 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for Using the QuantiFERON®-TB Test for Diagnosing Latent Mycobacterium tuberculosis InfectionPrepared by The material in this report originated in the National Center for HIV, STD, and TB Prevention, Harold W. Jaffe, M.D., Director, and the Division of Tuberculosis Elimination, Kenneth G. Castro, M.D., Director. Summary Until 2001, the only test used to diagnose latent tuberculosis infection (LTBI) was the tuberculin skin test (TST). However, in 2001, a new test (QuantiFERON®-TB or QFT; manufactured by Cellestis Limited, Carnegie, Victoria, Australia) that measures the release of interferon-gamma in whole blood in response to stimulation by purified protein derivative was approved by the Food and Drug Administration. This statement provides interim recommendations for using and interpreting QFT. As with TST, interpretation and indicated applications of QFT differ for persons according to their risk for LTBI and for developing tuberculosis (TB). This report provides guidance for public health officials, health-care providers, and laboratorians with responsibility for TB control activities in the United States in their efforts to incorporate QFT testing for detecting and treating LTBI. Regardless of the test used to identify LTBI, testing should be primarily targeted at diagnosing infected patients who will benefit from treatment. IntroductionIn 2001, the QuantiFERON®-TB test (QFT) (manufactured by Cellestis Limited, Carnegie, Victoria, Australia)
was approved by the Food and Drug Administration (FDA) as an aid for detecting latent
Mycobacterium tuberculosis infection
(1). This test is an in vitro diagnostic aid that measures a component of cell-mediated immune reactivity to
M. tuberculosis. The test is based on the quantification of interferon-gamma
(IFN- Tuberculin skin testing (TST) has been used for years as an aid in diagnosing latent tuberculosis infection (LTBI) and includes measurement of the delayed type hypersensitivity response 48--72 hours after intradermal injection of PPD. TST and QFT do not measure the same components of the immunologic response and are not interchangeable. Assessment of the accuracy of these tests is limited by lack of a standard for confirming LTBI. As a diagnostic test, QFT 1) requires phlebotomy, 2) can be accomplished after a single patient visit, 3) assesses responses to multiple antigens simultaneously, and 4) does not boost anamnestic immune responses. Compared with TST, QFT results are less subject to reader bias and error. In a CDC-sponsored multicenter trial, QFT and TST results were moderately concordant (overall kappa value = 0.60). The level of concordance was adversely affected by prior bacille Calmette-Guérin (BCG) vaccination, immune reactivity to nontuberculous mycobacteria (NTM), and a prior positive TST (2). In addition to the multicenter study, two other published studies have demonstrated moderate concordance between TST and QFT (3,4). However, one of the five sites involved in the CDC study reported less agreement (5). Limitations of QFT include the need to draw blood and process it within 12 hours after collection and limited laboratory and clinical experience with the assay. The utility of QFT in predicting the progression to active tuberculosis has not been evaluated. This report provides interim recommendations for using and interpreting QFT results based on available data. As with TST, interpretation and indicated applications of QFT differ between those persons at low risk and those at increased risk for LTBI. This report should assist public health officials, health-care providers, and laboratorians who are responsible for TB control activities in the United States in their efforts to incorporate QFT testing for detecting and treating LTBI. QFT Performance, Interpretation, and UseTuberculin testing is performed for persons who are 1) suspected as having active TB; 2) at increased risk for progression to active TB; 3) at increased risk for LTBI; or 4) at low risk for LTBI, but are tested for other reasons (Table 1). QFT PerformanceAliquots of heparinized whole blood are incubated with the test antigens for 16--24 hours.* The antigens included in the
test kits are PPD from M. tuberculosis
(tuberculin)† and PPD from Mycobacterium
avium (avian sensitin). The kits also include phytohemaglutinin (a mitogen used as a positive
assay control), and saline (negative control or nil). After incubation,
the concentration of IFN- QFT results are based on the proportion of IFN- QFT InterpretationInterpretation of QFT results (Table 2) is stratified by estimated risk for infection with M. tuberculosis, in a manner similar to that used for interpreting TST with different cut-off values. QFT results indicative of M. tuberculosis infection include the following three criteria:
Selection of different cut-offs affect the number of persons classified as having positive test results. Using 15 as the percentage tuberculin response cut-off for interpreting a QFT test as positive identifies approximately the same number of persons compared with using a TST induration cut-off of 10 mm. Using 30 as the percentage tuberculin response cut-off for interpreting a QFT test as positive identifies approximately the same number of persons compared with using a TST induration cut-off of 15 mm. The test is considered negative if (mitogen -- nil) > 1.5 IU but (tuberculin -- nil) < 15% (mitogen -- nil). Results are considered indeterminate if (mitogen -- nil) < 1.5 IU, which might be observed among anergic persons. Using QFT for Persons at Increased Risk for LTBIQFT can aid in detecting M. tuberculosis infections among certain populations who are at increased risk for LTBI (6). These populations include recent immigrants (i.e., immigrated within the previous 5 years) from high-prevalence countries where tuberculosis case rates are >30/100,000, injection-drug users, residents and employees of prisons and jails, and health-care workers who, after their preemployment assessment, are considered at increased risk for exposure to tuberculosis. For these populations, a percentage tuberculin response of >15 should be considered a positive QFT result. Using QFT for Persons at Low Risk for LTBICDC discourages use of diagnostic tests for LTBI among populations at low risk for infection with M. tuberculosis (6). However, initial testing is occasionally performed among certain population groups for surveillance purposes or where cases of active, infectious tuberculosis might result in extensive transmission to highly susceptible populations. These populations include military personnel, hospital staff and health-care workers whose risk of prior exposure to TB was low, and U.S.-born students at higher education institutions (e.g., as a requirement for admission to U.S. colleges and universities). For these populations, a percentage tuberculin response of >30 should be considered a positive QFT result. RecommendationsThe highest priority of targeted tuberculin testing programs remains one that identifies persons at increased risk for TB who will benefit from treatment for LTBI. Following that principle, targeted tuberculin testing should be conducted among groups at risk for recent infection with M. tuberculosis and those who, regardless of duration of infection, are at increased risk for progression to active TB. The role of QFT in targeted testing has not yet been defined, but QFT can be considered for LTBI screening as follows:
Before QFT testing is contemplated, arrangements should be made with a qualified laboratory. Those arrangements should include quality assurance and collection and transport of blood within the required 12 hours. Confirmation of QFT results with TST is possible because performance of QFT does not affect subsequent QFT or TST results. The probability of LTBI is greatest when both the QFT and TST are positive. Considerations for confirmation are as follows:
ContraindicationsBecause of insufficient data on which to base recommendations, QFT is not recommended for
ConclusionsThese interim recommendations are intended to achieve a high rate of acceptance and completion of testing for LTBI among groups who have been identified for targeted testing. Testing programs using TST or QFT should only be implemented if plans are also in place for the necessary follow-up medical evaluation and treatment (e.g., chest radiograph or LTBI treatment) of persons who are diagnosed with LTBI and quality laboratory services are ensured. References
* Additional technical information is available from the manufacturer at
http:// www.cellestis.com. Table 1 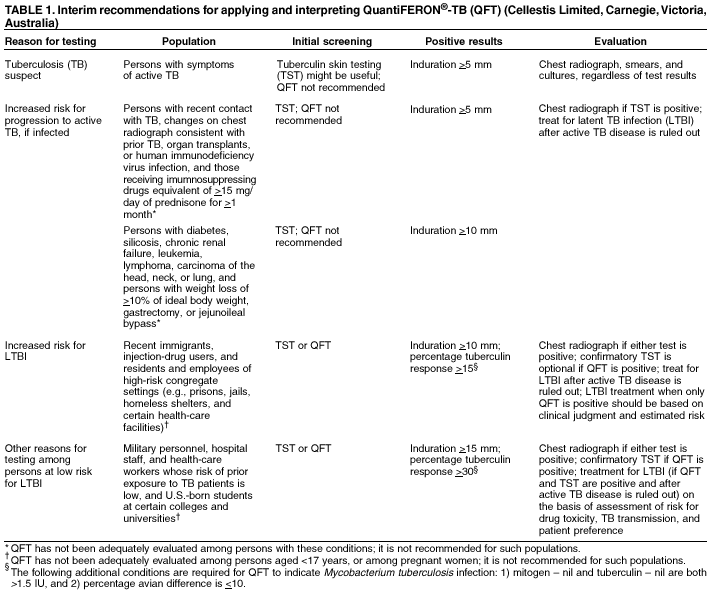 Return to top. Table 2 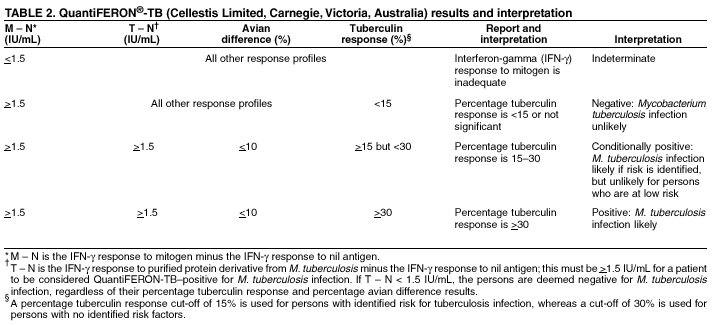 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/13/2003 |
|||||||||
This page last reviewed 1/13/2003
|