 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Monitoring Progress Toward Achieving Maternal and Infant Healthy People 2010 Objectives --- 19 States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000--2003Katherine Suellentrop, MPH1
Corresponding address: Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC, 4770 Buford Highway, N.E., MS K-22, Atlanta, GA 30341. Telephone: 770-488-6260; Fax: 770-488-6291; E-mail: LMWilliams@cdc.gov. AbstractProblem/Condition: Certain modifiable maternal behaviors and experiences before, during, and after pregnancy are associated with adverse health outcomes for the mother and her infant (e.g., physical abuse, insufficient folic acid consumption, smoking during pregnancy, and improper infant sleep position). Information about these behaviors and experiences is needed to monitor trends in maternal and infant health, enhance understanding of the relation between maternal behaviors and infant health outcomes, plan and evaluate maternal and infant health programs, direct policy decisions, and monitor progress toward achieving the national Healthy People 2010 [HP 2010] objectives (US Department of Health and Human Services. Healthy people 2010. 2nd ed. With understanding and improving health and objectives for improving health [2 vols.]. Washington, DC: US Department of Health and Human Services; 2000). Reporting Period Covered: 2000--2003. Description of System: The Pregnancy Risk Assessment Monitoring System (PRAMS) is an ongoing, state- and population-based surveillance system designed to monitor selected maternal behaviors and experiences that occur before, during, and after pregnancy among women who deliver live-born infants. PRAMS employs a mixed mode data-collection methodology; up to three self-administered surveys are mailed to a sample of mothers; nonresponders are followed up with telephone interviews. Self-reported survey data are linked to selected birth certificate data and weighted for sample design, nonresponse, and noncoverage to create annual PRAMS analysis data sets that can be used to produce statewide estimates of perinatal health behaviors and experiences among women delivering live infants. This report summarizes data for 2000--2003 from 19 states (Alabama, Alaska, Arkansas, Colorado, Florida, Hawaii, Illinois, Louisiana, Maine, Nebraska, New Mexico, New York, North Carolina, Ohio, Oklahoma, South Carolina, Utah, Washington, and West Virginia) that measured progress toward achieving HP 2010 objectives for eight perinatal indicators: 1) pregnancy intention, 2) multivitamin use, 3) physical abuse, 4) cigarette smoking during pregnancy, 5) cigarette smoking cessation, 6) drinking alcohol during pregnancy, 7) breastfeeding initiation, and 8) infant sleep position. Results: In 2003, prevalence of intended pregnancy among women having a live birth ranged from 48.1% in Louisiana to 66.5% in Maine; during 2000--2003, no state experienced a statistically significant (p<0.05) increase in prevalence of intended pregnancy, and one state experienced a significant decrease. In 2003, prevalence of multivitamin use at least four times per week during the month before pregnancy ranged from 23.0% in Arkansas to 45.2% in Maine; during 2000--2003, multivitamin use increased significantly in three states (Illinois, North Carolina, and Utah). In 2003, prevalence of physical abuse by a husband or partner during the 12 months before pregnancy ranged from 2.2% in Maine to 7.6% in New Mexico; during 2000--2003, significant decreases were recorded in three states (Alaska, Hawaii, and Nebraska). In 2003, prevalence of abstinence from cigarette smoking during the last 3 months of pregnancy ranged from 72.5% in West Virginia to 96.1% in Utah; during 2000--2003, a significant increase was recorded in Utah. In 2003, prevalence of smoking cessation during pregnancy ranged from 30.2% in West Virginia to 65.8% in Utah; during 2000--2003, a significant increase was recorded in Utah. In 2003, prevalence of abstinence from alcohol during the last 3 months of pregnancy ranged from 91.3% in Colorado to 98.0% in Utah; during 2000--2003, abstinence increased significantly in Louisiana and Utah but decreased significantly in Florida and Nebraska. In 2003, prevalence of mothers who breastfed their babies in the early postpartum period ranged from 51.2% in Louisiana to 90.3% in Alaska; during 2000--2003, significant increases were recorded in six states (Arkansas, Illinois, Louisiana, Nebraska, North Carolina, and South Carolina). In 2003, prevalence of healthy full-term infants who were placed to sleep on their backs ranged from 50.0% in Arkansas to 78.7% in Washington; during 2000--2003, significant increases were recorded in eight states (Alaska, Colorado, Illinois, Louisiana, Maine, Nebraska, North Carolina, and West Virginia). In 2003, all 19 states achieved or exceeded the HP 2010 objective for smoking cessation during pregnancy, and 16 states achieved the HP 2010 objective for abstinence from alcohol during the last 3 months of pregnancy. In addition, nearly half of the states achieved the objectives for breastfeeding in the early postpartum period and infant back sleep position. However, no state achieved the HP 2010 objectives for intended pregnancy, multivitamin use before pregnancy, absence of physical abuse before pregnancy, or abstinence from smoking during pregnancy. Interpretation: PRAMS data indicate variability among states regarding progress toward achieving HP 2010 objectives in the area of maternal and child health. More progress has been made in achieving objectives focused on the period during and after pregnancy (e.g., smoking cessation and proper infant sleep position); less progress has been made in achieving objectives related to behaviors and experiences in the preconception period (e.g., pregnancy intention and multivitamin use). Public Health Action: State maternal and child health programs can use these state- and population-based data to monitor progress toward achieving HP 2010 objectives, identify indicators to target for intervention, and plan and evaluate programs that promote positive maternal and infant health behaviors, experiences, and outcomes. These data also can be used to guide policy decisions that could affect the health of mothers and infants. Introduction Healthy People 2010 (HP 2010) serves as the national comprehensive guide for disease prevention and health promotion (1). The objectives outlined in HP 2010 cover a broad spectrum of health topics and aim to increase the quality and years of healthy life and to eliminate health disparities among persons living in the United States. With respect to maternal, infant, and child health, the overall goal is to improve the health and well-being of women, infants, children, and families. This report focuses on perinatal indicators associated with the following eight HP 2010 objectives regarding behaviors and experiences before, during, and after pregnancy: 1) pregnancy intention, 2) multivitamin use, 3) physical abuse, 4) cigarette smoking during pregnancy, 5) cigarette smoking cessation, 6) drinking alcohol during pregnancy, 7) breastfeeding initiation, and 8) infant sleep position (Table 1). In the preconception period, multiple factors (e.g., pregnancy intention, folic acid consumption, and physical abuse) affect maternal and infant health status during and after pregnancy. Women who experience an unintended pregnancy resulting in a live birth are more likely than those with an intended pregnancy to delay entry into prenatal care, have poor maternal nutrition, use alcohol during pregnancy, and have adverse maternal and infant outcomes (2). Folic acid consumption before pregnancy reduces the incidence of neural tube defects (NTDs) (3). NTDs affect an estimated 3,000 pregnancies annually, and 95% of children born with an NTD are born to couples with no history of these birth defects (4,5). Folic acid intake of >400 µg daily can reduce the incidence of NTDs by 50% (6). The U.S. Public Health Service recommends that all women of childbearing age who are capable of becoming pregnant should consume 400 µg of folic acid daily through either supplementation or fortified foods (6). Physical abuse before pregnancy is associated with late entry into prenatal care, especially among older women of higher socioeconomic status (7). Abuse also is related to an increased risk for low birthweight and to increased mortality and morbidity for mothers and infants (8). Physical abuse before pregnancy often is a strong predictor of physical abuse during pregnancy (9,10). Certain maternal behaviors and experiences during pregnancy (e.g., cigarette smoking and alcohol consumption) also affect maternal and infant health outcomes. Smoking during pregnancy contributes to multiple complications in pregnancy and poor infant health outcomes, including placenta previa, abruptio placentae, preterm birth, low birthweight, and sudden infant death syndrome (SIDS) (11--13). After delivery, maternal smoking continues to affect the health of the infant negatively, and environmental tobacco smoke exposure among children is associated with an increased risk for respiratory tract infections (e.g., bronchitis and pneumonia, otitis media, and childhood asthma) (14). The two causes of infant death most strongly associated with maternal smoking are respiratory infections and SIDS (13--15). Smoking cessation, especially early in pregnancy, has been determined to improve poor infant health outcomes associated with smoking during pregnancy (16--19). Drinking alcohol during pregnancy is associated with multiple birth defects, including fetal alcohol syndrome, mental retardation, neurodevelopment disorders, and increased spontaneous abortions (20,21). Because no threshold of alcohol consumption during pregnancy is recognized as safe, and because research indicating that susceptibility to adverse effects from prenatal alcohol exposure varies among children, the American Academy of Pediatrics (AAP), the American College of Obstetricians and Gynecologists, and the U.S. Surgeon General recommend that pregnant women abstain from alcohol consumption (22,23). Certain postpartum maternal behaviors (e.g., breastfeeding and positioning of an infant during sleep) also can affect infant health. Breastfeeding, the preferred method of infant feeding recommended by AAP, is associated with multiple health benefits, including reduced risk for infectious illnesses, reduced incidence of coughing or wheezing, reduced risk for ear infections (among those infants without older siblings), and improved immunity, growth, and cognitive function for the infant (24--27). In addition, breastfeeding is associated with less postpartum bleeding and a reduced risk for ovarian and premenopausal breast cancer for the mother (24,28). Infant sleep position has been recognized as a major modifiable risk factor for SIDS, a leading cause of infant mortality (29--31). To reduce the risk for SIDS, AAP recommends that infants be placed to sleep in the supine position (i.e., on their backs) (31). MethodsProject DescriptionThe Pregnancy Risk Assessment Monitoring System (PRAMS) is an ongoing, state- and population-based surveillance system designed to monitor selected self-reported maternal behaviors and experiences that occur before, during, and after pregnancy among women who deliver live-born infants. PRAMS is administered by CDC in collaboration with state health departments. The project supports the activities of CDC's Safe Motherhood Initiative, which aims to reduce infant mortality and low infant birthweight. PRAMS data can be used in planning and evaluating programs, directing policy decisions, and monitoring progress toward achieving national health objectives. PRAMS was developed to monitor low birthweight and preterm birth and to understand the relation between maternal behaviors and these outcomes, including maternal and child health and vital statistics. Since its inception in 1987, the program has expanded from six sites to 39 participating health departments (38 states and New York City) (Figure 1). This represents approximately 75% of all live births in the United States. An additional eight states (Delaware, Massachusetts, Missouri, Pennsylvania, Tennessee, Virginia, Wisconsin, and Wyoming) and one tribal area (South Dakota) were funded in 2006 to begin data collection in 2007. The PRAMS questionnaire collects information on multiple maternal behaviors and experiences. This report uses PRAMS data to assess the status during 2000--2003 of 19 states (Alabama, Alaska, Arkansas, Colorado, Florida, Hawaii, Illinois, Louisiana, Maine, Nebraska, New Mexico, New York, North Carolina, Ohio, Oklahoma, South Carolina, Utah, Washington, and West Virginia) with respect to achieving eight HP 2010 objectives related to maternal and child health (Table 1). State trends during 2000--2003 were analyzed to monitor progress toward achieving these objectives. The results from this analysis can assist states in setting priorities for policy and program planning related to making progress toward achieving the eight HP 2010 objectives. Data CollectionConsistent with previous and ongoing PRAMS procedure, all participating health departments use a standardized data collection method developed by CDC. In each reporting area, a monthly stratified sample of 100--300 new mothers is selected from birth certificates. PRAMS employs a mixed-mode data collection methodology in which a self- administered survey is mailed to mothers in the sample, typically 2--3 months after delivery to permit collection of information about postpartum maternal and infant experiences. Mothers who do not complete the first survey are mailed a second; if they do not complete the second survey, they are mailed a third. Mothers who do not complete any of the three mail surveys are contacted by telephone, for a total data collection period of 95 days. To minimize recall bias, efforts to contact women end 9 months postpartum. Self-reported survey data are linked to selected birth certificate data and weighted for sample design, nonresponse, and noncoverage. The PRAMS questionnaire is revised periodically to reflect changing priorities and emerging issues. Each revision is referred to as a phase, and all new questions are tested thoroughly through cognitive interviewing and written feedback before full-scale implementation. All data highlighted in this report were collected with the Phase Four version of the questionnaire, which was implemented with the 2000 birth cohort and continued through the 2003 cohort. Additional details regarding the PRAMS methodology have been published previously (32). Data AnalysisThis report includes results from 19 states that collected data during 2000--2003 and achieved weighted response rates of >70% in 1 year. To prevent nonresponse bias, a threshold of 70% was determined by an internal working group to ensure reasonable representativeness of the population of interest. Those states that did not achieve this threshold were excluded from the analysis. The weighted response rate indicates the proportion of women sampled who completed a survey, adjusted for sample design. Data are presented for eight self-reported maternal behaviors and experiences (Table 1). New York data exclude New York City, which has its own vital records agency separate from the state's. The 2003 prevalence estimates are presented by state together with the corresponding HP 2010 objective (Table 2). Trend data for 2000--2003 are presented by state for each indicator. All tables in this report were produced using weighted PRAMS data. Percentages were calculated for the characteristic using SUDAAN (33). An estimate is noted when the percentage of missing values is >10%. In tables with trend data, the p value indicates a test for linear trend and was calculated using SUDAAN (33). ResultsIntended PregnancyThe HP 2010 objective for this indicator (objective no. 9-1) is that 70% of all pregnancies will be intended. In 2003, state prevalence of intended pregnancy among women delivering a live-born infant ranged from 48.1% in Louisiana to 66.5% in Maine (Table 2). Although no state achieved the objective for intended pregnancy, four were within 10% of doing so. Trend analysis indicated that prevalence of intended pregnancy declined significantly in Nebraska during 2000--2003; among the other states, prevalence of intended pregnancy remained relatively unchanged (Table 3). Multivitamin UseThe HP 2010 objective for this indicator (objective no. 16-16a) is that 80% of nonpregnant women aged 15--44 years will consume >400 µg of folic acid daily. Multivitamin use at least four times per week has been demonstrated to provide the recommended amount of folic acid (34). In 2003, state prevalence of multivitamin use at least four times per week during the month before pregnancy ranged from 23.0% in Arkansas to 45.2% in Maine, much lower than the 80% goal for the objective (Table 2). In three states (Illinois, North Carolina, and Utah), prevalence of multivitamin use increased significantly during 2000--2003 (Table 4); for the other states, trend analysis indicated that prevalence of multivitamin use before pregnancy remained relatively unchanged. Physical AbuseThe HP 2010 objective for this indicator (objective no. 15-34) is to reduce to 0.33% the rate of physical assault on persons aged >12 years by a current or former intimate partner. In 2003, prevalence of physical abuse by a husband or partner during the 12 months before pregnancy ranged from 2.2% in Maine to 7.6% in New Mexico (Table 2). During 2000--2003, prevalence of physical abuse by a husband or partner during the 12 months before pregnancy decreased significantly in three states (Alaska, Hawaii, and Maine) (Table 5); for the other states, trend analysis indicated that prevalence of physical abuse during the 12 months before pregnancy remained relatively unchanged. Abstinence from Smoking During PregnancyThe HP 2010 objective for this indicator (objective no. 16-17c) is that 99% of pregnant women will abstain from cigarette smoking. Because PRAMS does not collect data on tobacco use during the first or second trimester of pregnancy, for this analysis, abstinence from smoking during pregnancy was defined as abstinence from smoking during the last 3 months of pregnancy. In 2003, prevalence of abstinence from cigarette smoking during the last 3 months of pregnancy ranged from 72.5% in West Virginia to 96.1% in Utah (Table 2). No state achieved the objective for abstinence from smoking during pregnancy. During 2000--2003, prevalence of abstinence from cigarette smoking during pregnancy increased significantly only in Utah (Table 6); for the other states, prevalence of abstinence from smoking during the last 3 months of pregnancy remained relatively unchanged. Smoking Cessation During PregnancyThe HP 2010 objective for this indicator (objective no. 27-6) is that 30% of smokers will stop smoking during pregnancy. For this analysis, smoking cessation was defined as the report of any cigarette smoking during the 3 months before pregnancy but no cigarette smoking reported during the last 3 months of pregnancy. In 2003, prevalence of smoking cessation during pregnancy ranged from 30.2% in West Virginia to 65.8% in Utah (Table 2). All states achieved the health objective for smoking cessation. During 2000--2003, prevalence of smoking cessation during pregnancy increased significantly (Table 7) in Utah; for the other states, trend analysis indicated that prevalence of smoking cessation remained relatively unchanged. Abstinence from Alcohol Use During PregnancyThe HP 2010 objective for this indicator (objective no. 16-17a) is that 94% of all women will abstain from drinking alcohol during pregnancy. Because PRAMS does not collect data on alcohol use during the first or second trimester of pregnancy, for this analysis, abstinence from alcohol use during pregnancy was defined as abstinence from drinking alcohol during the last 3 months of pregnancy. In 2003, prevalence of abstinence from alcohol during the last 3 months of pregnancy ranged from 91.3% in Colorado to 98.0% in Utah (Table 2). Sixteen states achieved the objective for this indicator. During 2000--2003, prevalence of abstinence from alcohol during the last 3 months of pregnancy increased significantly in two states (Louisiana and Utah) and decreased significantly in two states (Florida and Nebraska) (Table 8); for the other states, prevalence of abstinence from alcohol during pregnancy remained relatively unchanged. Breastfeeding in the Early Postpartum PeriodThe HP 2010 objective for this indicator (objective no. 16-19a) is that 75% of women with an infant will initiate breastfeeding in the early postpartum period. For this analysis, breastfeeding in the early postpartum period was defined as the report of having ever breastfed after delivery. In 2003, prevalence of mothers who breastfed their babies in the early postpartum period ranged from 51.2% in Louisiana to 90.3% in Alaska (Table 2). Eight states (Alaska, Colorado, Hawaii, Maine, Nebraska, New Mexico, Utah, and Washington) achieved the objective for this indicator. During 2000--2003, prevalence of mothers who breastfeed their babies in the early postpartum period increased significantly in six states (Arkansas, Illinois, Louisiana, Nebraska, North Carolina, and South Carolina) (Table 9); for the other states, trend analysis indicated that no change occurred in prevalence of breastfeeding in the early postpartum period. Infant Sleep PositionThe HP 2010 objective for this indicator (objective no. 16-13) is that 70% of all healthy full-term infants are placed to sleep on their backs. In 2003, prevalence of healthy full-term infants who were placed to sleep on their backs ranged from 50.0% in Arkansas to 78.7% in Washington (Table 2). Seven states (Alaska, Colorado, Maine, Nebraska, New York, Utah, and Washington) have achieved the objective for infant sleep position. During 2000--2003, prevalence of mothers who placed their healthy full-term infants to sleep on their backs a majority of the time increased significantly in eight states (Alaska, Colorado, Illinois, Louisiana, Maine, Nebraska, North Carolina and West Virginia) (Table 10); for the other states, trend analysis indicated that prevalence of infants who were placed to sleep on their backs a majority of the time remained relatively unchanged. SummaryNo state achieved the HP 2010 objectives for three indicators in the preconception period that affect maternal and child health outcomes (intended pregnancy, multivitamin use during the month before pregnancy, and physical abuse during the 12 months before pregnancy). For behaviors in the prenatal period, results were mixed. No state achieved the objective for abstinence from smoking during pregnancy. However, all states included in this analysis achieved the objective for smoking cessation during pregnancy, and more than three fourths have achieved or exceeded the objective for abstinence from alcohol during pregnancy. Nearly half of the states in this analysis achieved the objective for breastfeeding in the early postpartum period, and slightly more than one third achieved the objective for infant sleep position (Figure 2). DiscussionThe 19 states included in this report have made progress in achieving certain maternal and child health HP 2010 objectives. However, increased efforts are needed for states to achieve all eight HP 2010 objectives examined in this report. More progress has been made in the health indicators related to maternal behaviors during pregnancy (e.g., smoking cessation and abstinence from tobacco and alcohol) and after pregnancy (e.g., breastfeeding in the early postpartum period and infant sleep position) than for those related to behaviors before pregnancy (e.g., pregnancy intention and multivitamin use) (Figure 2). The preconception period is an important area of focus for future maternal and child health efforts. Recent recommendations for the preconception period include changing consumer knowledge about the importance of preconception health behaviors and services, improving clinical practice, developing and improving public health programs, improving health-care financing, and using data and research to identify new strategies for improvement and to monitor progress (35). Although PRAMS data are useful for assessing progress toward achieving the HP 2010 objectives, they cannot be used to determine causal agents or explain why certain intervention programs were more successful than others. Few published studies evaluate state level interventions specific to these objectives and offer concrete examples of ways to improve health outcomes. However, certain initiatives have proven successful in improving indicators discussed in this report. For example, effective interventions have been developed to encourage breastfeeding and placing infants to sleep on their backs. The Baby-Friendly Hospital Initiative, launched by the World Health Organization and the United Nations Children's Emergency Fund, improves breastfeeding rates in hospitals through a 10-step program to encourage successful breastfeeding. These steps include having a written breastfeeding policy, training staff in the skills needed to implement the policy, informing new mothers about the benefits of breastfeeding, helping new mothers initiate breastfeeding, allowing mothers and infants to remain together 24 hours a day, and fostering the development of support for breastfeeding after mothers leave the hospital (36). In one study, the Baby-Friendly Hospital Initiative improved breastfeeding initiation rates absolutely during a four-year period, from 58% to 86.5% (37). In 1992, AAP recommended that all healthy infants be placed to sleep on their backs to reduce the risk for SIDS (38). National and federal agencies joined together to launch the Back to Sleep campaign to educate parents and infant care givers about the importance of infants sleeping on their backs (39). An evaluation of that campaign, using PRAMS data for 1996--1998 from 15 states, determined that a significant reduction had occurred in prevalence of prone infant sleeping (40). As of 2003, seven PRAMS states had achieved the HP 2010 objective for infant sleep position. During 2000--2003, four other states made significant progress toward achieving the goal; seven states made no progress. Additional educational efforts might help the remaining states achieve the HP 2010 objective. LimitationsThe findings in this report are subject to at least five limitations. First, PRAMS data are not generalizable to other states, the entire United States, or all pregnant women, only those who delivered live-born infants. Second, because PRAMS reports only on unintended pregnancies resulting in a live birth, prevalence of unintended pregnancies is probably underestimated. Third, because PRAMS does not collect data on alcohol or tobacco use during the first or second trimesters of pregnancy, estimates do not capture prevalence of women who used alcohol or tobacco in early pregnancy. Fourth, smoking estimates are based on self-reported data, which likely underestimated the true rate of smoking (41). Finally, the indicator for folic acid consumption, multivitamin use, does not capture women's consumption for folic acid precisely, and PRAMS data therefore might not accurately reflect prevalence of women achieving this objective. ConclusionsPRAMS was established to provide state-level data on women's health before, during, and shortly after pregnancy to assist health agencies and researchers to monitor trends in maternal and infant health indicators. This report provides a snapshot of how PRAMS data can be used to monitor state progress toward achieving maternal and child HP 2010 objectives. Continued use of PRAMS data to monitor these maternal behaviors is important for implementing, evaluating, and setting priorities for future initiatives at the state level. PRAMS data can be used to gain support for specific programs and initiatives aimed at improving the health of women and infants (42). In April 2006, PRAMS added nine additional sites; the total number of sites collecting PRAMS data is 39 (38 states and New York City), representing approximately 75% of all U.S. live births. This expansion brings PRAMS closer to the goal of a nationwide maternal and child health surveillance system and provides the opportunity for more states to monitor progress toward achieving HP 2010 objectives. References
Pregnancy Risk Assessment Monitoring System (PRAMS) Working Group Alabama, Albert Woolbright, PhD; Alaska, Kathy Perham-Hester, MS, MPH; Arkansas, Gina Redford, MAP; Colorado, Alyson Shupe, PhD; Florida, Helen Marshall; Georgia, Carol Hoban, MS, MPH; Hawaii, Limin Song, MPH; Illinois, Theresa Sandidge, MA; Louisiana, Joan Wightkin; Maine, Kim Haggan; Maryland, Diana Cheng, MD; Michigan, Yasmina Bouraoui, MPH; Minnesota, Jan Jernell; Mississippi, Linda Pendleton; Montana, JoAnn Dotson; Nebraska, Jennifer Severe-Oforah; New Jersey, Lakota Kruse, MD; New Mexico, Ssu Weng, MD; New York, Anne Radigan-Garcia; New York City, Candace Mulready, MPH; North Carolina, Paul Buescher, PhD; North Dakota, Sandra Anseth; Ohio, Amy Davis; Oklahoma, Dick Lorenz; Oregon, Ken Rosenberg, MD; Rhode Island, Sam Viner-Brown; South Carolina, Jim Ferguson, DrPH; Texas, Tanya J. Guthrie, PhD; Utah, Laurie Baksh; Vermont, Peggy Brozicevic; Washington, Linda Lohdefinck; West Virginia, Melissa Baker, MA; PRAMS Team, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Table 1 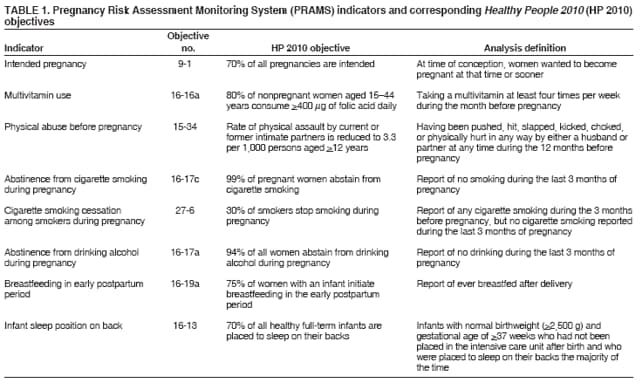 Return to top. Figure 1  Return to top. Table 2 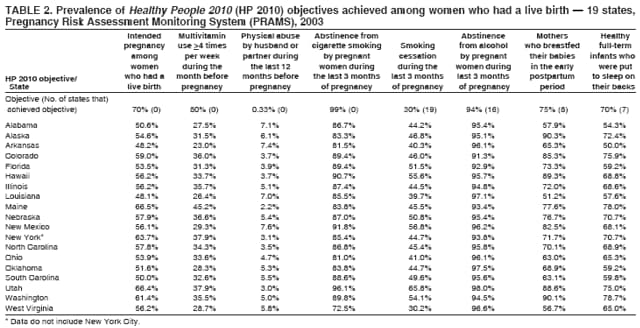 Return to top. Figure 2 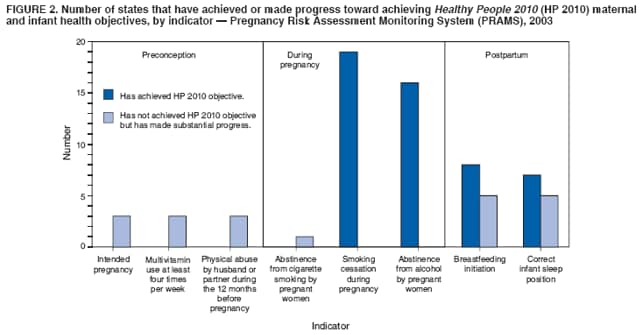 Return to top. Table 3 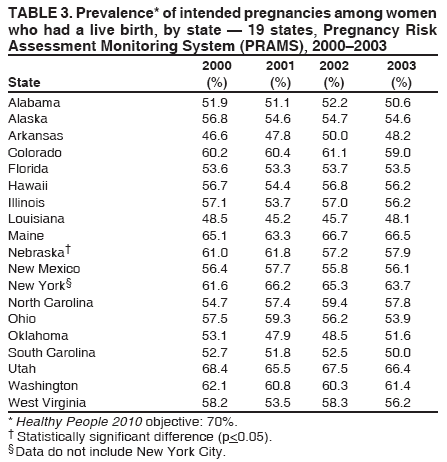 Return to top. Table 4 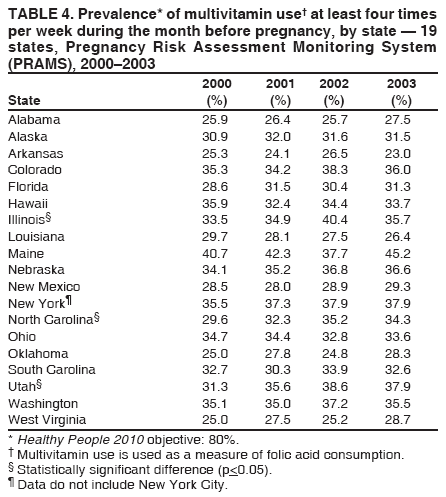 Return to top. Table 5 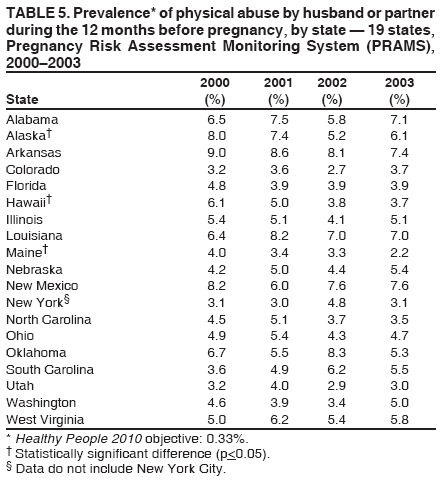 Return to top. Table 6 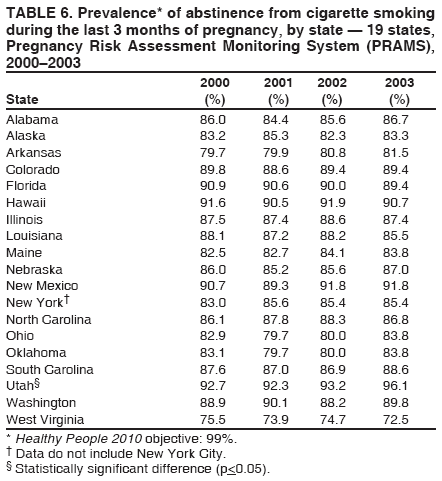 Return to top. Table 7 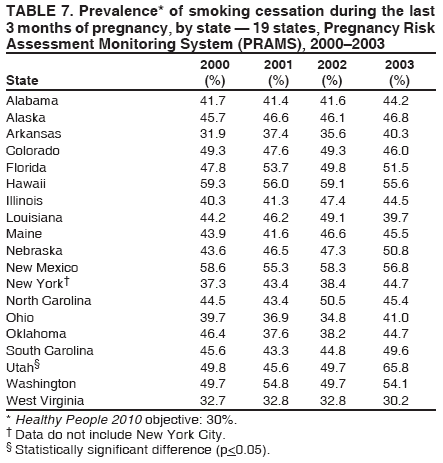 Return to top. Table 8 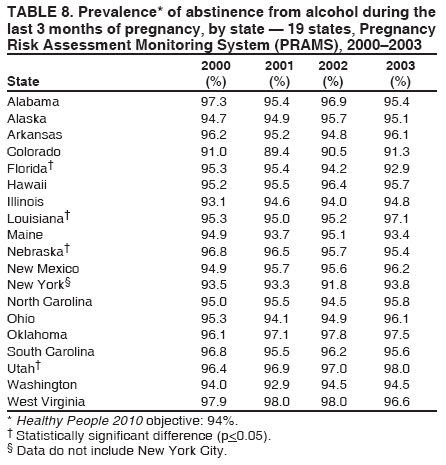 Return to top. Table 9 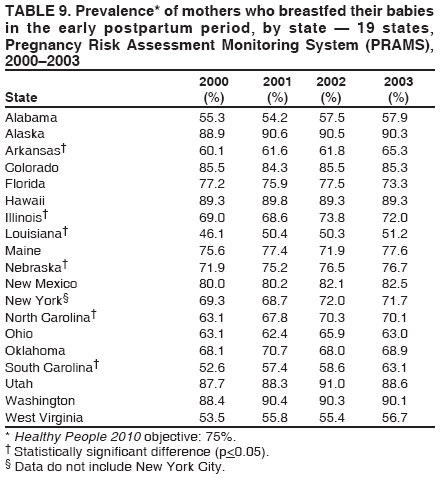 Return to top. Table 10 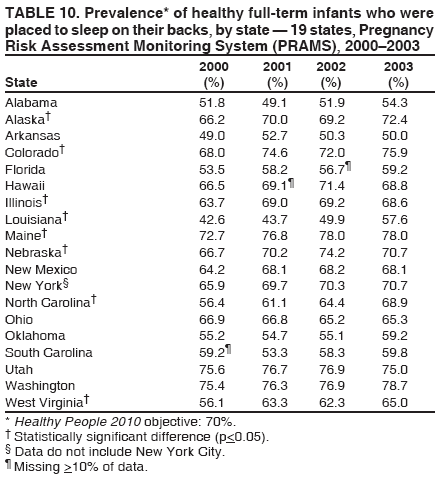 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/21/2006 |
|||||||||
|