 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Locally Acquired Mosquito-Transmitted Malaria: A Guide for Investigations in the United StatesPlease note: An erratum has been published for this article. To view the erratum, please click here. Prepared by
The material in this report originated in the National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed), Lonnie J. King, DVM, Acting Director; and the Division of Parasitic Diseases, Mark Eberhard, PhD, Director. Corresponding preparer: M. James Eliades, MD, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed), 4770 Buford Hwy., MS F-22, Atlanta, GA 30341. Telephone: 770-488-7793; Fax: 770-488-4206; E-mail: bvz9@cdc.gov. SummaryRecent outbreaks of locally acquired mosquito-transmitted malaria in the United States demonstrate the continued risk for reintroduction of the disease. Since 1957, when CDC's Malaria Branch started conducting malaria surveillance, 63 outbreaks have occurred, constituting 156 cases (annual range: 1--32) that were a result of locally acquired mosquitoborne transmission. This report describes the steps that should be taken to 1) investigate a case that might have been acquired locally, 2) prevent a small focus of malaria cases from becoming a source of sustained transmission, and 3) inform clinicians regarding the process of an investigation so they can effectively address concerns and questions from patients. Although these locally acquired mosquito-transmitted outbreaks frequently involve only a limited number of infected persons, they frequently raise concerns in the community and require substantial public health resources. For example, as a result of the most recent local outbreak of eight malaria cases in Florida in 2003, reverse 911 telephone calls (a community notification system) were made to approximately 300,000 residents; insect repellent, postcards, flyers, and posters in multiple languages were distributed; public announcements were made through the media and to schools and homeless shelters; and notifications were sent to local hospitals and physicians to inform residents of that community. When a local health department investigates a potential locally acquired mosquito-transmitted case, the systematic inquiry should include epidemiologic, environmental, and laboratory components. Local and state health departments inquiring about the proper approach to investigate and control a potential locally acquired case frequently request urgent assistance and tools from CDC. This report provides a starting point for such investigations to local and state health departments by providing them with the tools necessary to initiate an investigation. IntroductionMalaria is a major global public health problem. An estimated 300--500 million cases and approximately 1 million deaths occur annually (1). Malaria in humans is caused by four distinct protozoan species of the genus Plasmodium (P. falciparum, P. vivax, P. ovale, and P. malariae). All species are transmitted by the bite of an infective female Anopheles mosquito. Although malaria can be a fatal disease, severe illness and death are largely preventable when a prompt diagnosis is made and proper treatment is administered. During the 16th and 17th centuries, European colonists and African slaves introduced malaria into the continental United States. The disease spread, following patterns of settlement, where conducive environments for competent mosquito vectors existed. Malaria remained endemic throughout large parts of the United States until it was eradicated during the 1950s. The eradication of malaria has been attributed to increased urbanization and improved socioeconomic conditions, which resulted in decreased human-vector contact, increased access to medical care and effective treatment, and reduced Anopheles populations (2). In the United States, approximately 1,000--1,500 cases of malaria are reported annually to CDC (3). Nearly all of the cases diagnosed in the United States are imported from regions of the world where malaria is endemic. However, a limited number of cases also are acquired through local mosquitoborne transmission. From 1957, when the Malaria Branch started conducting malaria surveillance, to 2003, a total of 63 domestic outbreaks have occurred, constituting 156 cases (annual range: 1--32) that resulted from locally acquired mosquitoborne transmission (Figure 1) (4--11). Of the 63 outbreaks, the highest number of cases occurred in California (17 [27%]) (Figure 2). Outbreaks also have occurred in 23 states. Since approximately 1991, a trend has developed in which outbreaks have occurred in more populated areas (e.g., urban and suburban areas). P. vivax has been the predominant species involved (47 [74.6%] of 63), followed by P. falciparum (seven [11.1%] of 47), and P. malariae (five [10.6%] of 47) (Figure 3). The predominance of P. vivax is attributable to the ability of this species to develop relapsing infection weeks or months after the initial infection and to a high proportion of immigrants in the United States coming from areas where P. vivax is the predominant species transmitted. Recent outbreaks of locally acquired malaria demonstrate the continuing potential for reintroduction of malaria into the United States (Table 1). This report describes the steps that should be taken to 1) investigate a case that has been potentially acquired locally, 2) prevent a small focus of malaria cases from becoming a source of sustained transmission, and 3) inform clinicians regarding the process of an investigation so they can effectively address concerns and questions from patients (Box). SurveillanceThe National Malaria Surveillance System (NMSS) is CDC's oldest surveillance system. Before 1950, surveillance was conducted to evaluate the progress toward eradication of malaria. After eradication was achieved, surveillance activities continued to monitor trends in imported cases of malaria that helped guide prevention recommendations. The tracking of cases also identified possible outbreaks of local mosquitoborne transmission and assisted public health officials and clinicians in understanding other cases acquired in the United States (e.g., cases acquired through blood transfusions). Health-care providers or laboratories identify cases of blood-smear--confirmed malaria among civilians and military personnel. Each case is reported on a uniform case form that is sent to the local or state health department and CDC. Clinical, laboratory, and epidemiologic information is included on the form. CDC staff review all reports and request additional information, if necessary (e.g., when no recent travel to a country where malaria is transmitted is mentioned). Reports of other cases are made by telephone directly by health-care providers to CDC, usually when assistance with diagnosis or treatment is requested. Information concerning these cases are subsequently reported by CDC and sent to the states. Malaria case data are reported to NMSS and the National Notifiable Diseases Surveillance System (NNDSS). Both systems rely on passive reporting, but the numbers of reported cases might differ because of differences in collection and transmission of data. An additional difference in these two systems is that NMSS receives more detailed data regarding each case. InvestigationCase ConfirmationWhen a case of malaria is suspected to have been locally acquired, the initial step is to confirm the diagnosis. Individual hospitals might not have extensive experience in microscopic parasitic diagnoses, but the majority of state laboratories can assist in confirming diagnoses. For states that do not maintain such expertise or that would like further assistance, CDC can provide microscopy evaluation either by reviewing the slides or (in a near-real-time manner) through telediagnosis (submission of digital images through the Internet at http://www.dpd.cdc.gov/dpdx). The diagnosis should be confirmed in a timely manner to ensure that proper treatment is administered and that appropriate measures are initiated to prevent continued transmission. CDC also offers a confirmatory polymerase chain reaction (PCR) test, which is based on the amplification of a portion of the small subunit rRNA gene (ssrRNA) (12). PCR can provide a definitive parasite and species diagnosis when these diagnoses cannot be determined upon microscopic examination (e.g., cases in which the morphology of the parasites is atypical). Preliminary Patient InterviewThe investigation should begin with a careful interview with the patient, and risk factors for acquiring malaria should be explored (i.e., recent travel to an area where malaria is endemic, a history of previous malaria infection, recent blood transfusion or organ transplant, or contaminated needle use). If no link can be made to these risk factors, the index of suspicion for a locally acquired mosquito-transmitted illness increases. Even if one of the previously mentioned risk factors for malaria is present, locally acquired mosquitoborne transmission is not ruled out. The risk must be examined in the context of the species and the timing of the infection. For example, a P. falciparum infection will rarely present >2--3 years after primary exposure. Therefore, a case of P. falciparum identified with a remote travel history (>3 years) cannot easily be classified as an imported case (13). In contrast, an absence of risk factors does not always imply local acquisition. P. malariae has been reported to cause symptoms long after exposure, even up to 40 years later (14). Health departments can contact CDC's Malaria Hotline (770-488-7788) for assistance with species-specific information and to help determine whether local transmission should be considered. Initiation of InvestigationAfter a case has been confirmed by blood smear and the history suggests no alternative explanation, the full investigation of a locally acquired mosquito-transmitted case should be conducted. The investigation should include an epidemiologic, environmental, and laboratory component. During this phase of the investigation, the local health department should usually involve the state health department. This collaboration can assist in the allocation of resources and prepare the state in the event of further transmission across counties. The state health department can then request the assistance of CDC, which can provide malaria expertise and prepare for the unlikely event that the outbreak involves several states and requires a multistate coordinated effort. Epidemiologic InvestigationThe epidemiologic investigation has several components. The first step, ensuring that the case definition has been met, is critical. A malaria case is confirmed by demonstrating malaria parasites in a blood film. Suspect cases include patients with fevers of unclear etiology. In-Depth InterviewsCases identified during an outbreak require in-depth interviews to identify risk factors for malaria acquisition. If locally acquired mosquitoborne transmission is a possibility, the case-patients should be questioned about outdoor activities that might have placed them in contact with Anopheles vectors, which typically bite between dusk and dawn. An assessment of local travel, including to surrounding counties, might help identify possible sites of acquisition. Case ReportingA primary goal of an outbreak investigation is to promptly identify all cases of malaria in the local community, both retrospectively and prospectively. The health department should confirm that health-care providers or laboratories have reported all cases of malaria that have been diagnosed. Any cases identified should be carefully reviewed to assess their potential connection to the outbreak. Active Case FindingOne characteristic of malaria is that it causes an acute febrile illness with nonspecific symptoms. Because no distinctive clinical features are associated with malaria, the condition of patients who seek medical care through the health-care system might not be appropriately diagnosed, especially if history of travel to an area where malaria is endemic has not occurred. During an outbreak, the local health department can help identify patients whose conditions are undiagnosed by taking several steps. First, all health-care sites need to be identified, including hospitals, emergency departments, urgent-care clinics, and physicians' offices. Second, each of these points-of-care should collect all charts of patients with febrile illnesses for which a clear alternative explanation for their symptoms cannot be determined. The International Classification of Diseases (ICD) Ninth and Tenth Revision codes can be used to assist in identifying these charts, by targeting charts with certain diagnoses (e.g., fever, nonspecified; fever, unknown origin or viral syndrome; and fever, unspecified). A reasonable time frame to look retrospectively for undiagnosed cases is 8 weeks before the first recognized case. This time frame represents a biologically plausible time interval for one to two generations of local transmission, given the parasite and vector life cycles. Infection-control practitioners at the selected health facility can frequently help coordinate this process. After the chart review, the investigation team should communicate with health-care workers regarding each potential case to request that they contact their respective patients and assess whether the patients are still symptomatic. This contact should be done, if possible, through the health-care workers to protect the integrity of the patient-physician relationship. Patients who have been identified and who have persistent symptoms should be reexamined, and samples should be drawn for malaria evaluation (thick and thin blood films for diagnosis and whole blood for further testing, if required). Community SensitizationFinally, an additional component of enhanced case detection is to alert the medical community and the nonmedical population to the possibility that local transmission of malaria might be occurring. This community sensitization increases the likelihood of the identification of active or future cases that have not yet entered into the health-care system. Clinicians in the community need to be on alert to consider malaria in the differential diagnosis of patients who have a fever. Visits to select clinicians' offices might be helpful if a substantial likelihood exists that they might treat infected patients. The alert to health-care providers can be combined with a request for the reporting of confirmed cases and of cases in which persons with a nonspecific febrile illness might seek medical care. Alerting the nonmedical population to the possibility of being infected with malaria will encourage patients with symptoms consistent with malaria to seek care and educate others on how to avoid infection. The risk for infection will differ depending on characteristics of the outbreak (e.g., the number of persons infected and climatic changes). This alert needs to be tailored to the community in question. Local health departments have used reverse 911 calling (a community notification system) to reach persons who have a telephone (8) or have conducted door-to-door canvassing. Settings in the community where large numbers of persons congregate (e.g., church) should be targeted to communicate risk messages. Various media outlets can be considered for communicating messages regarding the possible risk for malaria, including television, radio, and newspapers. Every effort should be made to convey the information in the languages of the local community and to consider the educational level of the population. CDC has designed a malaria outbreak response tool kit that can help communicate the messages. The tool kit is available on CDC's website (http://www.cdc.gov/malaria/references.htm) (Table 2). Environmental InvestigationAn environmental investigation includes 1) a site visit to the home of each patient, 2) an entomologic assessment, 3) a review of meteorologic data, and 4) an investigation of any nearby airports or seaports that might be possible sources of infective persons or vectors. Site VisitA site visit to patients' homes to conduct a general review of the household, including a search for anopheline larval habitats, can help determine the probability of whether these persons had contact with a competent mosquito vector. This information can corroborate a history of time spent outdoors and demonstrate the possibility for vectors being in the home (e.g., a lack of screens and air conditioning in the home). EntomologyAn entomologic assessment during a locally acquired mosquito-transmitted outbreak serves multiple potential goals. Mosquito-trapping can identify the presence of competent vectors that would make local transmission possible. Data on mosquito densities and breeding locations can guide control of adult and larval mosquitoes and activities to reduce numbers of larval habitats. Local and state health departments might already have surveillance data on vectors in the area that is being assessed. Trapping with the intent of identifying malaria-infected mosquitoes has limited use. If infected mosquitoes are present, they are likely to be in a small focus, which would be difficult to find within the large uninfected background pool. Molecular and immunologic-based methods are available for identification of infected mosquitoes, and efforts are underway to address the utility of confirmation of positive mosquito pools using PCR-based methods (15--17). County mosquito abatement personnel can coordinate surveillance- and vector-control efforts. If these resources are not available, the following actions can be taken to seek needed resources: 1) entomologists at local universities can be contacted for assistance, 2) a private company can be contracted to conduct mosquito surveillance and control, or 3) the state department of health can request entomologic consultation from CDC. Climate and WeatherMeteorologic data are frequently available for the county where the outbreak has occurred. Temperature and rainfall levels throughout the year can be used to predict the viability and abundance of the competent vector. Analysis of weather data also can indicate whether climatic conditions are favorable for the maturation of Plasmodium species within the anopheline mosquitoes. This analysis might then be used to guide the decision to scale-back control activities, if the temperature is outside of a given range. For example, for P. vivax, the temperature window for sporogeny to occur during the life span of the anopheline mosquito is 60ºF--91ºF (16ºC--33ºC) (4). A local health department that wants to prioritize the use of resources might decide against the use of control measures requiring substantial logistic capacity (e.g., spraying of insecticide or reduction of breeding habitats) if the temperature has moved outside of this range. Airport MalariaA remote possibility of a source for a malaria outbreak is that an infective mosquito was transported on an aircraft (18) or in baggage (19) that arrived from an area where malaria is endemic. The distance from the airport to the patient's home should be ascertained and must be within a reasonable flight path distance of the competent vector to consider this mode of transmission as a possibility (i.e., approximately 1 mile), although this distance can vary substantially, depending on terrain, wind, and weather patterns. CDC has not documented any cases attributed to airport malaria in the United States. Laboratory InvestigationLaboratory tools can assist and complement the epidemiologic and environmental investigations. Techniques used in these investigations include microscopy of blood films, PCR rRNA gene analysis, molecular analysis of parasite DNA, and detection of antibodies produced against parasites in the patient's sera. Slide microscopy is an essential part of the case definition. If species identification is initially uncertain, a patient should receive treatment for P. falciparum while definitive speciation occurs. In addition to blood for microscopy, whole blood (preferably pretreatment) should be stored in case additional tests are required. After the species has been identified by microscopy or by PCR, additional molecular techniques (e.g., DNA sequencing) can elucidate the parasite genotype or strain. These data can be used to track and link the cases in an outbreak. The usefulness of this process has been demonstrated in the latest mosquito-transmitted locally acquired malaria outbreak in the United States, which occurred in Palm Beach County, Florida, in 2003 (8). All eight cases were linked by genetic analysis and were the same strain of P. vivax. Molecular analysis might also assist in determining the geographic origin of the parasite. However, the reliability of this approach depends on the genotypic database that is accumulated from strains obtained from specific geographic regions. This new technique is being developed further at CDC and will continue to provide a useful adjunct to these investigations. In the Palm Beach County outbreak, all eight P. vivax parasites matched genetic patterns consistent with a New World origin (the Americas), implicating this region as the original source of the parasite responsible for this outbreak. DiscussionPersons who travel to countries where malaria is endemic and return with the infection account for the majority of malaria cases diagnosed in the United States. However, small outbreaks of locally acquired mosquito-transmitted malaria continue to occur. This ongoing reintroduction occurs for multiple reasons. A steady increase in international travel and migration of persons from areas where malaria is endemic has occurred, which provides a parasitic reservoir for local transmission (4). This increase is coupled with the continued presence of competent vectors and conducive weather patterns for transmission. Whereas this introduction is probably unavoidable, ensuring that it does not lead to reestablishment of malaria is a challenge for the public health system. To properly address this challenge, this report has discussed epidemiologic, laboratory, and environmental approaches to investigate possible locally acquired mosquito-transmitted cases. A key element is the rapid reporting of cases through NMSS to allow for timely detection of locally acquired cases and implementation of control measures. For clinicians, malaria should be considered in the differential diagnosis of patients with unexplained persistent fevers. During malaria outbreaks, the primary goal is to identify all cases of malaria to provide effective therapy and to ensure that infected persons do not serve as reservoirs for establishing a transmission cycle. The team investigating the outbreak should ensure that the usual mechanisms for reporting are functioning properly and functioning in a timely manner, which will help ensure that all cases are detected. The team also should conduct active case finding and community sensitization. In the setting of a locally acquired mosquitoborne outbreak, the topic of mass screening for infective persons is frequently raised. Two techniques, blood films and serologic antibody tests, have been proposed to detect either the index case or other infective persons. A blood smear is the best tool to identify persons who have malaria parasitemia. However, in an outbreak setting, the evaluation of large numbers of asymptomatic persons in the community is typically low yield, expensive, and labor intensive. Community sensitization will alert the community to the risk for malaria and the symptoms of infection, helping to ensure that persons who have malaria parasitemia will seek medical attention when they have symptoms. Mass screening conducted by using serologic testing also is not routinely recommended. Antibody titers can remain positive for >1 year after infection. In one study, 41% of persons infected with P. vivax remained immunofluorescent antibody positive 12 months after treatment (20). Therefore, a positive serologic test signifies previous infection but does distinguish between active and remote infection. Locally acquired malaria outbreaks frequently occur in areas with large numbers of travelers and immigrants who have entered the United States from areas where malaria is endemic. A positive test in these persons would not differentiate who is infective from who was infected before entering the United States. Therefore, all positive serologic tests would require follow-up blood smears. As previously mentioned, this technique would be low yield, expensive, and labor intensive. The entomologic assessment and the appropriate level of mosquito-control activity need to be closely examined. Trapping can be used to identify the presence of competent vectors, but these data frequently are already available. Determining how a local team should adjust mosquito-control efforts in the setting of a local outbreak is difficult. An increase in spraying for mosquitoes and other control measures are frequently the immediate response to reduce adult mosquito populations because these vector-control activities are highly visible to the public. Whether additional activities above baseline-control efforts (e.g., the use of larvacides or reduction of vector breeding sites) have any value is unclear. The local health department will need to decide whether to conduct augmented control activities and should consider prioritization of resources in the decision-making process. The local health department also will have to decide when to make a statement that the outbreak is over. One approach has been to wait two complete parasite life cycles, which takes approximately 8 weeks. If this period has elapsed without evidence of further transmission, the likelihood is that no other cases will be identified. In certain settings, weather patterns can help facilitate the end point. The weather might be too cold to support the development of the parasite in the mosquito during the vector's life span. Malaria is not transmitted from female mosquitoes to their offspring, and the probability of an infective adult mosquito surviving the winter is low. Preventing the reestablishment of malaria transmission begins by preventing reintroduction. Barriers to certain groups getting prompt care, especially immigrant and migrant populations, decreases the likelihood that a malaria case will be diagnosed promptly and effectively treated. Persons can prevent mosquitoborne illnesses, in general, by using individual protective measures, including 1) using DEET (N,N-diethyl-m-toluamide)-based (i.e., DEET concentration <50%) insect repellents when outdoors; 2) wearing long-sleeved shirts and long trousers when outdoors, if possible; and 3) avoiding outdoor activity during early morning and evening hours because the peak biting time for many species of mosquitoes is from dusk to dawn. ConclusionTo prevent a locally acquired mosquito-transmitted malaria outbreak from becoming a source of sustained transmission, clinicians and public health officials at the local and state levels need to be aware of the steps involved in an investigation and how to implement appropriate control measures. A functional surveillance system is critical so that clinicians can rapidly report cases, and the information reported can be quickly acted upon. Case confirmation and assessment of risk factors are the first necessary steps. If a determination is made that local transmission is occurring, a thorough investigation, including epidemiologic, environmental, and laboratory components, should be conducted. References
Table 1 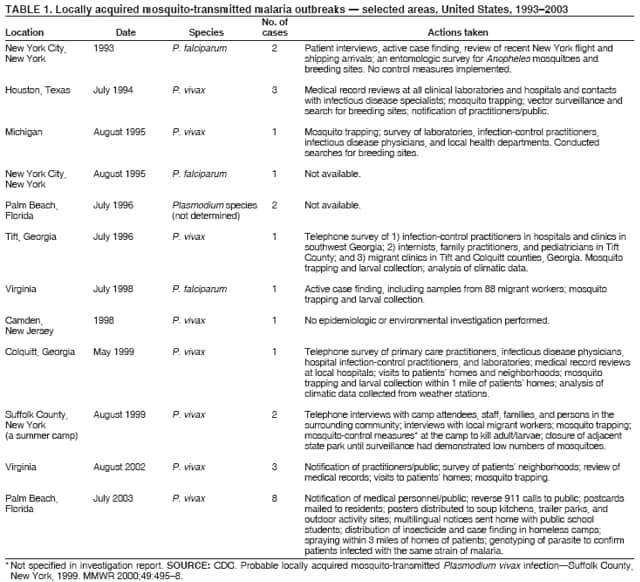 Return to top. Figure 1 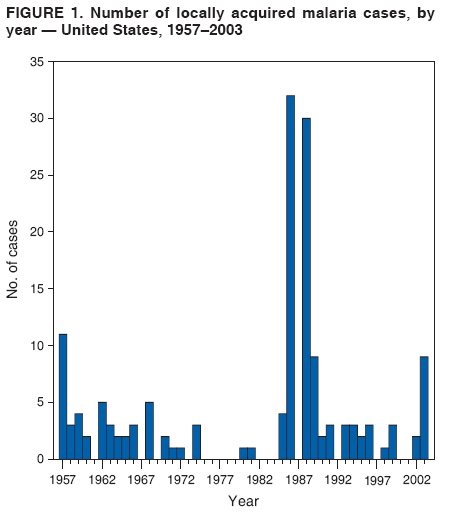 Return to top. Table 2 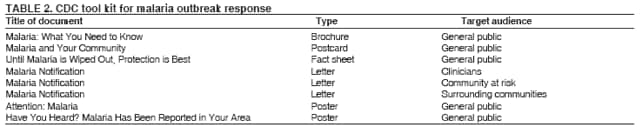 Return to top. Figure 2 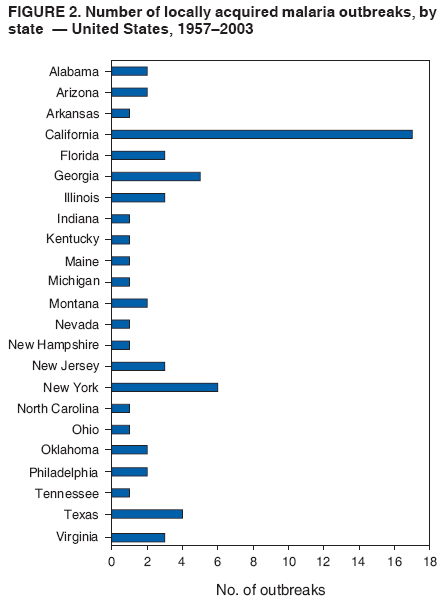 Return to top. Figure 3 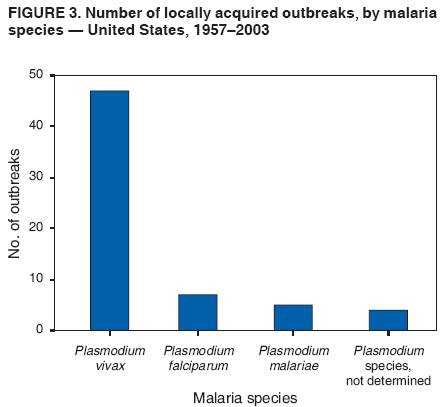 Return to top. Box 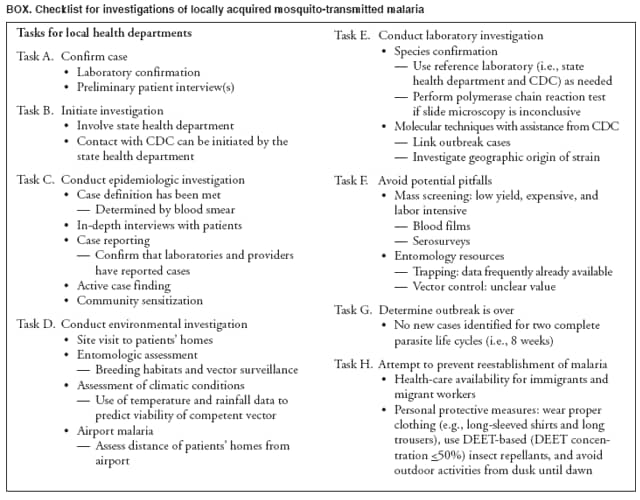 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/30/2006 |
|||||||||
|