dose of vaccine contains 4 µg each of capsular polysaccharide from serogroups A, C, Y, and W-135 conjugated to 48 µg of diphtheria toxoid. MCV4 is available only in single-dose vials. Immunologic Correlates of ProtectionStudies among U.S. military recruits conducted in the 1960s indicated that the absence of naturally acquired bactericidal antibodies, measured by a serum bactericidal antibody assay (SBA) using an intrinsic human complement source, was associated with susceptibility to meningococcal group C disease. SBA titers >4 using human serum as an exogenous complement source (hSBA) are considered the standard correlate of clinical protection against serogroup C meningococcal disease (91). Serogroup C conjugate meningococcal vaccines were licensed in the United Kingdom on the basis of data on safety and immunogenicity, without data on clinical efficacy (85). The immunologic data supporting the use of conjugate serogroup C vaccines were generated by serum bactericidal assay by using baby rabbit complement (rSBA). The threshold values were validated by comparing rSBA titers with those obtained by using hSBA (85,92). For licensure in the United Kingdom, rSBA titers of >128 were considered to predict protection; however, only 60% of rSBA titers in the range of 8--64 had hSBA titers of >4. For rSBA titers in this equivocal range, a fourfold rise in titers pre- to postvaccination was also proposed as a correlate of protection (92). Further evaluation of these threshold values was performed by using vaccine efficacy estimates from postlicensure surveillance, which indicated that these threshold values provided a conservative estimate of short-term clinical efficacy; rSBA threshold of >128 underestimated efficacy, with rSBA cutoffs of >4-->8 at 4 weeks after vaccination being most consistent with observed clinical efficacy (93). On the basis of these efficacy estimates, the proportion of responders in multiple clinical trials of meningococcal C conjugate vaccines, and the group C seroprevalence study conducted before introduction of group C conjugate vaccines (94), rSBA titers of <8 have been proposed to be predictive of susceptibility to invasive meningococcal disease, and rSBA titers of >8 have been proposed to correlate with short-term protection (95). Limited or no similar data exist to link immune response with clinical efficacy for serogroups A, Y, or W-135. In 1981, MPSV4 (Menomune) was licensed in the United States on the basis of data on safety and immunogenicity. Immunogenicity of this vaccine was compared with that of the vaccine then licensed for use in the United States, A/C meningococcal polysaccharide vaccine, which had demonstrated 97% efficacy against serogroup A and 90% efficacy against serogroup C (96). The immunologic criterion used for licensing was a fourfold or greater rise in SBA among 90% of adults at 3--4 weeks after vaccination. As a result, in 2005, MCV4 (Menactra) was licensed on the basis of findings indicating that it was not inferior to MPSV4 in terms of immunogenicity and safety (i.e., demonstrated noninferiority). A primary criterion in determining immunogenic noninferiority of the new vaccine was the percentage of vaccinees having a fourfold or greater increase in bactericidal antibody for MCV4 compared with MPSV4. ImmunogenicityImmunogenicity Among Persons Aged 11--18 Years A randomized controlled trial conducted among persons aged 11--18 years compared immunogenicity of MCV4 with that of MPSV4 at 28 days after vaccination. A similar percentage of subjects achieved at least a fourfold rise in rSBA titers in MCV4 and MPSV groups (Table 2). The percentage of subjects with at least a fourfold rise in rSBA was highest for serogroup W-135 (96.7% in MCV4 group and 95.3% in MPSV4 group), and lowest for serogroup Y (81.8% and 80.1%, respectively). The percentage of subjects achieving an rSBA geometric mean titer (GMT) of >128 was high (>98% for all serogroups) in both MCV4 and MPSV4 groups (97,98). Immunogenicity Among Persons Aged 18--55 Years Another randomized controlled trial conducted among persons aged 18--55 years compared immunogenicity of MCV4 and that of MPSV4 at 28 days after vaccination. Although the percentage of subjects achieving at least a fourfold increase in rSBA titer for each serogroup was higher in the MPSV4 group than in the MCV4 group (Table 2), the criteria for demonstrating immunologic noninferiority to MPSV4 were still achieved. As was the case among persons aged 11--18 years, this percentage was highest for serogroup W-135 (89.4% in the MCV4 group and 94.4% in the MPSV4 group) and lowest for serogroup Y (73.5% and 79.4%, respectively). The percentage of subjects achieving an rSBA GMT of >128 was high (>97% for all serogroups) in both MCV4 and MPSV4 groups (97,98). Persistence of Antibodies After 3 Years and Response to Revaccination MCV4 was administered to 76 subjects previously vaccinated with MCV4, 77 subjects previously vaccinated with MPSV4, and 88 age-matched vaccine-naïve subjects (97) (Sanofi Pasteur, Inc., unpublished data, 2004). Immunologic indices were measured before revaccination (day 0) and at days 8 and 28 after revaccination (Table 3). Subjects initially vaccinated with MCV4 had higher rSBA GMT at day 0 than those vaccinated with MPSV4 (Table 3); this difference was statistically significant for serogroups A (p<0.001) and W-135 (p<0.001). In addition, a higher percentage of those initially vaccinated with MCV4 had rSBA titers of >128 than those initially vaccinated with MPSV4 (Table 3). Vaccine-naïve subjects had lower rSBA on day 0 than subjects previously vaccinated with either MCV4 or MPSV4. Response to revaccination with MCV4 was assessed by administering MCV4 to subjects previously vaccinated with MPSV4 or MCV4 and to vaccine-naïve control subjects. All subjects in all three groups achieved rSBA titers of >128 at both 8 and 28 days after receiving MCV4 (Table 3). Subjects initially primed with MCV4 achieved higher rSBA GMTs than naïve control subjects for all serogroups except A. In contrast, rSBA GMTs of those primed with MPSV4 were lower than those of vaccine-naïve control subjects on both days 8 and 28 for all serogroups (Table 3). Concomitant Administration of MCV and Other Vaccines The concomitant administration of MCV4 and tetanus and diphtheria toxoids adsorbed for adult use (Td, manufactured by Sanofi Pasteur, Inc., Swiftwater, Pennsylvania) was evaluated in a double-blind, controlled trial of participants aged 11--17 years. One group received Td and MCV4 concomitantly at separate injection sites, followed by a saline placebo 28 days later; the other group received Td and a saline placebo at separate injection sites, followed 28 days later by MCV4. Concomitant administration of Td and MCV4 did not adversely affect immune response to either vaccine (97,98). When MCV4 and Td were administered concomitantly, antibody response to diphtheria antigen 28 days after vaccination was greater (diphtheria GMT 120.9 IU/mL) than when Td and MCV4 were administered sequentially, Td first (diphtheria GMT 8.4 IU/mL 28 days after Td dose) followed by MCV4 28 days after Td (diphtheria GMT 16.9 IU/mL 28 days after MCV4 dose) (97). The prelicensure data demonstrated comparable overall safety profiles among adolescents who received simultaneous and sequential vaccination (Td followed by MCV4 28 days later). The immunological and safety profiles among adolescents receiving MCV4 followed by Td on a later date were not evaluated during prelicensure trials (see "Safety of Concomitant Administration of MCV4 and Other Vaccines"). Among adults aged 18--55 years, a randomized controlled trial assessed immunogenicity of MCV4 and typhoid vaccine 1) when MCV4 and typhoid vaccine were administered concomitantly and 2) when typhoid vaccine was administered concomitantly with placebo and MCV4 was administered 28 days later. Concomitant administration did not adversely affect immune response to either typhoid vaccine or MCV4 (97,98). SafetySystemic and Local Adverse Reactions Among persons aged 11--18 years, safety of MCV4 and MPSV4 was assessed in two randomized controlled trials (97,98). The percentage of subjects reporting systemic adverse events was similar for persons who received either vaccine. In one study, approximately half of the participants experienced at least one systemic adverse reaction, and <5% experienced at least one severe systemic reaction. Fever (i.e., temperature >100ºF [>38ºC]) was reported by 5.1% of those who received MCV4 and by 3.0% of those who received MPSV4 (Table 4). Among persons aged 18--55 years, the safety of MCV4 and of MPSV4 also were compared in two randomized controlled trials. The percentage of subjects reporting systemic adverse events was similar for persons who received either vaccine. In one study, 62% of participants experienced at least one systemic adverse reaction, and <4% experienced severe systemic reaction after receiving MCV4. Fever was reported by 1.5% of those who received MCV4 and by 0.5% of those who received MPSV4 (Table 4). Local adverse reactions were more common among those persons aged 11--18 years who received MCV4 than among those who received MPSV4 (Table 5); 13% of those who received MCV4 reported pain that limited movement in the arm of injection, compared with 3% of those who received MPSV4. These differences in frequency of local reactions are related to the amount of diphtheria toxoid contained in each vaccine (99). The frequency of local adverse reactions reported after MCV4 was similar to that reported after Td vaccine (97,98). As with persons aged 11--18 years, local adverse reactions among persons aged 18--55 years were reported more commonly by those who received MCV4 than by those who received MPSV4 (Table 5). However, the frequency of local adverse reactions reported by adults after MCV4 was similar to that reported after typhoid vaccine (97,98). Safety of Concomitant Administration of MCV4 and Other Vaccines Among persons aged 11--17 years, frequency of reported local adverse effects at MCV4 injection site in the group for which MCV4 was administered concomitantly with Td was similar to those in which MCV4 was administered 28 days after Td. The percentage (58.6%) of subjects reporting at least one systemic adverse reaction after concomitant administration of MCV4 and Td was similar to the percentage (54.1%) of systemic reactions reported after Td was administered concomitantly with a placebo. Among persons aged 18--55 years, the frequency of local and systemic adverse effects was similar for those receiving concomitant administration of MCV4 and typhoid vaccine and those who received MCV4 28 days after receiving typhoid vaccine (97,98). Serious Adverse Events in All Safety Studies A total of 5,453 subjects aged 11--55 years who received MCV4 and 2,923 subjects in the same age group who received MPSV4 completed follow-up 6 months after vaccination. Serious adverse events reported within a 6-month period after vaccination occurred at the same rate (1.3%) in the MCV4 and MPSV4 groups. The events reported were consistent with events expected among healthy adolescent and adult populations (98). Cost-Effectiveness AnalysesCost-Effectiveness Analysis of MPSV4 Vaccine Among College StudentsFrom a societal perspective, the economic costs and benefits of vaccinating 1) a cohort of 591,587 freshmen who live in dormitories and 2) all freshmen enrolled in U.S. colleges, regardless of housing status (N = 2.4 million) were evaluated, on the basis of an assumption that the benefits of vaccination would last 4 years (100). Best- and worst-case scenarios were evaluated by varying the cost of vaccine and administration (range: $54--$88), costs per hospitalization ($10,924--$24,030), the value of premature death on the basis of lifetime productivity ($1.3 million--$4.8 million), the cost per case of vaccine side effects ($7,000--$24,540/1 million doses), and the average long-term cost of treating a case of sequelae of disease ($1,298--$14,600). Vaccination coverage (60% and 100%, respectively) and vaccine efficacy (80% and 90%, respectively) also were varied for evaluation purposes. Vaccination of freshmen who live in dormitories would result in the administration of approximately 354,950--591,590 doses of vaccine each year, preventing 16--30 cases of meningococcal disease and one to three deaths each year. The cost per case prevented would be an estimated $617,000--$1.85 million, at a cost per death prevented of $6.8--$20.4 million and a cost per life-year saved (LYS)* of $62,042--$489,185 (100). Vaccination of all freshmen would result in the administration of approximately 1,364,400--2,274,000 doses of vaccine each year, preventing 37--69 cases of meningococcal disease and two to five deaths each year. The cost per case prevented would be $1.4--$2.9 million, at a cost per death prevented of $22--$48 million (100). These data are similar to data derived from previous studies (101). Cost-Effectiveness Analysis of MCV4 Vaccine Among Adolescents Aged 11 YearsFrom a societal perspective, the economic costs and benefits of vaccinating a cohort of approximately 4,238,670 U.S. adolescents aged 11 years were evaluated, on the basis of an assumption that the benefits of vaccination would last 22 years (102). A multivariable (Monte Carlo) analysis was performed in which multiple parameters were varied simultaneously over specified probability distributions. These parameters included disease incidence (46%--120% of the 10-year average), case-fatality ratio (34%--131% of the 10-year average), rates of long-term sequelae, acute meningococcal disease costs (i.e., inpatient care, parents' work loss, and public health response), lifetime costs of meningococcal disease sequelae, and cost of vaccine and administration (range: $64--$114). Vaccination coverage (16%--95%) and vaccine efficacy (39%--99%) also were varied for evaluation purposes. Median program costs for vaccination of adolescents aged 11 years would be $227 million (5th--95th percentile: $158--$406 million). If a 3% discount rate were used for costs and benefits, during a 22-year period, vaccination among adolescents would prevent 270 cases and 36 deaths (21 cases and three deaths in the first year). The median cost would be $633,000 (5th--95th percentile: $329,000--$1,299,000)/case prevented; $5.0 million (5th--95th percentile: $2.4--$10.9 million)/death prevented; and $121,000 (5th--95th percentile: $69,000--$249,000)/LYS saved (102). Cost-Effectiveness Analysis of a Catch-Up Vaccination Campaign with MCV4The direct and indirect (herd immunity) benefits of a one-time catch-up vaccination campaign with MCV4 of adolescents aged 11--17 years followed by routine annual vaccination of adolescents aged 11 years were analyzed (CDC, unpublished data, 2005). For this purpose, a probabilistic model of disease burden and economic impacts was built for a 10-year period with and without an adolescent catch-up program. U.S. age- and serogroup-specific surveillance data on incidence and case fatality rates were used, as were hypothetical age-specific reductions in attack rates among unvaccinated persons obtained on the basis of U.K. data (86,103). Medical, work loss, and public response costs were estimated with and without a catch-up campaign, as were lifetime costs of meningococcal disease sequelae. After disease and vaccination program costs were projected, estimated costs per case averted, deaths prevented, LYS, and quality-adjusted life years (QALY)† saved were estimated. With herd immunity effects equivalent to recent experience in the United Kingdom, catch-up vaccination of adolescents plus an added routine program would prevent 5,263 cases during a 10-year period, a 32% reduction in the number of cases. Excluding program costs, the catch-up program would save $338 million in medical and public response costs and $591 million in time off from work, long-term disability, and premature death. At a hypothetical cost of $83 per vaccinee, a catch- up vaccination program (including 9 years of routine vaccination) would cost society approximately $3.6 billion (45% of this sum in the first year). At a 3% discount rate, the catch-up program would cost society $532,000/case averted, $5.9 million/death prevented, $138,000/LYS, and $64,000/QALY saved. A 20% reduction in herd immunity effects would increase the cost per LYS by $21,000; a $30 decrease in the cost of vaccination would decrease the cost per LYS by $55,000. On the basis of the assumption that herd immunity can be generated, targeting only those U.S. counties in which the disease is highly endemic would decrease the cost per LYS by two thirds. Catch-up vaccination of adolescents can have a substantial impact on disease burden and costs. However, these data demonstrate that catch-up and routine vaccination programs with MCV4 among adolescents are more costly per health outcome than existing vaccination strategies for Hib and S. pneumoniae (104,105). Compared with routine vaccination of children aged 11 years, catch-up vaccination could cost up to 20% more/LYS. Recommendations for Use of Meningococcal VaccinesRoutine Vaccination of AdolescentsACIP recommends routine vaccination of young adolescents (defined in this report as persons aged 11--12 years) with MCV4 at the preadolescent health-care visit (i.e., a visit to a health-care provider at age 11--12 years, at which time ACIP and other professional organizations [e.g., AAP and the American Medical Association] recommend that persons aged 11--12 years receive appropriate vaccinations and other preventive services [106--109]). Introducing a recommendation for MCV4 vaccination among persons aged 11--12 years might strengthen the role of the preadolescent health-care visit and have a positive effect on vaccine coverage during adolescence. For those adolescents who have not previously received MCV4, ACIP recommends vaccination before high school entry (at approximately age 15 years) as an effective strategy to reduce meningococcal disease incidence among adolescents and young adults. By 2008, the goal will be routine vaccination with MCV4 of all adolescents beginning at age 11 years. Other adolescents who wish to decrease their risk for meningococcal disease may elect to receive vaccine. Other Populations at Increased Risk for Meningococcal DiseaseRoutine vaccination also is recommended for certain persons who have increased risk for meningococcal disease (Table 6). Use of MCV4 is preferred among persons aged 11--55 years; however, use of MPSV4 is recommended among children aged 2--10 years and persons aged >55 years. If MCV4 is unavailable, MPSV4 is an acceptable alternative for persons aged 11--55 years. The following populations are at increased risk for meningococcal disease:
Because of feasibility constraints in targeting freshmen in dormitories, colleges can elect to target their vaccination campaigns to all matriculating freshmen. The risk for meningococcal disease among nonfreshmen college students is similar to that for the general population of similar age (age 18--24 years) (29). However, the vaccines are safe and immunogenic and therefore can be provided to nonfreshmen college students who want to reduce their risk for meningococcal disease. For travelers, vaccination is especially recommended to those visiting the parts of sub-Saharan Africa known as the "meningitis belt" (112) during the dry season (December--June). Vaccination is required by the government of Saudi Arabia for all travelers to Mecca during the annual Hajj. Advisories for travelers to other countries will be issued when epidemics of meningococcal disease caused by vaccine-preventable serogroups are detected. Travelers' health information is available from CDC at 877-FYI-TRIP (toll-free) or at http://www.cdc.gov/travel. Further information concerning geographic areas for which vaccination is recommended can be obtained from international health clinics for travelers and state health departments. Patients with human immunodeficiency virus (HIV) are likely at increased risk for meningococcal disease, although not to the extent that they are at risk for invasive S. pneumoniae infection (20,114). Although the efficacy of MCV4 among HIV-infected patients is unknown, HIV-infected patients may elect vaccination. For persons aged 11--55 years who have been previously vaccinated with MPSV4, revaccination with MCV4 is not indicated unless vaccination occurred 3--5 years previously and the person still remains at increased risk for meningococcal disease (see Revaccination). Adults Aged 20--55 YearsMCV4 is licensed for use among adults aged 20--55 years. It is safe, immunogenic (97,98,115,116), and likely to provide relatively long-lasting protection against meningococcal disease caused by serogroups A, C, Y, and W-135. The rates of meningococcal disease are low in this age group, and vaccination will decrease but not eliminate risk. Therefore, routine vaccination is not recommended; however, persons who wish to decrease their risk for meningococcal disease may elect to be vaccinated. Children Aged <11 Years and Adults Aged >55 YearsMCV 4 is not licensed for use among children aged <11 years or adults aged >55 years. Routine vaccination with MPSV4 is not recommended for children aged <2 years because it is relatively ineffective and offers a short duration of protection. Routine vaccination with MPSV4 is not recommended for children aged 2--10 years and adults aged >55 years who are not identified as being at increased risk for meningococcal disease. Outbreaks of Meningococcal DiseaseBoth MPSV4 (4) and MCV4 are recommended for use in control of meningococcal outbreaks caused by vaccine-preventable serogroups (A, C, W-135, and Y) of N. meningitdis. An outbreak is defined by the occurrence of at least three§ confirmed or probable primary¶ cases of serogroup C meningococcal disease in <3 months, with a resulting primary attack rate of >10 cases/100,000 population. For calculation of this threshold, population-based rates are used rather than age-specific attack rates. These recommendations are based on experience with serogroup C meningococcal outbreaks, but these principles might be applicable to outbreaks caused by the other vaccine-preventable meningococcal serogroups, including Y, W-135, and A. Both MCV4 and MPSV4 can be used for outbreak control, although use of MCV4 is preferred if the population targeted for vaccination includes age groups for which MCV4 is licensed. Detailed recommendations on evaluation and management of suspected outbreaks of meningococcal disease have been published previously (4). AdministrationFor persons aged 11--55 years, MCV4 is administered intramuscularly as a single 0.5-mL dose. MPSV4 is administered subcutaneously as a single 0.5-mL dose to persons aged >2 years. MCV4 and MPSV4 can be administered concomitantly with other vaccines, but at a different anatomic site (4,117). Protective levels of antibodies are usually achieved within 7--10 days of vaccination (60,118). RevaccinationRevaccination might be indicated for persons previously vaccinated with MPSV4 who remain at increased risk for infection (e.g., persons residing in areas in which disease is epidemic), particularly children who were first vaccinated at age <4 years. Such children should be considered for revaccination after 2--3 years if they remain at increased risk. Although the need for revaccination among adults and older children after receiving MPSV4 has not been determined, antibody levels decline rapidly after 2--3 years, and, if indications still exist for vaccination, revaccination might be considered after 5** years (4). Repeated vaccination with serogroup A and C polysaccharide vaccine might induce immunologic hyporesponsiveness (56--59), although clinical implications of such hyporesponsiveness are not known. Hyporesponsiveness to serogroup C polysaccharide can be overcome by vaccination with serogroup C conjugate vaccine (119,120). MCV4 is recommended for revaccination of persons aged 11--55 years; however, use of MSPV4 is acceptable. ACIP expects that MCV4 will provide longer protection than MPSV4; however, studies are needed to confirm this assumption (87). More data will likely become available within the next 5 years to guide recommendations on revaccination for persons who were previously vaccinated with MCV4. Precautions and ContraindicationsRecommended vaccinations can be administered to persons with minor acute illness (e.g., diarrhea or mild upper-respiratory tract infection with or without fever) (117). Vaccination should be deferred for persons with moderate or severe acute illness until the person's condition improves. Vaccination with MCV4 or MPSV4 is contraindicated among persons known to have a severe allergic reaction to any component of the vaccine, including dipththeria toxoid (for MCV4), or to dry natural rubber latex. Any adverse effect suspected to be associated with MCV4 or MPSV4 vaccine should be reported to the Vaccine Adverse Event Reporting System (VAERS). More information about VAERS is available at 800-822-7967 (toll-free) or from http://www.vaers.org. Because both MCV4 and MPSV4 are inactivated vaccines, they may be administered to persons who are immunosuppressed as a result of disease or medications; however, response to the vaccine might be less than optimal (117). Studies of vaccination with MPSV4 during pregnancy have not documented adverse effects among either pregnant women or newborns (121--123). On the basis of these data, pregnancy should not preclude vaccination with MPSV4, if indicated. MCV4 is safe and immunogenic among nonpregnant persons aged 11--55 years, but no data are available on the safety of MCV4 during pregnancy. Women of childbearing age who become aware that they were pregnant at the time of MCV4 vaccination should contact their health-care provider or the vaccine manufacturer. Future Meningococcal Vaccines, Areas for Research, and Public EducationMCV4 has been licensed on the basis of data regarding safety and short-term immunogenicity. Postmarketing studies are planned (98), including a study to evaluate the duration of the antibody response among participants who had received a single dose of MCV4 vaccine or MPSV4 vaccine 5 and 10 years earlier and a study to evaluate safety and immunogenicity when MCV4 is given concomitantly with tetanus and reduced diphtheria and acellular pertussis vaccine adsorbed (Tdap). However, immunogenicity data alone are insufficient to predict vaccine effectiveness and herd immunity effect, which depends largely on the ability of vaccine to alter transmission patterns. Additional studies are needed to evaluate vaccine effectiveness, vaccine impact on nasopharyngeal carriage of meningococci, and indirect effects of vaccine on disease rates among unvaccinated populations. Meningococcal conjugate vaccines might be considered for licensing in the United States among persons in other age groups, including infants and children aged <10 years (98). These vaccines are undergoing clinical trials and are likely to have better immunogenicity among infants and young children than MPSV4 (124--126), which is the only vaccine available for these age groups in the United States. Information on vaccine effectiveness, duration of protection, and herd immunity obtained from MCV4 evaluation studies will be valuable in guiding prevention policies and formulating recommendations for vaccination of persons in other age groups. Because serogroup B capsular polysaccharide is poorly immunogenic in humans, vaccine development for serogroup B meningococci have focused on common proteins, including the outer membrane proteins (OMP) of specific epidemic strains. Efficacy of OMP vaccines has been demonstrated among older children and adults but not among infants and young children, in whom rates of disease are highest (127--130). In addition, the variability in OMP strains causing endemic disease will likely limit their usefulness in the United States (131,132). Because of the potential limitations of these vaccines, other new approaches to serogroup B vaccines are being pursued, including the conjugation of a modified serogroup B polysaccharide (after substitution of the N-acetyl group with an N-propionyl group) to a recombinant serogroup B meningococcal porin protein. Although this vaccine is immunogenic in mice and nonhuman primates, concern exists that the vaccine might not be safe (132). In addition, with the recent sequencing of the serogroup B meningococcal genome, new genes encoding putative membrane proteins have been identified, indicating potential new targets for serogroup B vaccines (133--135). The availability of new meningococcal conjugate vaccines and the development of new vaccine strategies should lead to substantial improvements in global control and prevention of meningococcal disease. Although the signs and symptoms of meningococcal disease are frequently nonspecific, increasing awareness for meningococcal disease can result in earlier medical care-seeking behavior and improved clinical outcomes. In addition, educating adolescents and their parents about the benefits of receiving MCV4 is key to preventing a substantial number of cases of meningococcal disease. Finally, educating policy makers and the general public about the benefits of receiving MCV4 vaccine might improve vaccination coverage rates and substantially decrease the burden of meningococcal disease in the United States. Antimicrobial ChemoprophylaxisIn the United States, the primary means for prevention of sporadic meningococcal disease is antimicrobial chemoprophylaxis of close contacts of a patient with invasive meningococcal disease (Table 7). Close contacts include 1) household members (136,137), 2) child-care center contacts (136,138), and 3) anyone directly exposed to the patient's oral secretions (e.g., through kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management). For travelers, antimicrobial chemoprophylaxis should be considered for any passenger who had direct contact with respiratory secretions from an index-patient or for anyone seated directly next to an index-patient on a prolonged flight (i.e., one lasting >8 hours). Guidelines for chemoprophylaxis of travelers have been published previously (139). The attack rate for household contacts exposed to patients who have sporadic meningococcal disease was estimated to be four cases/1,000 persons exposed, which is 500--800 times greater than the rate for the total population (137). In the United Kingdom, the attack rate among health-care workers exposed to patients with meningococcal disease was determined to be 25 times higher than among the general population (140). Because the rate of secondary disease for close contacts is highest immediately after onset of disease in the index patient, antimicrobial chemoprophylaxis should be administered as soon as possible (ideally <24 hours after identification of the index patient). Conversely, chemoprophylaxis administered >14 days after onset of illness in the index patient is probably of limited or no value. Oropharyngeal or nasopharyngeal cultures are not helpful in determining the need for chemoprophylaxis and might unnecessarily delay institution of this preventive measure. Rifampin, ciprofloxacin, and ceftriaxone are 90%--95% effective in reducing nasopharyngeal carriage of N. meningitdis and are all acceptable antimicrobial agents for chemoprophylaxis (141--144). Systemic antimicrobial therapy of meningococcal disease with agents other than ceftriaxone or other third-generation cephalosporins might not reliably eradicate nasopharyngeal carriage of N. meningitdis. If other agents have been used for treatment, the index patient should receive chemoprophylactic antibiotics for eradication of nasopharyngeal carriage before being discharged from the hospital (145). One recent study has reported that a single 500-mg oral dose of azithromycin was effective in eradicating nasopharyngeal carriage of N. meningitdis (146). Azithromycin, in addition to being safe and easy to administer, is also available in a suspension form and is approved for use among children. Further evaluation is warranted of both the effectiveness of azithromycin in eradicating carriage of N. meningitdis and potential for development of microbial resistance to this drug if it is widely used for chemoprophylaxis. References
* The number of life-years saved as a result of a preventive intervention (i.e., the number of potential years of life expected if disease-specific events leading to premature death not occur [healthy life expectancy]). The number of life-years saved will be less or at the most equal to the number of potential years lost pre-intervention. Because life expectancy is age-specific, life-years saved is often calculated as the difference between the age-specific healthy life expectancy and the age when a disease-specific event leading to premature mortality could occur without the intervention. † A measure based on individual preferences for states of health that assigns a value of 1 to a year of perfect health and 0 to death. QALYs measure not only years of life saved but also functioning and health preserved. QALYs are highly relevant when disease-specific outcomes lead to both mortality (i.e., premature death) and substantial morbidity (i.e., temporal or permanent disability). Thus, effectiveness outcomes are expressed as change in health status. § Calculation of attack rates for organization-based outbreaks is most useful for sizable organizations (e.g., certain universities). However, for the majority of organization-based outbreaks with three cases of disease, the rate will be >10 cases/100,000 population. Thus, occurrence of three cases in these settings should prompt consideration of vaccination. In certain situations, public health officials also might consider vaccination after only two primary cases are identified. ¶ To calculate a primary attack rate, sum all confirmed cases; exclude secondary cases, and count each set of co-primary cases as one case. A primary case is one that occurs in the absence of previous known close contact with another patient. A secondary case is one that occurs among close contacts of a primary patient >24 hours after onset of illness in the primary patient. If two or more cases occur among a group of close contacts with onset of illness separated by <24 hours, these cases are considered to be co-primary. ** Certain sources recommend revaccination after 3 years (4).
Advisory Committee on Immunization Practices Membership List, February 2005 Chairman: Myron J. Levin, MD, University of Colorado Health Sciences Center, Denver, Colorado. Executive Secretary: Stephen C. Hadler, MD, Chief, Global Alliance Vaccine Initiative, CDC, Atlanta, Georgia. Members: Jon S. Abramson, MD, Wake Forest University School of Medicine, Winston-Salem, North Carolina; Ban Mishu Allos, MD, Vanderbilt University School of Medicine, Nashville, Tennessee; Guthrie S. Birkhead, MD, New York State Department of Health, Albany, New York; Judith Campbell, MD, Baylor College of Medicine, Houston, Texas; Reginald Finger, MD, Focus on the Family, Colorado Springs, Colorado; Janet R. Gilsdorf, MD, University of Michigan, Ann Arbor, Michigan; Tracy Lieu, MD, Harvard Medical School, Boston, Massachusetts; Edgar K. Marcuse, MD, Children's Hospital and Regional Medical Center, Seattle, Washington; Julia Morita, MD, Chicago Department of Public Health, Chicago, Illinois; Gregory Poland, MD, Mayo Medical School, Rochester, Minnesota; John B. Salamone, National Italian American Foundation, Washington, D.C.; Patricia, Stinchfield, Children's Hospitals and Clinics, St. Paul, Minnesota; John J. Treanor, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York; and Robin Womeodu, MD, University of Tennessee Health Sciences Center, Memphis, Tennessee. Ex-Officio Members: James E. Cheek, MD, Indian Health Service, Albuquerque, New Mexico; Stephen Phillips, DO, Department of Defense, Falls Church, Virginia; Geoffrey S. Evans, MD, Health Resources and Services Administration, Rockville, Maryland; Bruce Gellin, MD, National Vaccine Program Office, Washington, D.C..; Linda Murphy, Centers for Medicare and Medicaid Services, Baltimore, Maryland; George T. Curlin, MD, National Institutes of Health, Bethesda, Maryland; Norman Baylor, PhD, Office of Vaccines Research Review, Rockville, Maryland; and Kristin Lee Nichol, MD, University of Minnesota, Minneapolis, Minnesota. Liaison Representatives: American Academy of Family Physicians, Jonathan Temte, MD, Madison, Wisconsin, and Richard Clover, MD, Louisville, Kentucky; American Academy of Pediatrics, Margaret Rennels, MD, Baltimore, Maryland, and Carol Baker, MD, Houston, Texas; America's Health Insurance Plans, Robert Scalettar, MD, North Haven, Connecticut; American College Health Association, James C. Turner, MD, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Stanley Gall, MD, Louisville, Kentucky; American College of Physicians, Kathleen Neuzil, MD, Seattle, Washington; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Teachers of Preventive Medicine, W. Paul McKinney, MD, Louisville, Kentucky; Biotechnology Industry Organization, Clement Lewin, PhD, Orange, Connecticut; Canadian National Advisory Committee on Immunization, Monica Naus, MD, Vancouver, British Columbia; Healthcare Infection Control Practices Advisory Committee, Steve Gordon, MD, Cleveland, Ohio; Infectious Diseases Society of America, Samuel L. Katz, MD, Durham, North Carolina, and William Schaffner, MD, Nashville, Tennessee; London Department of Health, David M. Salisbury, MD, London, United Kingdom; National Association of County and City Health Officials, Nancy Bennett, MD, Rochester, New York; National Coalition for Adult Immunization, David A. Neumann, PhD, Alexandria, Virginia; National Immunization Council and Child Health Program, Mexico, Romeo Rodriguez, Mexico City, Mexico; National Medical Association, Dennis A. Brooks, MD, Baltimore, Maryland; National Vaccine Advisory Committee, Charles Helms, MD, Iowa City, Iowa; Society for Adolescent Medicine, Amy B. Middleman, MD, Houston, Texas; and the Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania, and Peter Paradiso, Collegeville, Pennsylvania. ACIP Meningococcal Working Group Chair: Reginald Finger, MD, Colorado Springs, Colorado. Members: Jon Abramson, MD, Winston-Salem, North Carolina; Carol Baker, MD, Houston, Texas; Guthrie Birkhead, MD, Albany, New York; Oleg Bilukha, MD, Atlanta, Georgia; Richard Clover, MD, Louisville, Kentucky; George Curlin, MD, Bethesda, Maryland; Geoffrey Evans, MD, Rockville, Maryland; Janet Gilsdorf, MD, Ann Arbor, Michigan; Lucia Lee, MD, Rockville, Maryland; Alison Mawle, MD, Atlanta, Georgia; Paul McKinney, MD, Louisville, Kentucky; Martin Meltzer, PhD, Atlanta, Georgia; John Moran, MD, Atlanta, Georgia; Paul Offit, MD, Philadelphia, Pennsylvania; Ismael Ortega-Sanchez, PhD, Atlanta, Georgia; Georges Peter, MD, Providence, Rhode Island; Nancy Rosenstein; MD, Atlanta, Georgia; David Salisbury, MD, London, United Kingdom; Jeanne Santoli, MD, Atlanta, Georgia; William Schaffner, MD, Nashville, Tennessee; R. Douglas Scott II, PhD, Atlanta, Georgia; Colin Shepard, MD, Atlanta, Georgia; Jonathan Temte, MD, Madison, Wisconsin; James Turner, MD, Charlottesville, Virginia; Gregory Wallace, MD, Atlanta, Georgia; and Kirk Winger, DVM, Atlanta, Georgia. Table 1 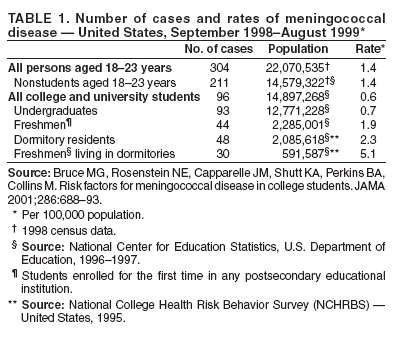 Return to top. Figure 1 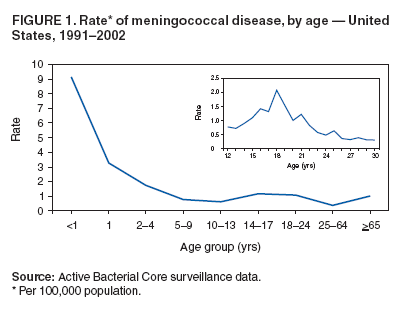 Return to top. Table 2 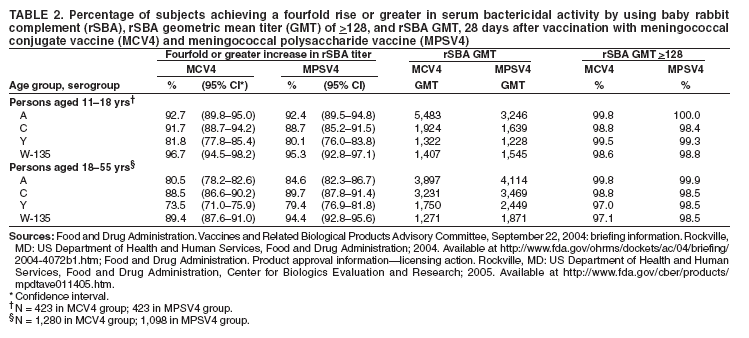 Return to top. Figure 2 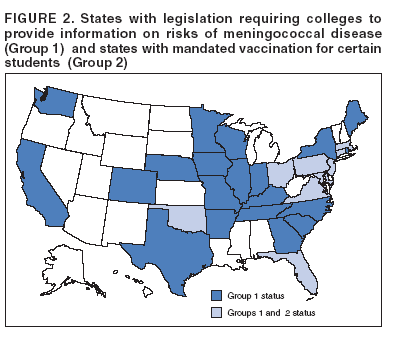 Return to top. Table 3 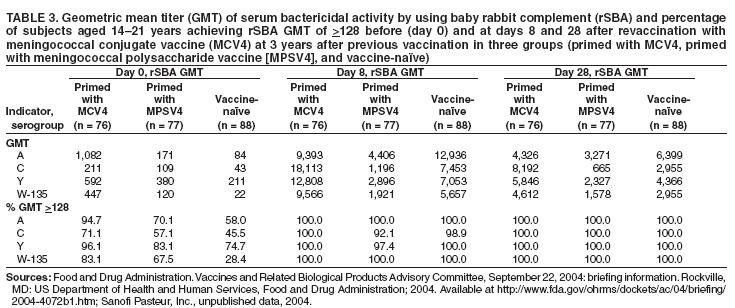 Return to top. Table 4 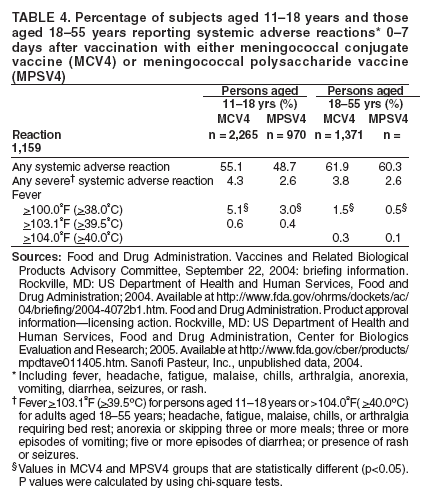 Return to top. Table 5 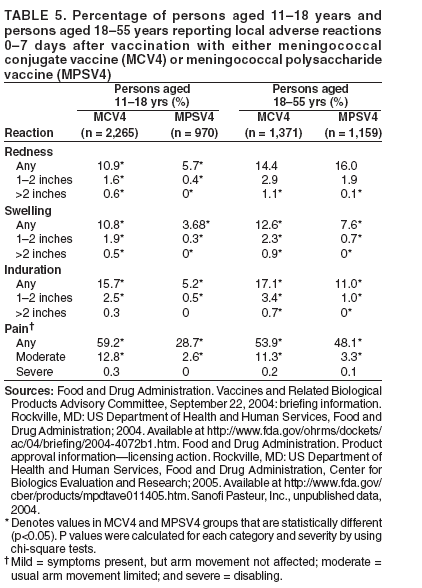 Return to top. Table 6 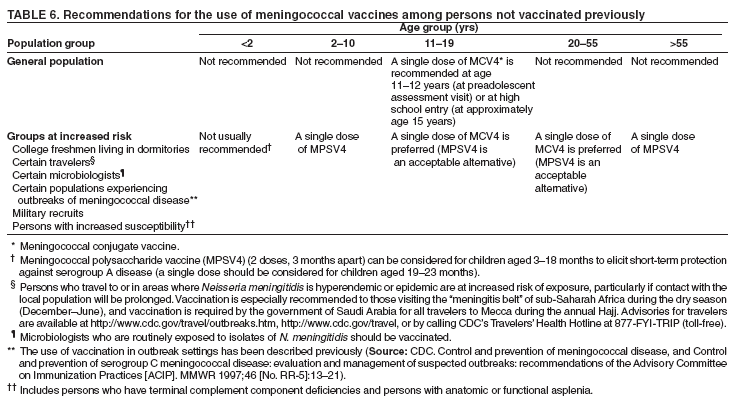 Return to top. Table 7 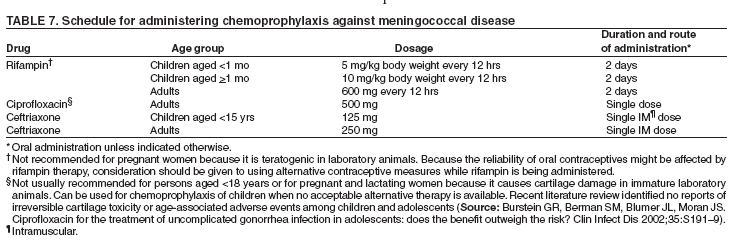 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/23/2005 |
|||||||||
|