 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Connecting Health Departments and Providers: Syndromic Surveillance's Last MileJames B. Daniel,1 D. Heisey-Grove,1 P.
Gadam,1 W. Yih,2 K. Mandl,3 A. DeMaria,
Jr.,1 R. Platt2,4
Corresponding author: James B. Daniel, Massachusetts Department of Public Health, State Laboratory Institute, 305 South Street, Jamaica Plain, MA 02130. Telephone: 617-983-6808; Fax: 617-983-6840; E-mail: James.Daniel@state.ma.us. Disclosure of relationship: The contributors of this report have disclosed that they have no financial interest, relationship, affiliation, or other association with any organization that might represent a conflict of interest. In addition, this report does not contain any discussion of unlabeled use of commercial products or products for investigational use. AbstractIntroduction: A critical need exists for mechanisms to identify and report acute illness clusters to health departments. The Massachusetts Department of Public Health (MDPH) works with partner organizations to conduct syndromic surveillance. This effort is based on CDC's Health Alert Network program and includes automated generation and notification of signals and a mechanism to obtain detailed clinical information when needed. Methods: Syndromic surveillance partners collect emergency department and ambulatory care data. The principal communications platform between syndromic surveillance partners and MDPH is the Massachusetts Homeland and Health Alert Network (HHAN). This Internet-based application serves as a portal for communication and collaboration and alerts predefined groups of users involved in emergency response. Syndromic surveillance partners' systems report to HHAN by using Public Health Information Network Messaging System events that meet thresholds selected by MDPH. Cluster summaries are automatically posted into a document library. HHAN notifies users by electronic mail, alphanumeric pager, facsimile, or voice communications; users decide how they want to be notified for each level of alert. Discussion threads permit real-time communication among all parties. Results: This automated alert system became operational in July 2004. During July--December 2004, HHAN facilitated communication and streamlined investigation of 15 alerts. Conclusion: The system allows rapid, efficient alerting and bidirectional communication among public health and private-sector partners and might be applicable to other public health agencies. IntroductionA critical need exists for mechanisms to report acute illness clusters and for public health personnel to obtain timely clinical information about persons who are part of these clusters. Timely identification of newly emerging pathogens and syndromes, as well as unusual clusters of illness, is difficult when health departments rely solely on traditional methods of surveillance (e.g., laboratory reporting). Identifying illnesses early facilitates treatment, prevention, and control of disease. Syndromic surveillance systems access and analyze data sources that are not normally accessible to departments of public health (e.g., symptoms and signs of illness captured by International Classification of Diseases, Ninth Revision [ICD-9] codes and chief-complaint data). As a result, syndromic surveillance might detect unusual clusters of illness before definitive diagnoses are made and thus potentially earlier than traditional disease reporting allows. In Massachusetts, two syndromic surveillance systems are in place, one capturing visit data from ambulatory-care settings and one using chief-complaint data from hospital emergency departments (EDs). Both systems were established in partnership with the Massachusetts Department of Public Health (MDPH). The Harvard Pilgrim Health Care/Harvard Vanguard Medical Associates (HPHC/HVMA) system collects ambulatory care data from an electronic medical record system at 14 clinic sites in eastern Massachusetts (1--4). The Children's Hospital Boston system (AEGIS) utilizes chief-complaint data from eight Massachusetts hospital EDs (5,6). In both systems, new visits are grouped into syndromes defined previously by a CDC-led working group (7) and aggregated by ZIP code. Statistical models are used to assess whether each day's syndrome counts are unusual. The first challenge to response to syndromic surveillance signals is to establish bidirectional communication among partners. MDPH uses the Massachusetts Health and Homeland Alert Network (HHAN) to automate communication of alerts. HHAN is an Internet-based application that serves as the principal platform permitting communication among Massachusetts' syndromic surveillance partners. This report describes the experience gained and outlines remaining challenges. MethodsData on HPHC/HVMA patient encounters (i.e., visits or calls), including demographic information and diagnostic codes, are recorded electronically as part of routine patient care, usually on the same day. Every 24 hours, encounters with codes of interest are extracted automatically from the clinical data system. The data are deidentified and aggregated by syndrome and ZIP code of residence; the resulting aggregate counts of illness are automatically uploaded to a datacoordinating center. During processing of the daily data file, a line list of the day's encounters is generated and kept at HPHC/HVMA. This list contains demographic information and the text of the diagnostic codes assigned during each encounter and allows a first-level epidemiologic assessment (short of consulting the full medical record). These methods have been described previously (1--4). The Small Area Regression and Testing (SMART) scores method (8) is used. This method uses generalized linear mixed models that adjust for day of the week, holidays, seasonal patterns, and any secular trends on the basis of historic data to determine the degree of statistical aberration associated with each date-syndrome--ZIP code count. The signal detection is automated and occurs at the data coordinating center. If the number of cases of a syndrome detected in a particular ZIP code on a particular date is higher than expected, an automatic alert is generated and sent to designated recipients at MDPH through HHAN by means of an interface between the data-coordinating center and HHAN. MDPH created three alert levels (low, medium, and high), corresponding to recurrence interval (i.e., the number of days of surveillance that would normally elapse between the chance occurrence of counts as unusual as the one observed) thresholds. MDPH uses recurrence-interval thresholds of 2 months (low), 6 months (medium), and 2 years (high), except for respiratory syndrome, for which the chosen thresholds are 6 months, 1 year, and 2 years, respectively. Syndromespecific alert levels are defined by MDPH staff and can be changed readily; for example, the alert threshold can be lowered during periods of heightened concern (e.g., during a political convention). HHAN functions as a secure collaboration portal that allows role-based alerting and access to a document library (Figure). Once a syndromic surveillance partner's system detects a signal, a document is posted in the library on HHAN through the Public Health Information Network Messaging System (PHIN-MS). Simultaneously, an automatic alert message is sent from PHIN-MS to HHAN, which then uses built-in functionality to distribute the alert further by electronic mail, facsimile, alphanumeric pager, or voice (e.g., cellular telephone). Each user decides how to be notified for each alert level (low, medium, or high). Simultaneously, the system automatically posts a document providing details about the cluster that generated the alert. Once alerts and documents have been sent, bidirectional communication between MDPH and clinical responders is facilitated by using discussion threads associated with the alert document in question. After the HPHC/HVMA system generates an alert, MDPH staff contact a designated clinical responder on call. Responders have been trained for this purpose and are available at all times according to an established schedule. Within the line list for the day in question, the clinician reviews the cases responsible for the alert, and, if a cluster of illness of public health importance is suspected (e.g., a substantial cluster within a single ZIP code of lower gastrointestinal illness that includes family members), MDPH staff proceed with further investigation. A response protocol guides MDPH epidemiologists in contacting on-call responders to obtain further details about cases contributing to suspect clusters of illness. Before full implementation of the HHAN alert system, this response protocol was pilot tested with clinical responders from HPHC/HVMA. ResultsPilot testing of HPHC/HVMA clinical response began in June 2004, and the automated alert system became operational in July 2004, in time for use during the Democratic National Convention held in Boston July 26--29. During July--December 2004, HHAN received 15 alerts from the HVMA/HPHC system. Two alerts required investigation beyond consultation of line lists (e.g., chart review), but no public health intervention was necessary. Case Scenario #1: Medium-Level Neurologic AlertIn September 2004, MDPH received an alert through HHAN about 23 persons who reported symptoms consistent with a neurologic syndrome. These 23 cases occurred in multiple areas with ZIP codes beginning with 021 (HVMA/HPHC population: 55,866 persons; U.S. Census population: 1,183,247 persons). The estimated recurrence interval was 405 days. Review of the line list determined that 20 (86.9%) of 23 patients had headaches. The clinical responder reviewed the patient medical records and determined that the patients' clinical presentations did not suggest a genuine cluster. The clinician posted the results of the chart review in the discussion thread, allowing MDPH to halt the investigation. Case Scenario #2: Medium-Level Lower Gastrointestinal Illness AlertIn October 2004, MDPH received an alert through HHAN about 37 persons (age range: <1-->80 years) who reported lower gastrointestinal illness. The cases occurred in multiple areas with ZIP codes beginning with 021 (HVMA/HPHC population: 55,866 persons; U.S. Census population: 1,183,247 persons). The estimated recurrence interval was 296 days. MDPH staff contacted the clinical responder, who posted a deidentified line list of cases in the discussion thread of the alert. Review of the ICD-9 codes associated with the visit indicated multiple symptoms and diagnoses, including abdominal pain, diarrhea, gastroenteritis, and Clostridium difficile. In the discussion thread, the clinical responder noted that among the 18 towns included in the alert, symptoms generally did not appear to be similar within each town. In one town, four patients had abdominal pain, but the characteristics of the pain varied. Epidemiologic review of the line-list information ruled out the need for further clinical response, and MDPH closed the investigation. Case Scenario #3: High-Level Respiratory AlertIn September 2004, MDPH received a high-level respiratory alert through HHAN involving five cases in a single ZIP code (HVMA/HPHC population: 56 persons; U.S. Census population: 7,480 persons). The estimated recurrence interval was >200 years. The clinical responder posted the deidentified line list in the discussion thread and noted that two of the five patients were members of the same family and that three patients had asthma. A second clinical responder viewed the discussion thread and concurred with this assessment. Epidemiologic review of the line-list information ruled out the need for further clinical response, and MDPH closed the investigation. DiscussionThe HHAN alert system meets multiple needs. It allows routine, timely, and automated aggregation of clinical information from a large, defined population; identification of unusual clusters of illness; and communication about these events to designated health department epidemiologists. It also establishes a repository of limited clinical information (line lists) about each case that contributes to a cluster and creates a formal protocol for obtaining additional information about cases at any time from a clinician in the delivery system who has access to full-text medical records. In implementing this system, MDPH recognized a need for a more timely response to HHAN alerts. To address this critical need, MDPH epidemiologists need access to the limited line-list information about cases that contribute to clusters. In each of the scenarios described in this report, initial clinical response time for review of line lists was approximately 2--4 hours, whereas epidemiologists were available to review HHAN alert information within 30 minutes. To address this delay in response time, the system is being revised so that deidentified line lists of cases will be provided automatically to HHAN along with the alert. This will allow MDPH staff to perform an initial epidemiologic evaluation of the cluster without contacting the clinical responder. The line list will include a unique identifier, age range, sex, ZIP code, or town of residence, ICD-9 codes, and a family identifier (to identify multiple cases occurring in the same household). Receiving the information in this way will reduce the need to involve clinical responders so responders can focus on performing chart reviews and conferring with health department personnel about additional follow-up (e.g., contacting patients or advising clinicians to be alert for additional cases). Only the first scenario described in this report required medical record review; epidemiologic review of the line-list information in the other two scenarios described ruled out the need for further clinical response. Although the HVMA/HPHC catchments encompass densely populated urban areas, implementing this system in rural areas might present a problem with regard to line lists being fully deidentified. Submitting patientidentifiable information only when the health department and clinical responders agree that a cluster is potentially of public health concern minimizes the total amount of personal health-care information provided routinely. Avoiding routine transfer of information for health-care encounters that are not part of clusters helps the delivery system assure patients about the confidentiality of their health-care data. In addition, data are protected by a secure web portal and by MDPH privacy and confidentiality standards. Once a potential cluster of public health concern is identified, obtaining a comprehensive line list that includes identifiable patient information remains problematic. Lists cannot be posted on HHAN because HHAN does not have a second-tier identification mechanism; second-tier authentication (e.g., a secure token or digital certificate) would give HHAN an additional layer of security and permit posting of identifiable information. MDPH is also exploring using PHIN-MS to securely transfer these data to a proposed Internet-based disease reporting system. Given the investments that have been made and the effort involved in responding to alerts, the stillunanswered question of the usefulness of syndromic surveillance to public health can and should be addressed even before the issues discussed in this report are fully resolved. As part of a multistate effort, MDPH and its partners are undertaking an evaluation of the sensitivity, predictive value-positive, timeliness, and cost-benefit of these alerts and establishing databases of alerts and outbreaks that include nontraditional data elements (e.g., person-time spent on investigation and interventions and assessment of costs and benefits of receiving and responding to each alert). ConclusionThe HHAN alert system allows rapid, efficient alerting and bidirectional communication among public health and private-sector partners. Automatic generation of alerts saves time because epidemiologists do not have to manually review data each day to define clusters. Issues identified in the implementation of the system include the need to generate and make accessible to public health signal-specific line lists, a problem that is being addressed. Future MDPH plans include building the ability to send alerts to local boards of health to gain local public health participation earlier in the investigation process. This experience with HHAN might be applicable to other public health agencies, including those with access to syndromic surveillance data. Evaluation of the effectiveness and utility of this surveillance and reporting system in improving public health is needed and is currently under way. AcknowledgmentThis project was funded in part by two grants from CDC cooperative agreements (UR8/CCU115079 and U90/CCU116997). References
Figure 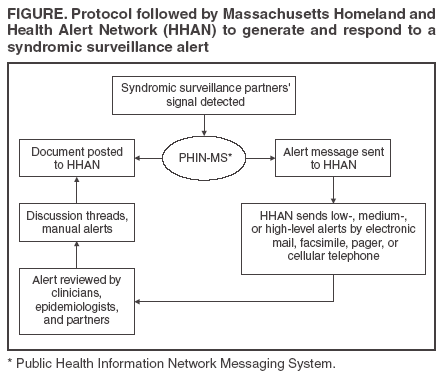 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/5/2005 |
|||||||||
|