 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. "Norwalk-Like Viruses"Public Health Consequences and Outbreak ManagementAn erratum has been published for this article. To view the erratum, please click here. Summary"Norwalk-like viruses" (NLVs) cause outbreaks of gastroenteritis and are spread frequently through contaminated food or water. Molecular diagnostics now enables detecting viruses in clinical and environmental specimens, linking of NLV strains causing outbreaks in multiple geographic locations, and tracing them to their sources in contaminated food or water. This report reviews recent advances in NLV detection and provides guidelines and recommendations for investigating NLV-related outbreaks, including specimen collection and disease prevention and control. This report also updates information provided in CDC's previously published, Viral Agents of Gastroenteritis: Public Health Importance and Outbreak Management (MMWR 1990;39 [No. RR-5]:1--24). These CDC recommendations are intended for public health professionals who investigate outbreaks of acute gastroenteritis but could be useful in academic and research settings as well. INTRODUCTIONDuring the early 1970s, before the discovery of diarrhea-causing viruses, an etiologic agent could be detected only among a limited proportion of persons with gastroenteritis. Subsequently, with the discovery of Norwalk virus (1), rotavirus (2), astrovirus (3,4), and enteric adenovirus (5), researchers began to recognize viruses as causative agents of gastroenteritis. However, progress in detecting and managing outbreaks of disease caused by these agents was hampered by the unavailability of sensitive and specific diagnostic tests that could be applied outside research settings. Although outbreaks of nonbacterial gastroenteritis were recognized as a public health concern, electron microscopy (EM) proved to be a tedious and insensitive method for routine examination for enteric viruses in stool specimens collected during outbreak investigations. Detection rates improved with the development of immunologic assays (6--11), and 19%--42% of nonbacterial outbreaks were attributed to Norwalk virus in targeted studies conducted during the late 1970s and 1980s (12,13). However, because reagents for these assays came from human volunteers, the reagents were available only in limited quantities and only at certain facilities. Consequently, outbreaks were not fully investigated, and a substantial number were still labeled as being of unknown etiology. Furthermore, because of the antigenic and genetic diversity of "Norwalk-like viruses"* (NLVs) and the inability to cultivate these viruses in cell lines, developing assays to detect the full spectrum of NLVs associated with outbreaks of gastroenteritis was not possible. To circumvent these obstacles to laboratory diagnosis, clinical and epidemiologic criteria were developed that correlate with the presence of NLVs in outbreaks of acute gastroenteritis (14). These criteria included a) stool specimens that are negative for bacterial and parasitic pathogens; b) percentage of cases with vomiting >50%; c) mean (or median) duration of illness of 12--60 hours; and d) if available, mean (or median) incubation period of 24--48 hours. During the early 1990s, breakthroughs in cloning and sequencing of Norwalk virus and Southampton virus (15--18) led to the development of sensitive molecular assays (e.g., reverse transcription-polymerase chain reaction [RT-PCR]), nucleotide hybridization probes, and enzyme-linked immunosorbent assays (ELISA) that used baculovirus-expressed viral antigens (19--33). Using these assays, researchers demonstrated that NLVs caused a majority of foodborne gastroenteritis outbreaks in Minnesota and approximately 96% of 90 outbreaks of nonbacterial gastroenteritis reported to CDC during January 1996--June 1997 (34,35). These data demonstrate that NLVs are a common cause of outbreaks of nonbacterial gastroenteritis in the United States and similar findings have been reported in other countries (36--42). In addition to improving detection rates of NLVs in outbreaks of gastroenteritis, advances in laboratory methods have also refined the epidemiologic investigation of these outbreaks. This progress is well-illustrated in reports of recent outbreaks linked to contaminated water, oysters, and other food items (43--49). In certain outbreaks, the detection of a genetically identical strain of NLV among patients from different geographic locations provided substantial evidence to support the link among the cases that was indicated by epidemiologic observations. In other outbreaks, the detection in the implicated vehicle of NLVs with a sequence identical to that of the strain detected from the patients confirmed the causal link. Although diagnostic advances have improved the investigation of gastroenteritis outbreaks, they have also required that investigators collect clinical and environmental specimens accurately and in a timely manner and collaborate with the laboratory during the investigation. This report reviews recent advances in NLV diagnosis, and provides CDC's guidelines and recommendations for investigating gastroenteritis outbreaks, including methods for collecting specimens and preventing and controlling outbreaks. BIOLOGY AND EPIDEMIOLOGY OF NLVsTaxonomy Norwalk virus is the prototype strain of genetically and antigenically diverse single-stranded RNA (ribonucleic acid) viruses, previously called small round-structured viruses (SRSVs), that are classified in the genus Norwalk-like viruses in the family Caliciviridae (50). Other genera in the Caliciviridae family include "Sapporo-like viruses," which also cause gastroenteritis among both children and adults, and Lagovirus and Vesivirus, neither of which are pathogenic for humans. NLVs can be divided into three distinct genogroups: GI, GII, and GIII (51). GI and GII NLVs infect humans and include 5 and 10 genetic clusters, respectively; GIII NLVs infect pigs and cows. Endemic Disease The burden of NLV-caused endemic disease is unknown because simple and sensitive diagnostic assays are not readily available. However, the potential burden of NLV disease can be understood by examining the disease burden of gastroenteritis of all causes with the insights obtained during the limited number of studies in which newer diagnostics for NLVs have been applied. Annually, approximately 267,000,000 episodes of diarrhea leading to 612,000 hospitalizations and 3,000 deaths occur among adults in the United States (52). An etiologic agent is identified in <10% of these cases, but studies to assess the disease burden of NLV among hospitalized adults are underway. In other countries (e.g., the Netherlands and England [53--55]), NLVs have been reported to account for 5%--17% of cases of diarrhea in the community and 5%--7% of cases requiring treatment by physicians. Although rotavirus is the leading cause of severe diarrhea among children (56), data from recent studies demonstrate that NLVs also might be a factor in childhood gastroenteritis (57--65). For example, NLVs were detected in 20% of 783 stool specimens collected during acute gastroenteritis episodes among Finnish children prospectively followed from age 2 months to 2 years (65). "Sapporo-like viruses" were detected in an additional 9% of cases. Clinical Features NLV-caused gastroenteritis has an average incubation period of 12--48 hours and lasts 12--60 hours. Illness is characterized by acute onset of nausea, vomiting, abdominal cramps, and diarrhea. Vomiting is relatively more prevalent among children, whereas a greater proportion of adults experience diarrhea. Patients can experience vomiting alone, a condition first identified as winter vomiting disease (66). Constitutional symptoms (e.g., headache, fever, chills, and myalgia) are frequently reported. Although rare, severe dehydration caused by NLV gastroenteritis can be fatal, with this outcome occurring among susceptible persons (e.g., older persons with debilitating health conditions). No long-term sequelae of NLV infection have been reported. Transmission Mode Fecal-oral spread is probably the primary NLV transmission mode, although airborne and fomite transmission might facilitate spread during outbreaks (67--71). Frequently during an outbreak, primary cases result from exposure to a fecally contaminated vehicle (e.g., food or water), whereas secondary and tertiary cases among contacts of primary cases result from person-to-person transmission (72). For 348 outbreaks of NLV gastroenteritis reported to CDC during January 1996--November 2000, food was implicated in 39%, person-to-person contact in 12%, and water in 3%; 18% could not be linked to a specific transmission mode (Figure 1). Previously, researchers believed that a person remained contagious 48--72 hours after recovery from NLV gastroenteritis (73). However, data from recent studies using more sensitive diagnostic assays demonstrate that this belief might require further evaluation. During a 1994 study of 50 volunteers exposed to NLV, 82% became infected; of these infections, 68% resulted in illness, whereas the remaining 32% were asymptomatic (74). Viral shedding in stool began 15 hours after virus administration and peaked 25--72 hours after virus administration. Unexpectedly, viral antigen could be detected by ELISA in stool specimens collected 7 days after inoculation in both symptomatic and asymptomatic persons. In a later study of infected volunteers, viral antigen in stool was detected <2 weeks after administration of virus (75). Anecdotal evidence from outbreak investigations also demonstrates that viral shedding can occur for a prolonged period and in the absence of clinical illness (46,48,76--80). However, the epidemiologic significance of these findings is unclear. Additional research is need to determine whether the viral antigen that is detectable for prolonged periods after recovery from illness is evidence of infectious virus or just a soluble antigen and to assess the time of maximal viral shedding so that control measures can focus on the period during which the person is most likely to be contagious. Characteristics of NLVs facilitate their spread during epidemics (Table 1). The low infectious dose of NLVs (i.e., <100 viral particles [81]) readily allows spread by droplets, fomites, person-to-person transmission, and environmental contamination, as evidenced by the increased rate of secondary and tertiary spread among contacts and family members. Prolonged duration of viral shedding that can occur among asymptomatic persons increases the risk for secondary spread and is of concern in foodhandler-related transmission. The ability of the virus to survive relatively high levels of chlorine (82) and varying temperatures (i.e., from freezing to 60 C) (81) facilitates spread through recreational and drinking water and food items, including steamed oysters (83). Because of the diversity of NLV strains, lack of complete cross-protection, and lack of long-term immunity, repeated infections can occur throughout life. Immunity Studies of NLV immunity have been hampered by the inability of these viruses to be cultivated in cell lines, and thus, in vitro neutralization assays are not available. Early studies indicated that approximately 50% of persons exposed to NLVs experience illness and acquire short-term homologous immunity (i.e., against the same strain) that is correlated with serum antibody levels (84). Certain studies also demonstrated, paradoxically, that persons with higher levels of preexisting NLV antibodies would probably experience illness if exposed to the virus (84,85). A recent study using molecular assays confirmed that approximately 50% of volunteers exposed to NLVs are susceptible to illness, but the study demonstrated also that approximately 80% become infected, with certain infections being asymptomatic (74). Although a trend for higher rates of viral shedding, seroconversion, and clinical illness was observed among those with higher levels of preexisting antibody, 60% of volunteers without preexisting antibody also demonstrated a seroconversion. Researchers hypothesized that certain persons might be genetically more susceptible to NLV infection and disease. If true, this hypothesis could explain why those with greater levels of preexisting antibody are more likely to experience NLV infection and disease after reexposure to virus. Outbreaks Outbreaks of NLV gastroenteritis occur in multiple settings. Of 348 such outbreaks reported to CDC during January 1996--November 2000, a total of 39% occurred in restaurants; 29% occurred in nursing homes and hospitals; 12% in schools and day care centers; 10% in vacation settings, including cruise ships; and 9% in other settings (Figure 2). Nursing Homes and Residential Institutions Protracted outbreaks of NLV disease have been reported among elderly persons living in institutional settings, (e.g., nursing homes) (86,87). In certain cases, the outbreak was initially caused by a common-source exposure to a fecally contaminated vehicle (e.g., food or water). Later, the outbreak spreads through person-to-person transmission among the residents; this spread is facilitated by the enclosed living quarters and reduced levels of personal hygiene that result from incontinence, immobility, or reduced mental alertness. Because of underlying medical conditions, the disease among these persons can be severe or fatal. Restaurants and Catered Events A report from Minnesota demonstrates the relevance of NLVs as a cause of foodborne outbreaks (34). The survey determined that 41% of 295 foodborne outbreaks reported in Minnesota during 1981--1998 met the epidemiologic criteria for NLV gastroenteritis. Further, NLVs were detected in 70% of 23 foodborne outbreaks investigated during 1996--1998 in which molecular diagnostics were used to test stool specimens. Investigations of foodborne NLV outbreaks have implicated multiple food items, including oysters, salads, sandwiches, cakes, frosting, raspberries, drinking water, and ice (43--49,88--91). In certain outbreaks, the implicated food is fecally contaminated with NLVs at its source (e.g., oysters harvested from fecally contaminated waters or raspberries irrigated with sewage-contaminated water). However, foodhandlers might contaminate food items during preparation. The risk for contamination through foodhandlers is increased when the food item is consumed without further cooking (e.g., ready-to-eat foods) and when a semiliquid food (e.g., cake frosting or salad dressing) is contaminated so that a small inoculum is mixed and spread to multiple persons. Cruise Ships Passengers and crew members on cruise ships and naval vessels are frequently affected by outbreaks of NLV gastroenteritis (35,92,93). These ships dock in countries where levels of sanitation might be inadequate, thus increasing the risk for contamination of water and food taken aboard or for having a passenger board with an active infection. After a passenger or crew member brings the virus on board, the close living quarters on ships amplify opportunities for person-to-person transmission. Furthermore, the arrival of new and susceptible passengers every 1 or 2 weeks on affected cruise ships provides an opportunity for sustained transmission during successive cruises. NLV outbreaks extending beyond 12 successive cruises have been reported (94). PREVENTION AND CONTROL OF NLV OUTBREAKSAlthough person-to-person spread might extend NLV gastroenteritis outbreaks, the initiating event is often the contamination of a common vehicle (e.g., food or water). Consequently, efforts to prevent both the initial contamination of the implicated vehicle and subsequent person-to-person NLV transmission will prevent the occurrence and spread of NLV gastroenteritis outbreaks. Foodborne Transmission Theoretically, any food item can potentially be infected with NLVs through fecal contamination. However, certain foods are implicated more often than others in outbreaks of NLV gastroenteritis. Shellfish (e.g., oysters or clams) tend to concentrate in their tissues NLVs that contaminate the waters from which they are harvested (95,96), and even harvests meeting bacteriologic standards of hygiene can contain NLVs. In addition, cooking (e.g., steaming) might not completely inactivate NLVs (83). Until reliable indicators for routine monitoring of viral contamination of harvest waters and shellfish are available, measures to prevent the contamination of harvest waters with human waste (e.g., surveillance of the shoreline for potential sources of fecal contamination and restricting boaters from dumping waste overboard) are probably a useful means of preventing shellfish-associated NLV gastroenteritis outbreaks. Food contamination by infectious foodhandlers is another frequent cause of NLV gastroenteritis outbreaks. Because of the low infectious dose of NLVs and the high concentration of virus in stool, even a limited contamination can result in substantial outbreaks. Ready-to-eat foods that require handling but no subsequent cooking (e.g., salads and deli sandwiches) pose greater risk. Previously, the exclusion of ill foodhandlers for 48--72 hours after resolution of illness was recommended to prevent outbreaks caused by foodhandlers (97). Data from recent human volunteer and epidemiologic studies demonstrate that viral antigen can be shed for a longer duration after recovery from illness and in the absence of clinical disease. Although data are limited regarding whether this detectable viral antigen represents infectious virus, foodhandlers should be required to maintain strict personal hygiene at all times. Waterborne Transmission Although waterborne outbreaks are far less common than foodborne outbreaks, NLV gastroenteritis outbreaks have been associated with sources of contaminated water, including municipal water, well water, stream water, commercial ice, lake water, and swimming pool water. Because current analytic methods do not permit direct monitoring of NLVs in water, indicator organisms (e.g., coliform bacteria) have been used as proxy indicators of fecal contamination. However, because the size, physiology, and susceptibility to physical treatment and disinfection of bacterial indicators differ from those of NLVs, inherent limitations of this approach exist. Until reliable methods for assessing the occurrence and susceptibility to treatment of NLVs are available, prevention methods should focus on reducing human waste contamination of water supplies. If drinking or recreational water is suspected as being an outbreak source, high-level chlorination (i.e., 10 ppm or 10 mg/L for >30 minutes) might be required for adequate disinfection; however, even this method might be insufficient in certain cases (82). Person-to-Person Transmission Person-to-person spread of NLVs occurs by direct fecal-oral and airborne transmission. Such transmission plays a role in propagating NLV disease outbreaks, notably in institutional settings (e.g., nursing homes and day care centers) and on cruise ships. Although interruption of person-to-person transmission can be difficult, certain measures might help. Frequent handwashing with soap and water is an effective means of prevention. The recommended procedure is to rub all surfaces of lathered hands together vigorously for >10 seconds and then thoroughly rinse the hands under a stream of water. Because spattering or aerosols of infectious material might be involved in disease transmission, wearing masks should be considered for persons who clean areas substantially contaminated by feces or vomitus (e.g., hospital or nursing home personnel). Soiled linens and clothes should be handled as little as possible and with minimum agitation. They should be laundered with detergent at the maximum available cycle length and then machine dried. Because environmental surfaces have been implicated in the transmission of enteric viruses, surfaces that have been soiled should be cleaned with an appropriate germicidal product (e.g., 10% solution of household bleach) according to the manufacturer's instructions. In situations in which the epidemic is extended by periodic renewal of the susceptible population (e.g., camps and cruise ships), the facility or institution might have to be closed until it can be cleaned appropriately. DIAGNOSTIC METHODSAdvances in methods for detecting NLVs have changed our understanding of the epidemiology of these viruses. The following sections provide a summary of the commonly available diagnostic methods, which are extensively reviewed elsewhere (98). Electron Microscopy and Immune Electron Microscopy Under the electron microscope, NLVs can be identified by their characteristic morphology. Approximately 106--107/ml of virus in stool is required for visualization by EM; therefore, this technique is useful only for specimens collected during the early stages of illness when substantial quantities of virus are shed. Even among experimentally infected volunteers, the virus can be found in only 10%--20% of fecal specimens collected on days 2 or 3 of illness. Immune electron microscopy (IEM) can improve the sensitivity of EM by 10- to 100-fold. In one type of IEM, convalescent-phase serum from patients is coated on the examination grid of the microscope before stool specimens are applied. The antibody on the grid traps homologous virus, thereby increasing diagnostic yield. However, IEM has certain disadvantages, the greatest of which is that success is highly dependant on the skill and expertise of the microscopist. Furthermore, the virus might be totally masked if a large excess of antibody is present, resulting in a false-negative test. Enzyme Immunoassays The expression in baculoviruses of the capsid proteins of NLVs that self-assemble into stable virus-like particles has allowed the detection of these viruses by ELISAs. To develop assays to detect virus in fecal specimens, the expressed capsid antigens have been used to generate hyperimmune antibodies in laboratory animals. These assays have been reported to detect the presence of 104--106 viral particles/ml in clinical specimens. To date, these assays have been type-specific, but broadly reactive tests are under development. The baculovirus-expressed viral antigen can be directly used for detection of antibodies to NLVs in patient's sera by enzyme immunoassay. Because certain adults have preexisting immunoglobulin G (IgG) antibodies to NLVs, a single serum specimen is insufficient to indicate recent infection. Seroconversion, defined as a >4-fold rise in IgG antibody titer during acute- and convalescent-phase sera, is indicative of a recent infection. In outbreak settings, if at least half of affected persons seroconvert to a specific NLV, that viral strain can be designated as etiologic. Titers can begin to rise by the fifth day after onset of symptoms, peak at approximately the third week, and begin to fall by the sixth week. Hence, for IgG assays, the acute-phase serum should be drawn within the first 5 days and the convalescent-phase serum during the third to sixth weeks. In certain cases where diagnosis is critical (e.g., when a foodhandler is implicated as the source of an outbreak), single assays of serum immunoglobulin A (IgA) antibody can be successful if specimens are collected 7--14 days after exposure. In addition to potential difficulties in obtaining an adequate number of serum specimens during outbreaks, serologic assays are currently limited by the fact that the available array of expressed NLV antigens is insufficient to detect all antigenic types of NLVs. Nucleic Acid Hybridization Assays and RT-PCR Nucleic acid hybridization assays and RT-PCR assays to detect NLV genome in clinical and environmental specimens have provided a sensitive and specific tool for NLV-outbreak investigations. High sensitivity of these assays (i.e., ability to detect 102--104 viral particles/ml in stool) is both an asset and a liability because extreme care is required to avoid contamination in the laboratory. In addition, although the available primers for RT-PCR assays detect multiple strains of NLVs, certain strains can escape detection. Efforts are ongoing to develop universal or degenerate primers that would detect the majority of NLV strains that cause gastroenteritis outbreaks. Applying New Diagnostics in Outbreak Investigations Application of new molecular diagnostics has expanded the scope of outbreak investigations, as demonstrated in recent outbreaks (Table 2), because outbreak source vehicles (e.g., food or water) can be definitively implicated by detecting NLVs in environmental specimens. However, these methods are not sufficiently developed to be routinely applied. Through nucleotide sequencing, establishing an irrefutable genetic link between outbreaks that occur through a single contaminated vehicle that is distributed in multiple geographic locations is possible. RECOMMENDATIONS REGARDING SPECIMEN COLLECTION FOR DIAGNOSIS OF NLVs*Clinical Specimens Stool Timing. Specimen collection for viral testing should begin on day 1 of the epidemiologic investigation. Any delays to await testing results for bacterial or parasitic agents could preclude establishing a viral diagnosis. Ideally, specimens should be obtained during the acute phase of illness (i.e., within 48--72 hours after onset) while the stools are still liquid or semisolid because the level of viral excretion is greatest then. With the development of sensitive molecular assays, the ability to detect viruses in specimens collected later in the illness has been improved. In specific cases, specimens might be collected later during the illness (i.e., 7--10 days after onset), if the testing is necessary for either determining the etiology of the outbreak or for epidemiologic purposes (e.g., a specimen obtained from an ill foodhandler who might be the source of infection). If specimens are collected late in the illness, the utility of viral diagnosis and interpretation of the results should be discussed with laboratory personnel before tests are conducted. Number and Quantity. Ideally, specimens from >10 ill persons should be obtained during the acute phase of illness. Bulk samples (i.e., 10--50 ml of stool placed in a stool cup or urine container) are preferred, as are acute diarrhea specimens that are loose enough to assume the shape of their containers. Serial specimens from persons with acute, frequent, high-volume diarrhea are useful as reference material for the development of assays. The smaller the specimen and the more formed the stool, the lower the diagnostic yield. Rectal swabs are of limited or no value because they contain insufficient quantity of nucleic acid for amplification. Storage and Transport. Because freezing can destroy the characteristic viral morphology that permits a diagnosis by EM, specimens should be kept refrigerated at 4 C. At this temperature, specimens can be stored without compromising diagnostic yield for 2--3 weeks, during which time testing for other pathogens can be completed. If the specimens have to be transported to a laboratory for testing, they should be bagged and sealed and kept on ice or frozen refrigerant packs in an insulated, waterproof container. If facilities for testing specimens within 2--3 weeks are not available, specimens can be frozen for antigen or PCR testing. Vomitus Vomiting is the predominant symptom among children, and specimens of vomitus can be collected to supplement the diagnostic yield from stool specimens during an investigation. Recommendations for collection, storage, and shipment of vomitus specimens are the same as those for stool specimens. Serum Timing. If feasible, acute- and convalescent-phase serum specimens should be obtained to test for a diagnostic >4-fold rise in IgG titer to NLVs. Acute-phase specimens should be obtained during the first 5 days of symptoms, and the convalescent-phase specimen should be collected from the third to sixth week after resolution of symptoms. Number and Quantity. Ideally, 10 pairs of specimens from ill persons (i.e., the same persons submitting stool specimens) and 10 pairs from well persons (controls) should be obtained. Adults should provide 5--7 ml of blood, and children should provide 3--4 ml. Storage. Specimens should be collected in tubes containing no anticoagulant, and the sera should be spun off and frozen. If a centrifuge is not available, a clot should be allowed to form, and the serum should be decanted and frozen. If this step cannot be accomplished, the whole blood should be refrigerated but not frozen. Environmental Specimens NLVs cannot be detected routinely in water, food, or environmental specimens. Nevertheless, during recent outbreaks (33--36), NLVs have been detected successfully in vehicles epidemiologically implicated as the source of infection. If a food or water item is strongly suspected as the source of an outbreak, then a sample should be obtained as early as possible and stored at 4 C. If the epidemiologic investigation confirms the link, a laboratory with the capacity to test these specimens should be contacted for further testing. If drinking water is suspected, special filtration (45) of large volumes (i.e., 5--100 liters) of water can concentrate virus to facilitate its detection. CONSULTATION AND ASSISTANCEDuring any outbreak, CDC's National Center for Infectious Diseases, Division of Viral and Rickettsial Diseases, Respiratory and Enteric Viruses Branch, Viral Gastroenteritis Section, (Telephone: [404] 639-3607) is available to provide assistance. If, after consultation, viral diagnostic services would be useful, specimens may be shipped to CDC's Viral Gastroenteritis Section with the following provisions:
NCID/DVRD/REVB/VGS Reporting of Outbreaks to CDC All suspected foodborne outbreaks of viral gastroenteritis for which specimens are sent to CDC for laboratory testing should be reported to CDC on a standard form. This form and instructions for completing it are available on the Internet at <http://www.cdc.gov/ncidod/dbmd/outbreak/report_f.htm> (accessed May 1, 2001). References
* Taxonomy nomenclature source: van Regenmortel MHV, Fauquet CM, Bishop DHL, et al., eds. Virus taxonomy: seventh report of the International Committee on Taxonomy of Viruses. New York, NY: Academic Press, Inc., 1999. **A summary table with instructions for collecting clinical specimens during outbreaks to test for bacteria, viruses, and parasites is available at <http://www.cdc.gov/ncidod/dbmd/outbreak/guide_sc.htm> (accessed May 1, 2001). Table 1 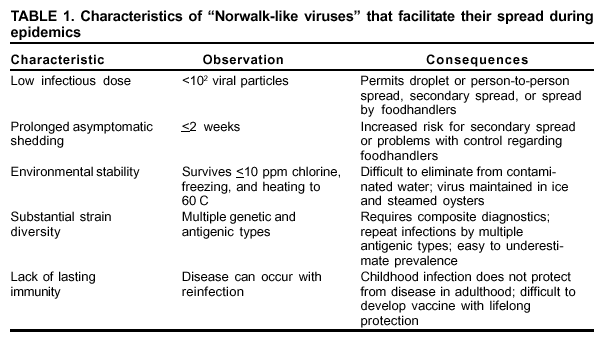 Return to top. Figure 1 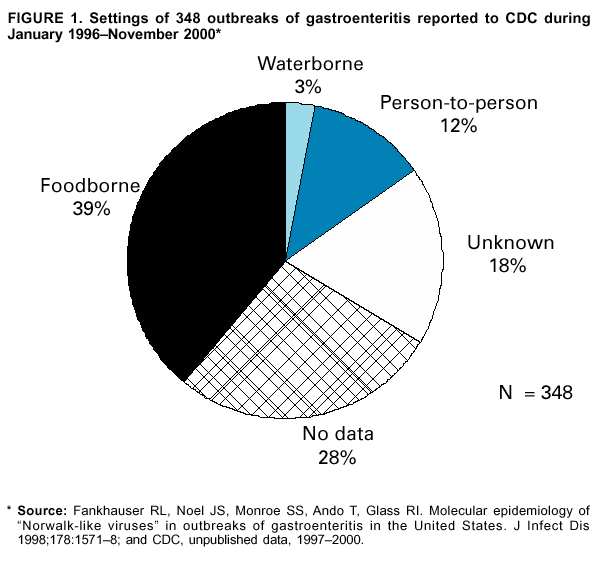 Return to top. Table 2 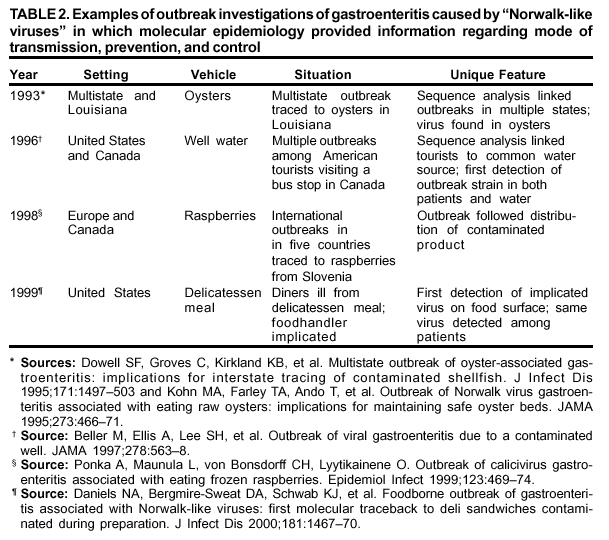 Return to top. Figure 2 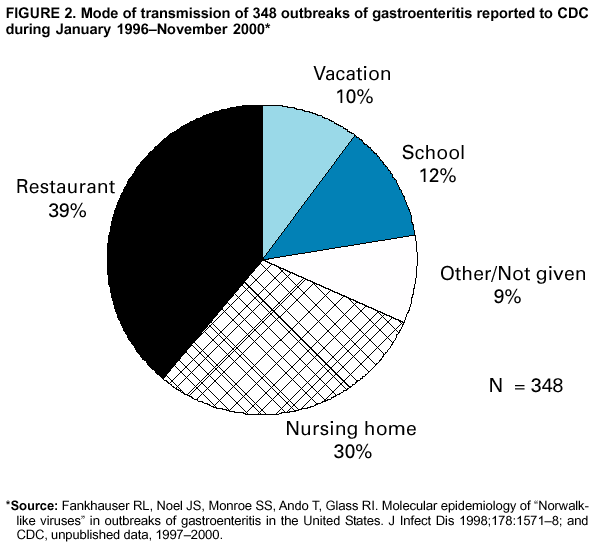 Return to top. All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/8/2001 |
|||||||||
This page last reviewed 6/8/2001
|