 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for Infection Control in Dental Health-Care Settings --- 2003Prepared by The material in this report originated in the National Center for Chronic Disease Prevention and Health Promotion, James S. Marks, M.D., M.P.H., Director; and the Division of Oral Health, William R. Maas, D.D.S., M.P.H., Director. SummaryThis report consolidates previous recommendations and adds new ones for infection control in dental settings. Recommendations are provided regarding 1) educating and protecting dental health-care personnel; 2) preventing transmission of bloodborne pathogens; 3) hand hygiene; 4) personal protective equipment; 5) contact dermatitis and latex hypersensitivity; 6) sterilization and disinfection of patient-care items; 7) environmental infection control; 8) dental unit waterlines, biofilm, and water quality; and 9) special considerations (e.g., dental handpieces and other devices, radiology, parenteral medications, oral surgical procedures, and dental laboratories). These recommendations were developed in collaboration with and after review by authorities on infection control from CDC and other public agencies, academia, and private and professional organizations. IntroductionThis report consolidates recommendations for preventing and controlling infectious diseases and managing personnel health and safety concerns related to infection control in dental settings. This report 1) updates and revises previous CDC recommendations regarding infection control in dental settings (1,2); 2) incorporates relevant infection-control measures from other CDC guidelines; and 3) discusses concerns not addressed in previous recommendations for dentistry. These updates and additional topics include the following:
These guidelines were developed by CDC staff members in collaboration with other authorities on infection control. Draft documents were reviewed by other federal agencies and professional organizations from the fields of dental health care, public health, and hospital epidemiology and infection control. A Federal Register notice elicited public comments that were considered in the decision-making process. Existing guidelines and published research pertinent to dental infection-control principles and practices were reviewed. Wherever possible, recommendations are based on data from well-designed scientific studies. However, only a limited number of studies have characterized risk factors and the effectiveness of prevention measures for infections associated with dental health-care practices. Some infection-control practices routinely used by health-care practitioners cannot be rigorously examined for ethical or logistical reasons. In the absence of scientific evidence for such practices, certain recommendations are based on strong theoretical rationale, suggestive evidence, or opinions of respected authorities based on clinical experience, descriptive studies, or committee reports. In addition, some recommendations are derived from federal regulations. No recommendations are offered for practices for which insufficient scientific evidence or lack of consensus supporting their effectiveness exists. BackgroundIn the United States, an estimated 9 million persons work in health-care professions, including approximately 168,000 dentists, 112,000 registered dental hygienists, 218,000 dental assistants (3), and 53,000 dental laboratory technicians (4). In this report, dental health-care personnel (DHCP) refers to all paid and unpaid personnel in the dental health-care setting who might be occupationally exposed to infectious materials, including body substances and contaminated supplies, equipment, environmental surfaces, water, or air. DHCP include dentists, dental hygienists, dental assistants, dental laboratory technicians (in-office and commercial), students and trainees, contractual personnel, and other persons not directly involved in patient care but potentially exposed to infectious agents (e.g., administrative, clerical, housekeeping, maintenance, or volunteer personnel). Recommendations in this report are designed to prevent or reduce potential for disease transmission from patient to DHCP, from DHCP to patient, and from patient to patient. Although these guidelines focus mainly on outpatient, ambulatory dental health-care settings, the recommended infection-control practices are applicable to all settings in which dental treatment is provided. Dental patients and DHCP can be exposed to pathogenic microorganisms including cytomegalovirus (CMV), HBV, HCV, herpes simplex virus types 1 and 2, HIV, Mycobacterium tuberculosis, staphylococci, streptococci, and other viruses and bacteria that colonize or infect the oral cavity and respiratory tract. These organisms can be transmitted in dental settings through 1) direct contact with blood, oral fluids, or other patient materials; 2) indirect contact with contaminated objects (e.g., instruments, equipment, or environmental surfaces); 3) contact of conjunctival, nasal, or oral mucosa with droplets (e.g., spatter) containing microorganisms generated from an infected person and propelled a short distance (e.g., by coughing, sneezing, or talking); and 4) inhalation of airborne microorganisms that can remain suspended in the air for long periods (5). Infection through any of these routes requires that all of the following conditions be present:
Occurrence of these events provides the chain of infection (6). Effective infection-control strategies prevent disease transmission by interrupting one or more links in the chain. Previous CDC recommendations regarding infection control for dentistry focused primarily on the risk of transmission of bloodborne pathogens among DHCP and patients and use of universal precautions to reduce that risk (1,2,7,8). Universal precautions were based on the concept that all blood and body fluids that might be contaminated with blood should be treated as infectious because patients with bloodborne infections can be asymptomatic or unaware they are infected (9,10). Preventive practices used to reduce blood exposures, particularly percutaneous exposures, include 1) careful handling of sharp instruments, 2) use of rubber dams to minimize blood spattering; 3) handwashing; and 4) use of protective barriers (e.g., gloves, masks, protective eyewear, and gowns). The relevance of universal precautions to other aspects of disease transmission was recognized, and in 1996, CDC expanded the concept and changed the term to standard precautions. Standard precautions integrate and expand the elements of universal precautions into a standard of care designed to protect HCP and patients from pathogens that can be spread by blood or any other body fluid, excretion, or secretion (11). Standard precautions apply to contact with 1) blood; 2) all body fluids, secretions, and excretions (except sweat), regardless of whether they contain blood; 3) nonintact skin; and 4) mucous membranes. Saliva has always been considered a potentially infectious material in dental infection control; thus, no operational difference exists in clinical dental practice between universal precautions and standard precautions. In addition to standard precautions, other measures (e.g., expanded or transmission-based precautions) might be necessary to prevent potential spread of certain diseases (e.g., TB, influenza, and varicella) that are transmitted through airborne, droplet, or contact transmission (e.g., sneezing, coughing, and contact with skin) (11). When acutely ill with these diseases, patients do not usually seek routine dental outpatient care. Nonetheless, a general understanding of precautions for diseases transmitted by all routes is critical because 1) some DHCP are hospital-based or work part-time in hospital settings; 2) patients infected with these diseases might seek urgent treatment at outpatient dental offices; and 3) DHCP might become infected with these diseases. Necessary transmission-based precautions might include patient placement (e.g., isolation), adequate room ventilation, respiratory protection (e.g., N-95 masks) for DHCP, or postponement of nonemergency dental procedures. DHCP should be familiar also with the hierarchy of controls that categorizes and prioritizes prevention strategies (12). For bloodborne pathogens, engineering controls that eliminate or isolate the hazard (e.g., puncture-resistant sharps containers or needle-retraction devices) are the primary strategies for protecting DHCP and patients. Where engineering controls are not available or appropriate, work-practice controls that result in safer behaviors (e.g., one-hand needle recapping or not using fingers for cheek retraction while using sharp instruments or suturing), and use of personal protective equipment (PPE) (e.g., protective eyewear, gloves, and mask) can prevent exposure (13). In addition, administrative controls (e.g., policies, procedures, and enforcement measures targeted at reducing the risk of exposure to infectious persons) are a priority for certain pathogens (e.g., M. tuberculosis), particularly those spread by airborne or droplet routes. Dental practices should develop a written infection-control program to prevent or reduce the risk of disease transmission. Such a program should include establishment and implementation of policies, procedures, and practices (in conjunction with selection and use of technologies and products) to prevent work-related injuries and illnesses among DHCP as well as health-care--associated infections among patients. The program should embody principles of infection control and occupational health, reflect current science, and adhere to relevant federal, state, and local regulations and statutes. An infection-control coordinator (e.g., dentist or other DHCP) knowledgeable or willing to be trained should be assigned responsibility for coordinating the program. The effectiveness of the infection-control program should be evaluated on a day-to-day basis and over time to help ensure that policies, procedures, and practices are useful, efficient, and successful (see Program Evaluation). Although the infection-control coordinator remains responsible for overall management of the program, creating and maintaining a safe work environment ultimately requires the commitment and accountability of all DHCP. This report is designed to provide guidance to DHCP for preventing disease transmission in dental health-care settings, for promoting a safe working environment, and for assisting dental practices in developing and implementing infection-control programs. These programs should be followed in addition to practices and procedures for worker protection required by the Occupational Safety and Health Administration's (OSHA) standards for occupational exposure to bloodborne pathogens (13), including instituting controls to protect employees from exposure to blood or other potentially infectious materials (OPIM), and requiring implementation of a written exposure-control plan, annual employee training, HBV vaccinations, and postexposure follow-up (13). Interpretations and enforcement procedures are available to help DHCP apply this OSHA standard in practice (14). Also, manufacturer's Material Safety Data Sheets (MSDS) should be consulted regarding correct procedures for handling or working with hazardous chemicals (15). Previous RecommendationsThis report includes relevant infection-control measures from the following previously published CDC guidelines and recommendations:
Selected DefinitionsAlcohol-based hand rub: An alcohol-containing preparation designed for reducing the number of viable microorganisms on the hands. Antimicrobial soap: A detergent containing an antiseptic agent. Antiseptic: A germicide used on skin or living tissue for the purpose of inhibiting or destroying microorganisms (e.g., alcohols, chlorhexidine, chlorine, hexachlorophene, iodine, chloroxylenol [PCMX], quaternary ammonium compounds, and triclosan). Bead sterilizer: A device using glass beads 1.2--1.5 mm diameter and temperatures 217ºC--232ºC for brief exposures (e.g., 45 seconds) to inactivate microorganisms. (This term is actually a misnomer because it has not been cleared by the Food and Drug Administration [FDA] as a sterilizer). Bioburden: Microbiological load (i.e., number of viable organisms in or on an object or surface) or organic material on a surface or object before decontamination, or sterilization. Also known as bioload or microbial load. Colony-forming unit (CFU): The minimum number (i.e., tens of millions) of separable cells on the surface of or in semisolid agar medium that give rise to a visible colony of progeny. CFUs can consist of pairs, chains, clusters, or as single cells and are often expressed as colony-forming units per milliliter (CFUs/mL). Decontamination: Use of physical or chemical means to remove, inactivate, or destroy pathogens on a surface or item so that they are no longer capable of transmitting infectious particles and the surface or item is rendered safe for handling, use, or disposal. Dental treatment water: Nonsterile water used during dental treatment, including irrigation of nonsurgical operative sites and cooling of high-speed rotary and ultrasonic instruments. Disinfectant: A chemical agent used on inanimate objects (e.g., floors, walls, or sinks) to destroy virtually all recognized pathogenic microorganisms, but not necessarily all microbial forms (e.g., bacterial endospores). The U.S. Environmental Protection Agency (EPA) groups disinfectants on the basis of whether the product label claims limited, general, or hospital disinfectant capabilities. Disinfection: Destruction of pathogenic and other kinds of microorganisms by physical or chemical means. Disinfection is less lethal than sterilization, because it destroys the majority of recognized pathogenic microorganisms, but not necessarily all microbial forms (e.g., bacterial spores). Disinfection does not ensure the degree of safety associated with sterilization processes. Droplet nuclei: Particles <5 µm in diameter formed by dehydration of airborne droplets containing microorganisms that can remain suspended in the air for long periods of time. Droplets: Small particles of moisture (e.g., spatter) generated when a person coughs or sneezes, or when water is converted to a fine mist by an aerator or shower head. These particles, intermediate in size between drops and droplet nuclei, can contain infectious microorganisms and tend to quickly settle from the air such that risk of disease transmission is usually limited to persons in close proximity to the droplet source. Endotoxin: The lipopolysaccharide of gram-negative bacteria, the toxic character of which resides in the lipid protein. Endotoxins can produce pyrogenic reactions in persons exposed to their bacterial component. Germicide: An agent that destroys microorganisms, especially pathogenic organisms. Terms with the same suffix (e.g., virucide, fungicide, bactericide, tuberculocide, and sporicide) indicate agents that destroy the specific microorganism identified by the prefix. Germicides can be used to inactivate microorganisms in or on living tissue (i.e., antiseptics) or on environmental surfaces (i.e., disinfectants). Hand hygiene: General term that applies to handwashing, antiseptic handwash, antiseptic hand rub, or surgical hand antisepsis. Health-care--associated infection: Any infection associated with a medical or surgical intervention. The term health-care--associated replaces nosocomial, which is limited to adverse infectious outcomes occurring in hospitals. Hepatitis B immune globulin (HBIG): Product used for prophylaxis against HBV infection. HBIG is prepared from plasma containing high titers of hepatitis B surface antibody (anti-HBs) and provides protection for 3--6 mos. Hepatitis B surface antigen (HBsAg): Serologic marker on the surface of HBV detected in high levels during acute or chronic hepatitis. The body normally produces antibodies to surface antigen as a normal immune response to infection. Hepatitis B e antigen (HBeAg): Secreted product of the nucleocapsid gene of HBV found in serum during acute and chronic HBV infection. Its presence indicates that the virus is replicating and serves as a marker of increased infectivity. Hepatitis B surface antibody (anti-HBs): Protective antibody against HBsAg. Presence in the blood can indicate past infection with, and immunity to, HBV, or immune response from hepatitis B vaccine. Heterotrophic bacteria: Those bacteria requiring an organic carbon source for growth (i.e., deriving energy and carbon from organic compounds). High-level disinfection: Disinfection process that inactivates vegetative bacteria, mycobacteria, fungi, and viruses but not necessarily high numbers of bacterial spores. FDA further defines a high-level disinfectant as a sterilant used for a shorter contact time. Hospital disinfectant: Germicide registered by EPA for use on inanimate objects in hospitals, clinics, dental offices, and other medical-related facilities. Efficacy is demonstrated against Salmonella choleraesuis, Staphylococcus aureus, and Pseudomonas aeruginosa. Iatrogenic: Induced inadvertently by HCP, medical (including dental) treatment, or diagnostic procedures. Used particularly in reference to an infectious disease or other complication of treatment. Immunization: Process by which a person becomes immune, or protected against a disease. Vaccination is defined as the process of administering a killed or weakened infectious organism or a toxoid; however, vaccination does not always result in immunity. Implantable device: Device placed into a surgically or naturally formed cavity of the human body and intended to remain there for >30 days. Independent water reservoir: Container used to hold water or other solutions and supply it to handpieces and air and water syringes attached to a dental unit. The independent reservoir, which isolates the unit from the public water system, can be provided as original equipment or as a retrofitted device. Intermediate-level disinfection: Disinfection process that inactivates vegetative bacteria, the majority of fungi, mycobacteria, and the majority of viruses (particularly enveloped viruses) but not bacterial spores. Intermediate-level disinfectant: Liquid chemical germicide registered with EPA as a hospital disinfectant and with a label claim of potency as tuberculocidal (Appendix A). Latex: Milky white fluid extracted from the rubber tree Hevea brasiliensis that contains the rubber material cis-1,4 polyisoprene. Low-level disinfection: Process that inactivates the majority of vegetative bacteria, certain fungi, and certain viruses, but cannot be relied on to inactivate resistant microorganisms (e.g., mycobacteria or bacterial spores). Low-level disinfectant: Liquid chemical germicide registered with EPA as a hospital disinfectant. OSHA requires low-level hospital disinfectants also to have a label claim for potency against HIV and HBV if used for disinfecting clinical contact surfaces (Appendix A). Microfilter: Membrane filter used to trap microorganisms suspended in water. Filters are usually installed on dental unit waterlines as a retrofit device. Microfiltration commonly occurs at a filter pore size of 0.03--10 µm. Sediment filters commonly found in dental unit water regulators have pore sizes of 20--90 µm and do not function as microbiological filters. Nosocomial: Infection acquired in a hospital as a result of medical care. Occupational exposure: Reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or OPIM that can result from the performance of an employee's duties. OPIM: Other potentially infectious materials. OPIM is an OSHA term that refers to 1) body fluids including semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures; any body fluid visibly contaminated with blood; and all body fluids in situations where differentiating between body fluids is difficult or impossible; 2) any unfixed tissue or organ (other than intact skin) from a human (living or dead); and 3) HIV-containing cell or tissue cultures, organ cultures; HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV. Parenteral: Means of piercing mucous membranes or skin barrier through such events as needlesticks, human bites, cuts, and abrasions. Persistent activity: Prolonged or extended activity that prevents or inhibits proliferation or survival of microorganisms after application of a product. This activity can be demonstrated by sampling a site minutes or hours after application and demonstrating bacterial antimicrobial effectiveness when compared with a baseline level. Previously, this property was sometimes termed residual activity. Prion: Protein particle lacking nucleic acid that has been implicated as the cause of certain neurodegenerative diseases (e.g., scrapie, CJD, and bovine spongiform encephalopathy [BSE]). Retraction: Entry of oral fluids and microorganisms into waterlines through negative water pressure. Seroconversion: The change of a serological test from negative to positive indicating the development of antibodies in response to infection or immunization. Sterile: Free from all living microorganisms; usually described as a probability (e.g., the probability of a surviving microorganism being 1 in 1 million). Sterilization: Use of a physical or chemical procedure to destroy all microorganisms including substantial numbers of resistant bacterial spores. Surfactants: Surface-active agents that reduce surface tension and help cleaning by loosening, emulsifying, and holding soil in suspension, to be more readily rinsed away. Ultrasonic cleaner: Device that removes debris by a process called cavitation, in which waves of acoustic energy are propagated in aqueous solutions to disrupt the bonds that hold particulate matter to surfaces. Vaccination: See immunization. Vaccine: Product that induces immunity, therefore protecting the body from the disease. Vaccines are administered through needle injections, by mouth, and by aerosol. Washer-disinfector: Automatic unit that cleans and thermally disinfects instruments, by using a high-temperature cycle rather than a chemical bath. Wicking: Absorption of a liquid by capillary action along a thread or through the material (e.g., penetration of liquids through undetected holes in a glove). Review of Science Related to Dental Infection ControlPersonnel Health Elements of an Infection-Control ProgramA protective health component for DHCP is an integral part of a dental practice infection-control program. The objectives are to educate DHCP regarding the principles of infection control, identify work-related infection risks, institute preventive measures, and ensure prompt exposure management and medical follow-up. Coordination between the dental practice's infection-control coordinator and other qualified health-care professionals is necessary to provide DHCP with appropriate services. Dental programs in institutional settings, (e.g., hospitals, health centers, and educational institutions) can coordinate with departments that provide personnel health services. However, the majority of dental practices are in ambulatory, private settings that do not have licensed medical staff and facilities to provide complete on-site health service programs. In such settings, the infection-control coordinator should establish programs that arrange for site-specific infection-control services from external health-care facilities and providers before DHCP are placed at risk for exposure. Referral arrangements can be made with qualified health-care professionals in an occupational health program of a hospital, with educational institutions, or with health-care facilities that offer personnel health services. Education and Training Personnel are more likely to comply with an infection-control program and exposure-control plan if they understand its rationale (5,13,16). Clearly written policies, procedures, and guidelines can help ensure consistency, efficiency, and effective coordination of activities. Personnel subject to occupational exposure should receive infection-control training on initial assignment, when new tasks or procedures affect their occupational exposure, and at a minimum, annually (13). Education and training should be appropriate to the assigned duties of specific DHCP (e.g., techniques to prevent cross-contamination or instrument sterilization). For DHCP who perform tasks or procedures likely to result in occupational exposure to infectious agents, training should include 1) a description of their exposure risks; 2) review of prevention strategies and infection-control policies and procedures; 3) discussion regarding how to manage work-related illness and injuries, including PEP; and 4) review of work restrictions for the exposure or infection. Inclusion of DHCP with minimal exposure risks (e.g., administrative employees) in education and training programs might enhance facilitywide understanding of infection-control principles and the importance of the program. Educational materials should be appropriate in content and vocabulary for each person's educational level, literacy, and language, as well as be consistent with existing federal, state, and local regulations (5,13). Immunization Programs DHCP are at risk for exposure to, and possible infection with, infectious organisms. Immunizations substantially reduce both the number of DHCP susceptible to these diseases and the potential for disease transmission to other DHCP and patients (5,17). Thus, immunizations are an essential part of prevention and infection-control programs for DHCP, and a comprehensive immunization policy should be implemented for all dental health-care facilities (17,18). The Advisory Committee on Immunization Practices (ACIP) provides national guidelines for immunization of HCP, which includes DHCP (17). Dental practice immunization policies should incorporate current state and federal regulations as well as recommendations from the U.S. Public Health Service and professional organizations (17) (Appendix B). On the basis of documented health-care--associated transmission, HCP are considered to be at substantial risk for acquiring or transmitting hepatitis B, influenza, measles, mumps, rubella, and varicella. All of these diseases are vaccine-preventable. ACIP recommends that all HCP be vaccinated or have documented immunity to these diseases (5,17). ACIP does not recommend routine immunization of HCP against TB (i.e., inoculation with bacille Calmette-Guérin vaccine) or hepatitis A (17). No vaccine exists for HCV. ACIP guidelines also provide recommendations regarding immunization of HCP with special conditions (e.g., pregnancy, HIV infection, or diabetes) (5,17). Immunization of DHCP before they are placed at risk for exposure remains the most efficient and effective use of vaccines in health-care settings. Some educational institutions and infection-control programs provide immunization schedules for students and DHCP. OSHA requires that employers make hepatitis B vaccination available to all employees who have potential contact with blood or OPIM. Employers are also required to follow CDC recommendations for vaccinations, evaluation, and follow-up procedures (13). Nonpatient-care staff (e.g., administrative or housekeeping) might be included, depending on their potential risk of coming into contact with blood or OPIM. Employers are also required to ensure that employees who decline to accept hepatitis B vaccination sign an appropriate declination statement (13). DHCP unable or unwilling to be vaccinated as required or recommended should be educated regarding their exposure risks, infection-control policies and procedures for the facility, and the management of work-related illness and work restrictions (if appropriate) for exposed or infected DHCP. Exposure Prevention and Postexposure Management Avoiding exposure to blood and OPIM, as well as protection by immunization, remain primary strategies for reducing occupationally acquired infections, but occupational exposures can still occur (19). A combination of standard precautions, engineering, work practice, and administrative controls is the best means to minimize occupational exposures. Written policies and procedures to facilitate prompt reporting, evaluation, counseling, treatment, and medical follow-up of all occupational exposures should be available to all DHCP. Written policies and procedures should be consistent with federal, state, and local requirements addressing education and training, postexposure management, and exposure reporting (see Preventing Transmission of Bloodborne Pathogens). DHCP who have contact with patients can also be exposed to persons with infectious TB, and should have a baseline tuberculin skin test (TST), preferably by using a two-step test, at the beginning of employment (20). Thus, if an unprotected occupational exposure occurs, TST conversions can be distinguished from positive TST results caused by previous exposures (20,21). The facility's level of TB risk will determine the need for routine follow-up TSTs (see Special Considerations). Medical Conditions, Work-Related Illness, and Work Restrictions DHCP are responsible for monitoring their own health status. DHCP who have acute or chronic medical conditions that render them susceptible to opportunistic infection should discuss with their personal physicians or other qualified authority whether the condition might affect their ability to safely perform their duties. However, under certain circumstances, health-care facility managers might need to exclude DHCP from work or patient contact to prevent further transmission of infection (22). Decisions concerning work restrictions are based on the mode of transmission and the period of infectivity of the disease (5) (Table 1). Exclusion policies should 1) be written, 2) include a statement of authority that defines who can exclude DHCP (e.g., personal physicians), and 3) be clearly communicated through education and training. Policies should also encourage DHCP to report illnesses or exposures without jeopardizing wages, benefits, or job status. With increasing concerns regarding bloodborne pathogens and introduction of universal precautions, use of latex gloves among HCP has increased markedly (7,23). Increased use of these gloves has been accompanied by increased reports of allergic reactions to natural rubber latex among HCP, DHCP, and patients (24--30), as well as increased reports of irritant and allergic contact dermatitis from frequent and repeated use of hand-hygiene products, exposure to chemicals, and glove use. DHCP should be familiar with the signs and symptoms of latex sensitivity (5,31--33). A physician should evaluate DHCP exhibiting symptoms of latex allergy, because further exposure could result in a serious allergic reaction. A diagnosis is made through medical history, physical examination, and diagnostic tests. Procedures should be in place for minimizing latex-related health problems among DHCP and patients while protecting them from infectious materials. These procedures should include 1) reducing exposures to latex-containing materials by using appropriate work practices, 2) training and educating DHCP, 3) monitoring symptoms, and 4) substituting nonlatex products where appropriate (32) (see Contact Dermatitis and Latex Hypersensitivity). Maintenance of Records, Data Management, and Confidentiality The health status of DHCP can be monitored by maintaining records of work-related medical evaluations, screening tests, immunizations, exposures, and postexposure management. Such records must be kept in accordance with all applicable state and federal laws. Examples of laws that might apply include the Privacy Rule of the Health Insurance Portability and Accountability Act (HIPAA) of 1996, 45 CFR 160 and 164, and the OSHA Occupational Exposure to Bloodborne Pathogens; Final Rule 29 CFR 1910.1030(h)(1)(i--iv) (34,13). The HIPAA Privacy Rule applies to covered entities, including certain defined health providers, health-care clearinghouses, and health plans. OSHA requires employers to ensure that certain information contained in employee medical records is 1) kept confidential; 2) not disclosed or reported without the employee's express written consent to any person within or outside the workplace except as required by the OSHA standard; and 3) maintained by the employer for at least the duration of employment plus 30 years. Dental practices that coordinate their infection-control program with off-site providers might consult OSHA's Bloodborne Pathogen standard and employee Access to Medical and Exposure Records standard, as well as other applicable local, state, and federal laws, to determine a location for storing health records (13,35). Preventing Transmission of Bloodborne PathogensAlthough transmission of bloodborne pathogens (e.g., HBV, HCV, and HIV) in dental health-care settings can have serious consequences, such transmission is rare. Exposure to infected blood can result in transmission from patient to DHCP, from DHCP to patient, and from one patient to another. The opportunity for transmission is greatest from patient to DHCP, who frequently encounter patient blood and blood-contaminated saliva during dental procedures. Since 1992, no HIV transmission from DHCP to patients has been reported, and the last HBV transmission from DHCP to patients was reported in 1987. HCV transmission from DHCP to patients has not been reported. The majority of DHCP infected with a bloodborne virus do not pose a risk to patients because they do not perform activities meeting the necessary conditions for transmission. For DHCP to pose a risk for bloodborne virus transmission to patients, DHCP must 1) be viremic (i.e., have infectious virus circulating in the bloodstream); 2) be injured or have a condition (e.g., weeping dermatitis) that allows direct exposure to their blood or other infectious body fluids; and 3) enable their blood or infectious body fluid to gain direct access to a patient's wound, traumatized tissue, mucous membranes, or similar portal of entry. Although an infected DHCP might be viremic, unless the second and third conditions are also met, transmission cannot occur. The risk of occupational exposure to bloodborne viruses is largely determined by their prevalence in the patient population and the nature and frequency of contact with blood and body fluids through percutaneous or permucosal routes of exposure. The risk of infection after exposure to a bloodborne virus is influenced by inoculum size, route of exposure, and susceptibility of the exposed HCP (12). The majority of attention has been placed on the bloodborne pathogens HBV, HCV, and HIV, and these pathogens present different levels of risk to DHCP. Hepatitis B Virus HBV is a well-recognized occupational risk for HCP (36,37). HBV is transmitted by percutaneous or mucosal exposure to blood or body fluids of a person with either acute or chronic HBV infection. Persons infected with HBV can transmit the virus for as long as they are HBsAg-positive. The risk of HBV transmission is highly related to the HBeAg status of the source person. In studies of HCP who sustained injuries from needles contaminated with blood containing HBV, the risk of developing clinical hepatitis if the blood was positive for both HBsAg and HBeAg was 22%--31%; the risk of developing serologic evidence of HBV infection was 37%--62% (19). By comparison, the risk of developing clinical hepatitis from a needle contaminated with HBsAg-positive, HBeAg-negative blood was 1%--6%, and the risk of developing serologic evidence of HBV infection, 23%--37% (38). Blood contains the greatest proportion of HBV infectious particle titers of all body fluids and is the most critical vehicle of transmission in the health-care setting. HBsAg is also found in multiple other body fluids, including breast milk, bile, cerebrospinal fluid, feces, nasopharyngeal washings, saliva, semen, sweat, and synovial fluid. However, the majority of body fluids are not efficient vehicles for transmission because they contain low quantities of infectious HBV, despite the presence of HBsAg (19). The concentration of HBsAg in body fluids can be 100--1,000-fold greater than the concentration of infectious HBV particles (39). Although percutaneous injuries are among the most efficient modes of HBV transmission, these exposures probably account for only a minority of HBV infections among HCP. In multiple investigations of nosocomial hepatitis B outbreaks, the majority of infected HCP could not recall an overt percutaneous injury (40,41), although in certain studies, approximately one third of infected HCP recalled caring for a patient who was HBsAg-positive (42,43). In addition, HBV has been demonstrated to survive in dried blood at room temperature on environmental surfaces for <1 week (44). Thus, HBV infections that occur in HCP with no history of nonoccupational exposure or occupational percutaneous injury might have resulted from direct or indirect blood or body fluid exposures that inoculated HBV into cutaneous scratches, abrasions, burns, other lesions, or on mucosal surfaces (45--47). The potential for HBV transmission through contact with environmental surfaces has been demonstrated in investigations of HBV outbreaks among patients and HCP in hemodialysis units (48--50). Since the early 1980s, occupational infections among HCP have declined because of vaccine use and adherence to universal precautions (51). Among U.S. dentists, >90% have been vaccinated, and serologic evidence of past HBV infection decreased from prevaccine levels of 14% in 1972 to approximately 9% in 1992 (52). During 1993--2001, levels remained relatively unchanged (Chakwan Siew, Ph.D., American Dental Association, Chicago, Illinois, personal communication, June 2003). Infection rates can be expected to decline further as vaccination rates remain high among young dentists and as older dentists with lower vaccination rates and higher rates of infection retire. Although the potential for transmission of bloodborne infections from DHCP to patients is considered limited (53--55), precise risks have not been quantified by carefully designed epidemiologic studies (53,56,57). Reports published during 1970--1987 describe nine clusters in which patients were thought to be infected with HBV through treatment by an infected DHCP (58--67). However, transmission of HBV from dentist to patient has not been reported since 1987, possibly reflecting such factors as 1) adoption of universal precautions, 2) routine glove use, 3) increased levels of immunity as a result of hepatitis B vaccination of DHCP, 4) implementation of the 1991 OSHA bloodborne pathogen standard (68), and 5) incomplete ascertainment and reporting. Only one case of patient-to-patient transmission of HBV in the dental setting has been documented (CDC, unpublished data, 2003). In this case, appropriate office infection-control procedures were being followed, and the exact mechanism of transmission was undetermined. Because of the high risk of HBV infection among HCP, DHCP who perform tasks that might involve contact with blood, blood-contaminated body substances, other body fluids, or sharps should be vaccinated (2,13,17,19,69). Vaccination can protect both DHCP and patients from HBV infection and, whenever possible, should be completed when dentists or other DHCP are in training and before they have contact with blood. Prevaccination serological testing for previous infection is not indicated, although it can be cost-effective where prevalence of infection is expected to be high in a group of potential vacinees (e.g., persons who have emigrated from areas with high rates of HBV infection). DHCP should be tested for anti-HBs 1--2 months after completion of the 3-dose vaccination series (17). DHCP who do not develop an adequate antibody response (i.e., anti-HBs <10 mIU/mL) to the primary vaccine series should complete a second 3-dose vaccine series or be evaluated to determine if they are HBsAg-positive (17). Revaccinated persons should be retested for anti-HBs at the completion of the second vaccine series. Approximately half of nonresponders to the primary series will respond to a second 3-dose series. If no antibody response occurs after the second series, testing for HBsAg should be performed (17). Persons who prove to be HBsAg-positive should be counseled regarding how to prevent HBV transmission to others and regarding the need for medical evaluation. Nonresponders to vaccination who are HBsAg-negative should be considered susceptible to HBV infection and should be counseled regarding precautions to prevent HBV infection and the need to obtain HBIG prophylaxis for any known or probable parenteral exposure to HBsAg-positive blood. Vaccine-induced antibodies decline gradually over time, and 60% of persons who initially respond to vaccination will lose detectable antibodies over 12 years. Even so, immunity continues to prevent clinical disease or detectable viral infection (17). Booster doses of vaccine and periodic serologic testing to monitor antibody concentrations after completion of the vaccine series are not necessary for vaccine responders (17). Hepatitis D Virus An estimated 4% of persons with acute HBV infection are also infected with hepatitis Delta virus (HDV). Discovered in 1977, HDV is a defective bloodborne virus requiring the presence of HBV to replicate. Patients coinfected with HBV and HDV have substantially higher mortality rates than those infected with HBV alone. Because HDV infection is dependent on HBV for replication, immunization to prevent HBV infection, through either pre- or postexposure prophylaxis, can also prevent HDV infection (70). Hepatitis C Virus Hepatitis C virus appears not to be transmitted efficiently through occupational exposures to blood. Follow-up studies of HCP exposed to HCV-infected blood through percutaneous or other sharps injuries have determined a low incidence of seroconversion (mean: 1.8%; range, 0%--7%) (71--74). One study determined transmission occurred from hollow-bore needles but not other sharps (72). Although these studies have not documented seroconversion associated with mucous membrane or nonintact skin exposure, at least two cases of HCV transmission from a blood splash to the conjunctiva (75,76) and one case of simultaneous transmission of HCV and HIV after nonintact skin exposure have been reported (77). Data are insufficient to estimate the occupational risk of HCV infection among HCP, but the majority of studies indicate the prevalence of HCV infection among dentists, surgeons, and hospital-based HCP is similar to that among the general population, approximately 1%--2% (78--86). In a study that evaluated risk factors for infection, a history of unintentional needlesticks was the only occupational risk factor independently associated with HCV infection (80). No studies of transmission from HCV-infected DHCP to patients have been reported, and the risk for such transmission appears limited. Multiple reports have been published describing transmission from HCV-infected surgeons, which apparently occurred during performance of invasive procedures; the overall risk for infection averaged 0.17% (87--90). Human Immunodeficiency Virus In the United States, the risk of HIV transmission in dental settings is extremely low. As of December 2001, a total of 57 cases of HIV seroconversion had been documented among HCP, but none among DHCP, after occupational exposure to a known HIV-infected source (91). Transmission of HIV to six patients of a single dentist with AIDS has been reported, but the mode of transmission could not be determined (2,92,93). As of September 30, 1993, CDC had information regarding test results of >22,000 patients of 63 HIV-infected HCP, including 33 dentists or dental students (55,93). No additional cases of transmission were documented. Prospective studies worldwide indicate the average risk of HIV infection after a single percutaneous exposure to HIV-infected blood is 0.3% (range: 0.2%--0.5%) (94). After an exposure of mucous membranes in the eye, nose, or mouth, the risk is approximately 0.1% (76). The precise risk of transmission after skin exposure remains unknown but is believed to be even smaller than that for mucous membrane exposure. Certain factors affect the risk of HIV transmission after an occupational exposure. Laboratory studies have determined if needles that pass through latex gloves are solid rather than hollow-bore, or are of small gauge (e.g., anesthetic needles commonly used in dentistry), they transfer less blood (36). In a retrospective case-control study of HCP, an increased risk for HIV infection was associated with exposure to a relatively large volume of blood, as indicated by a deep injury with a device that was visibly contaminated with the patient's blood, or a procedure that involved a needle placed in a vein or artery (95). The risk was also increased if the exposure was to blood from patients with terminal illnesses, possibly reflecting the higher titer of HIV in late-stage AIDS. Exposure Prevention Methods Avoiding occupational exposures to blood is the primary way to prevent transmission of HBV, HCV, and HIV, to HCP in health-care settings (19,96,97). Exposures occur through percutaneous injury (e.g., a needlestick or cut with a sharp object), as well as through contact between potentially infectious blood, tissues, or other body fluids and mucous membranes of the eye, nose, mouth, or nonintact skin (e.g., exposed skin that is chapped, abraded, or shows signs of dermatitis). Observational studies and surveys indicate that percutaneous injuries among general dentists and oral surgeons occur less frequently than among general and orthopedic surgeons and have decreased in frequency since the mid-1980s (98--102). This decline has been attributed to safer work practices, safer instrumentation or design, and continued DHCP education (103,104). Percutaneous injuries among DHCP usually 1) occur outside the patient's mouth, thereby posing less risk for recontact with patient tissues; 2) involve limited amounts of blood; and 3) are caused by burs, syringe needles, laboratory knives, and other sharp instruments (99--102,105,106). Injuries among oral surgeons might occur more frequently during fracture reductions using wires (104,107). Experience, as measured by years in practice, does not appear to affect the risk of injury among general dentists or oral surgeons (100,104,107). The majority of exposures in dentistry are preventable, and methods to reduce the risk of blood contacts have included use of standard precautions, use of devices with features engineered to prevent sharp injuries, and modifications of work practices. These approaches might have contributed to the decrease in percutaneous injuries among dentists during recent years (98--100,103). However, needlesticks and other blood contacts continue to occur, which is a concern because percutaneous injuries pose the greatest risk of transmission. Standard precautions include use of PPE (e.g., gloves, masks, protective eyewear or face shield, and gowns) intended to prevent skin and mucous membrane exposures. Other protective equipment (e.g., finger guards while suturing) might also reduce injuries during dental procedures (104). Engineering controls are the primary method to reduce exposures to blood and OPIM from sharp instruments and needles. These controls are frequently technology-based and often incorporate safer designs of instruments and devices (e.g., self-sheathing anesthetic needles and dental units designed to shield burs in handpieces) to reduce percutaneous injuries (101,103,108). Work-practice controls establish practices to protect DHCP whose responsibilities include handling, using, assembling, or processing sharp devices (e.g., needles, scalers, laboratory utility knives, burs, explorers, and endodontic files) or sharps disposal containers. Work-practice controls can include removing burs before disassembling the handpiece from the dental unit, restricting use of fingers in tissue retraction or palpation during suturing and administration of anesthesia, and minimizing potentially uncontrolled movements of such instruments as scalers or laboratory knives (101,105). As indicated, needles are a substantial source of percutaneous injury in dental practice, and engineering and work-practice controls for needle handling are of particular importance. In 2001, revisions to OSHA's bloodborne pathogens standard as mandated by the Needlestick Safety and Prevention Act of 2000 became effective. These revisions clarify the need for employers to consider safer needle devices as they become available and to involve employees directly responsible for patient care (e.g., dentists, hygienists, and dental assistants) in identifying and choosing such devices (109). Safer versions of sharp devices used in hospital settings have become available (e.g., blunt suture needles, phlebotomy devices, and butterfly needles), and their impact on reducing injuries has been documented (110--112). Aspirating anesthetic syringes that incorporate safety features have been developed for dental procedures, but the low injury rates in dentistry limit assessment of their effect on reducing injuries among DHCP. Work-practice controls for needles and other sharps include placing used disposable syringes and needles, scalpel blades, and other sharp items in appropriate puncture-resistant containers located as close as feasible to where the items were used (2,7,13,113--115). In addition, used needles should never be recapped or otherwise manipulated by using both hands, or any other technique that involves directing the point of a needle toward any part of the body (2,7,13,97,113,114). A one-handed scoop technique, a mechanical device designed for holding the needle cap to facilitate one-handed recapping, or an engineered sharps injury protection device (e.g., needles with resheathing mechanisms) should be employed for recapping needles between uses and before disposal (2,7,13,113,114). DHCP should never bend or break needles before disposal because this practice requires unnecessary manipulation. Before attempting to remove needles from nondisposable aspirating syringes, DHCP should recap them to prevent injuries. For procedures involving multiple injections with a single needle, the practitioner should recap the needle between injections by using a one-handed technique or use a device with a needle-resheathing mechanism. Passing a syringe with an unsheathed needle should be avoided because of the potential for injury. Additional information for developing a safety program and for identifying and evaluating safer dental devices is available at
Postexposure Management and Prophylaxis Postexposure management is an integral component of a complete program to prevent infection after an occupational exposure to blood. During dental procedures, saliva is predictably contaminated with blood (7,114). Even when blood is not visible, it can still be present in limited quantities and therefore is considered a potentially infectious material by OSHA (13,19). A qualified health-care professional should evaluate any occupational exposure incident to blood or OPIM, including saliva, regardless of whether blood is visible, in dental settings (13). Dental practices and laboratories should establish written, comprehensive programs that include hepatitis B vaccination and postexposure management protocols that 1) describe the types of contact with blood or OPIM that can place DHCP at risk for infection; 2) describe procedures for promptly reporting and evaluating such exposures; and 3) identify a health-care professional who is qualified to provide counseling and perform all medical evaluations and procedures in accordance with current recommendations of the U.S. Public Health Service (PHS), including PEP with chemotherapeutic drugs when indicated. DHCP, including students, who might reasonably be considered at risk for occupational exposure to blood or OPIM should be taught strategies to prevent contact with blood or OPIM and the principles of postexposure management, including PEP options, as part of their job orientation and training. Educational programs for DHCP and students should emphasize reporting all exposures to blood or OPIM as soon as possible, because certain interventions have to be initiated promptly to be effective. Policies should be consistent with the practices and procedures for worker protection required by OSHA and with current PHS recommendations for managing occupational exposures to blood (13,19). After an occupational blood exposure, first aid should be administered as necessary. Puncture wounds and other injuries to the skin should be washed with soap and water; mucous membranes should be flushed with water. No evidence exists that using antiseptics for wound care or expressing fluid by squeezing the wound further reduces the risk of bloodborne pathogen transmission; however, use of antiseptics is not contraindicated. The application of caustic agents (e.g., bleach) or the injection of antiseptics or disinfectants into the wound is not recommended (19). Exposed DHCP should immediately report the exposure to the infection-control coordinator or other designated person, who should initiate referral to the qualified health-care professional and complete necessary reports. Because multiple factors contribute to the risk of infection after an occupational exposure to blood, the following information should be included in the exposure report, recorded in the exposed person's confidential medical record, and provided to the qualified health-care professional:
Each occupational exposure should be evaluated individually for its potential to transmit HBV, HCV, and HIV, based on the following:
All of these factors should be considered in assessing the risk for infection and the need for further follow-up (e.g., PEP). During 1990--1998, PHS published guidelines for PEP and other management of health-care worker exposures to HBV, HCV, or HIV (69,116--119). In 2001, these recommendations were updated and consolidated into one set of PHS guidelines (19). The new guidelines reflect the availability of new antiretroviral agents, new information regarding the use and safety of HIV PEP, and considerations regarding employing HIV PEP when resistance of the source patient's virus to antiretroviral agents is known or suspected. In addition, the 2001 guidelines provide guidance to clinicians and exposed HCP regarding when to consider HIV PEP and recommendations for PEP regimens (19). Hand HygieneHand hygiene (e.g., handwashing, hand antisepsis, or surgical hand antisepsis) substantially reduces potential pathogens on the hands and is considered the single most critical measure for reducing the risk of transmitting organisms to patients and HCP (120--123). Hospital-based studies have demonstrated that noncompliance with hand hygiene practices is associated with health-care--associated infections and the spread of multiresistant organisms. Noncompliance also has been a major contributor to outbreaks (123). The prevalence of health-care--associated infections decreases as adherence of HCP to recommended hand hygiene measures improves (124--126). The microbial flora of the skin, first described in 1938, consist of transient and resident microorganisms (127). Transient flora, which colonize the superficial layers of the skin, are easier to remove by routine handwashing. They are often acquired by HCP during direct contact with patients or contaminated environmental surfaces; these organisms are most frequently associated with health-care--associated infections. Resident flora attached to deeper layers of the skin are more resistant to removal and less likely to be associated with such infections. The preferred method for hand hygiene depends on the type of procedure, the degree of contamination, and the desired persistence of antimicrobial action on the skin (Table 2). For routine dental examinations and nonsurgical procedures, handwashing and hand antisepsis is achieved by using either a plain or antimicrobial soap and water. If the hands are not visibly soiled, an alcohol-based hand rub is adequate. The purpose of surgical hand antisepsis is to eliminate transient flora and reduce resident flora for the duration of a procedure to prevent introduction of organisms in the operative wound, if gloves become punctured or torn. Skin bacteria can rapidly multiply under surgical gloves if hands are washed with soap that is not antimicrobial (127,128). Thus, an antimicrobial soap or alcohol hand rub with persistent activity should be used before surgical procedures (129--131). Agents used for surgical hand antisepsis should substantially reduce microorganisms on intact skin, contain a nonirritating antimicrobial preparation, have a broad spectrum of activity, be fast-acting, and have a persistent effect (121,132--135). Persistence (i.e., extended antimicrobial activity that prevents or inhibits survival of microorganisms after the product is applied) is critical because microorganisms can colonize on hands in the moist environment underneath gloves (122). Alcohol hand rubs are rapidly germicidal when applied to the skin but should include such antiseptics as chlorhexidine, quaternary ammonium compounds, octenidine, or triclosan to achieve persistent activity (130). Factors that can influence the effectiveness of the surgical hand antisepsis in addition to the choice of antiseptic agent include duration and technique of scrubbing, as well as condition of the hands, and techniques used for drying and gloving. CDC's 2002 guideline on hand hygiene in health-care settings provides more complete information (123). Selection of Antiseptic Agents Selecting the most appropriate antiseptic agent for hand hygiene requires consideration of multiple factors. Essential performance characteristics of a product (e.g., the spectrum and persistence of activity and whether or not the agent is fast-acting) should be determined before selecting a product. Delivery system, cost per use, reliable vendor support and supply are also considerations. Because HCP acceptance is a major factor regarding compliance with recommended hand hygiene protocols (122,123,147,148), considering DHCP needs is critical and should include possible chemical allergies, skin integrity after repeated use, compatibility with lotions used, and offensive agent ingredients (e.g., scent). Discussing specific preparations or ingredients used for hand antisepsis is beyond the scope of this report. DHCP should choose from commercially available HCP handwashes when selecting agents for hand antisepsis or surgical hand antisepsis. Storage and Dispensing of Hand Care Products Handwashing products, including plain (i.e., nonantimicrobial) soap and antiseptic products, can become contaminated or support the growth of microorganisms (122). Liquid products should be stored in closed containers and dispensed from either disposable containers or containers that are washed and dried thoroughly before refilling. Soap should not be added to a partially empty dispenser, because this practice of topping off might lead to bacterial contamination (149,150). Store and dispense products according to manufacturers' directions. Lotions The primary defense against infection and transmission of pathogens is healthy, unbroken skin. Frequent handwashing with soaps and antiseptic agents can cause chronic irritant contact dermatitis among DHCP. Damage to the skin changes skin flora, resulting in more frequent colonization by staphylococci and gram-negative bacteria (151,152). The potential of detergents to cause skin irritation varies considerably, but can be reduced by adding emollients. Lotions are often recommended to ease the dryness resulting from frequent handwashing and to prevent dermatitis from glove use (153,154). However, petroleum-based lotion formulations can weaken latex gloves and increase permeability. For that reason, lotions that contain petroleum or other oil emollients should only be used at the end of the work day (122,155). Dental practitioners should obtain information from lotion manufacturers regarding interaction between lotions, gloves, dental materials, and antimicrobial products. Fingernails and Artificial Nails Although the relationship between fingernail length and wound infection is unknown, keeping nails short is considered key because the majority of flora on the hands are found under and around the fingernails (156). Fingernails should be short enough to allow DHCP to thoroughly clean underneath them and prevent glove tears (122). Sharp nail edges or broken nails are also likely to increase glove failure. Long artificial or natural nails can make donning gloves more difficult and can cause gloves to tear more readily. Hand carriage of gram-negative organisms has been determined to be greater among wearers of artificial nails than among nonwearers, both before and after handwashing (157--160). In addition, artificial fingernails or extenders have been epidemiologically implicated in multiple outbreaks involving fungal and bacterial infections in hospital intensive-care units and operating rooms (161--164). Freshly applied nail polish on natural nails does not increase the microbial load from periungual skin if fingernails are short; however, chipped nail polish can harbor added bacteria (165,166). Jewelry Studies have demonstrated that skin underneath rings is more heavily colonized than comparable areas of skin on fingers without rings (167--170). In a study of intensive-care nurses, multivariable analysis determined rings were the only substantial risk factor for carriage of gram-negative bacilli and Staphylococcus aureus, and the concentration of organisms correlated with the number of rings worn (170). However, two other studies demonstrated that mean bacterial colony counts on hands after handwashing were similar among persons wearing rings and those not wearing rings (169,171). Whether wearing rings increases the likelihood of transmitting a pathogen is unknown; further studies are needed to establish whether rings result in higher transmission of pathogens in health-care settings. However, rings and decorative nail jewelry can make donning gloves more difficult and cause gloves to tear more readily (142,143). Thus, jewelry should not interfere with glove use (e.g., impair ability to wear the correct-sized glove or alter glove integrity). Personal Protective EquipmentPPE is designed to protect the skin and the mucous membranes of the eyes, nose, and mouth of DHCP from exposure to blood or OPIM. Use of rotary dental and surgical instruments (e.g., handpieces or ultrasonic scalers) and air-water syringes creates a visible spray that contains primarily large-particle droplets of water, saliva, blood, microorganisms, and other debris. This spatter travels only a short distance and settles out quickly, landing on the floor, nearby operatory surfaces, DHCP, or the patient. The spray also might contain certain aerosols (i.e., particles of respirable size, <10 µm). Aerosols can remain airborne for extended periods and can be inhaled. However, they should not be confused with the large-particle spatter that makes up the bulk of the spray from handpieces and ultrasonic scalers. Appropriate work practices, including use of dental dams (172) and high-velocity air evacuation, should minimize dissemination of droplets, spatter, and aerosols (2). Primary PPE used in oral health-care settings includes gloves, surgical masks, protective eyewear, face shields, and protective clothing (e.g., gowns and jackets). All PPE should be removed before DHCP leave patient-care areas (13). Reusable PPE (e.g., clinician or patient protective eyewear and face shields) should be cleaned with soap and water, and when visibly soiled, disinfected between patients, according to the manufacturer's directions (2,13). Wearing gloves, surgical masks, protective eyewear, and protective clothing in specified circumstances to reduce the risk of exposures to bloodborne pathogens is mandated by OSHA (13). General work clothes (e.g., uniforms, scrubs, pants, and shirts) are neither intended to protect against a hazard nor considered PPE. Masks, Protective Eyewear, Face Shields A surgical mask that covers both the nose and mouth and protective eyewear with solid side shields or a face shield should be worn by DHCP during procedures and patient-care activities likely to generate splashes or sprays of blood or body fluids. Protective eyewear for patients shields their eyes from spatter or debris generated during dental procedures. A surgical mask protects against microorganisms generated by the wearer, with >95% bacterial filtration efficiency, and also protects DHCP from large-particle droplet spatter that might contain bloodborne pathogens or other infectious microorganisms (173). The mask's outer surface can become contaminated with infectious droplets from spray of oral fluids or from touching the mask with contaminated fingers. Also, when a mask becomes wet from exhaled moist air, the resistance to airflow through the mask increases, causing more airflow to pass around edges of the mask. If the mask becomes wet, it should be changed between patients or even during patient treatment, when possible (2,174). When airborne infection isolation precautions (expanded or transmission-based) are necessary (e.g., for TB patients), a National Institute for Occupational Safety and Health (NIOSH)-certified particulate-filter respirator (e.g., N95, N99, or N100) should be used (20). N95 refers to the ability to filter 1-µm particles in the unloaded state with a filter efficiency of >95% (i.e., filter leakage <5%), given flow rates of <50 L/min (i.e., approximate maximum airflow rate of HCP during breathing). Available data indicate infectious droplet nuclei measure 1--5 µm; therefore, respirators used in health-care settings should be able to efficiently filter the smallest particles in this range. The majority of surgical masks are not NIOSH-certified as respirators, do not protect the user adequately from exposure to TB, and do not satisfy OSHA requirements for respiratory protection (174,175). However, certain surgical masks (i.e., surgical N95 respirator) do meet the requirements and are certified by NIOSH as respirators. The level of protection a respirator provides is determined by the efficiency of the filter material for incoming air and how well the face piece fits or seals to the face (e.g., qualitatively or quantitatively tested in a reliable way to obtain a face-seal leakage of <10% and to fit the different facial sizes and characteristics of HCP). When respirators are used while treating patients with diseases requiring airborne-transmission precautions (e.g., TB), they should be used in the context of a complete respiratory protection program (175). This program should include training and fit testing to ensure an adequate seal between the edges of the respirator and the wearer's face. Detailed information regarding respirator programs, including fit-test procedures are available at http://www.cdc.gov/niosh/99-143.html (174,176). Protective Clothing Protective clothing and equipment (e.g., gowns, lab coats, gloves, masks, and protective eyewear or face shield) should be worn to prevent contamination of street clothing and to protect the skin of DHCP from exposures to blood and body substances (2,7,10,11,13,137). OSHA bloodborne pathogens standard requires sleeves to be long enough to protect the forearms when the gown is worn as PPE (i.e., when spatter and spray of blood, saliva, or OPIM to the forearms is anticipated) (13,14). DHCP should change protective clothing when it becomes visibly soiled and as soon as feasible if penetrated by blood or other potentially infectious fluids (2,13,14,137). All protective clothing should be removed before leaving the work area (13). Gloves and Gloving DHCP wear gloves to prevent contamination of their hands when touching mucous membranes, blood, saliva, or OPIM, and also to reduce the likelihood that microorganisms present on the hands of DHCP will be transmitted to patients during surgical or other patient-care procedures (1,2,7,10). Medical gloves, both patient examination and surgeon's gloves, are manufactured as single-use disposable items that should be used for only one patient, then discarded. Gloves should be changed between patients and when torn or punctured. Wearing gloves does not eliminate the need for handwashing. Hand hygiene should be performed immediately before donning gloves. Gloves can have small, unapparent defects or can be torn during use, and hands can become contaminated during glove removal (122,177--187). These circumstances increase the risk of operative wound contamination and exposure of the DHCP's hands to microorganisms from patients. In addition, bacteria can multiply rapidly in the moist environments underneath gloves, and thus, the hands should be dried thoroughly before donning gloves and washed again immediately after glove removal. Types of Gloves Because gloves are task-specific, their selection should be based on the type of procedure to be performed (e.g., surgery or patient examination) (Table 3). Sterile surgeon's gloves must meet standards for sterility assurance established by FDA and are less likely than patient examination gloves to harbor pathogens that could contaminate an operative wound (188). Appropriate gloves in the correct size should be readily accessible (13). Glove Integrity Limited studies of the penetrability of different glove materials under conditions of use have been conducted in the dental environment. Consistent with observations in clinical medicine, leakage rates vary by glove material (e.g., latex, vinyl, and nitrile), duration of use, and type of procedure performed (182,184,186,189--191), as well as by manufacturer (192--194). The frequency of perforations in surgeon's gloves used during outpatient oral surgical procedures has been determined to range from 6% to 16% (181,185,195,196). Studies have demonstrated that HCP and DHCP are frequently unaware of minute tears in gloves that occur during use (186,190,191,197). These studies determined that gloves developed defects in 30 minutes--3 hours, depending on type of glove and procedure. Investigators did not determine an optimal time for changing gloves during procedures. During dental procedures, patient examination and surgeon's gloves commonly contact multiple types of chemicals and materials (e.g., disinfectants and antiseptics, composite resins, and bonding agents) that can compromise the integrity of latex as well as vinyl, nitrile, and other synthetic glove materials (198--206). In addition, latex gloves can interfere with the setting of vinyl polysiloxane impression materials (207--209), although the setting is apparently not adversely affected by synthetic vinyl gloves (207,208). Given the diverse selection of dental materials on the market, dental practitioners should consult glove manufacturers regarding the chemical compatibility of glove materials. If the integrity of a glove is compromised (e.g., punctured), it should be changed as soon as possible (13,210,211). Washing latex gloves with plain soap, chlorhexidine, or alcohol can lead to the formation of glove micropunctures (177,212,213) and subsequent hand contamination (138). Because this condition, known as wicking, can allow penetration of liquids through undetected holes, washing gloves is not recommended. After a hand rub with alcohol, the hands should be thoroughly dried before gloving, because hands still wet with an alcohol-based hand hygiene product can increase the risk of glove perforation (192). FDA regulates the medical glove industry, which includes gloves marketed as sterile surgeon's and sterile or nonsterile patient examination gloves. General-purpose utility gloves are also used in dental health-care settings but are not regulated by FDA because they are not promoted for medical use. More rigorous standards are applied to surgeon's than to examination gloves. FDA has identified acceptable quality levels (e.g., maximum defects allowed) for glove manufacturers (214), but even intact gloves eventually fail with exposure to mechanical (e.g., sharps, fingernails, or jewelry) and chemical (e.g., dimethyacrylates) hazards and over time. These variables can be controlled, ultimately optimizing glove performance, by 1) maintaining short fingernails, 2) minimizing or eliminating hand jewelry, and 3) using engineering and work-practice controls to avoid injuries with sharps. Sterile Surgeon's Gloves and Double-Gloving During Oral Surgical Procedures Certain limited studies have determined no difference in postoperative infection rates after routine tooth extractions when surgeons wore either sterile or nonsterile gloves (215,216). However, wearing sterile surgeon's gloves during surgical procedures is supported by a strong theoretical rationale (2,7,137). Sterile gloves minimize transmission of microorganisms from the hands of surgical DHCP to patients and prevent contamination of the hands of surgical DHCP with the patient's blood and body fluids (137). In addition, sterile surgeon's gloves are more rigorously regulated by FDA and therefore might provide an increased level of protection for the provider if exposure to blood is likely. Although the effectiveness of wearing two pairs of gloves in preventing disease transmission has not been demonstrated, the majority of studies among HCP and DHCP have demonstrated a lower frequency of inner glove perforation and visible blood on the surgeon's hands when double gloves are worn (181,185,195,196,198,217--219). In one study evaluating double gloves during oral surgical and dental hygiene procedures, the perforation of outer latex gloves was greater during longer procedures (i.e., >45 minutes), with the highest rate (10%) of perforation occurring during oral surgery procedures (196). Based on these studies, double gloving might provide additional protection from occupational blood contact (220). Double gloving does not appear to substantially reduce either manual dexterity or tactile sensitivity (221--223). Additional protection might also be provided by specialty products (e.g., orthopedic surgical gloves and glove liners) (224). Contact Dermatitis and Latex HypersensitivityOccupationally related contact dermatitis can develop from frequent and repeated use of hand hygiene products, exposure to chemicals, and glove use. Contact dermatitis is classified as either irritant or allergic. Irritant contact dermatitis is common, nonallergic, and develops as dry, itchy, irritated areas on the skin around the area of contact. By comparison, allergic contact dermatitis (type IV hypersensitivity) can result from exposure to accelerators and other chemicals used in the manufacture of rubber gloves (e.g., natural rubber latex, nitrile, and neoprene), as well as from other chemicals found in the dental practice setting (e.g., methacrylates and glutaraldehyde). Allergic contact dermatitis often manifests as a rash beginning hours after contact and, similar to irritant dermatitis, is usually confined to the area of contact. Latex allergy (type I hypersensitivity to latex proteins) can be a more serious systemic allergic reaction, usually beginning within minutes of exposure but sometimes occurring hours later and producing varied symptoms. More common reactions include runny nose, sneezing, itchy eyes, scratchy throat, hives, and itchy burning skin sensations. More severe symptoms include asthma marked by difficult breathing, coughing spells, and wheezing; cardiovascular and gastrointestinal ailments; and in rare cases, anaphylaxis and death (32,225). The American Dental Association (ADA) began investigating the prevalence of type I latex hypersensitivity among DHCP at the ADA annual meeting in 1994. In 1994 and 1995, approximately 2,000 dentists, hygienists, and assistants volunteered for skin-prick testing. Data demonstrated that 6.2% of those tested were positive for type I latex hypersensitivity (226). Data from the subsequent 5 years of this ongoing cross-sectional study indicated a decline in prevalence from 8.5% to 4.3% (227). This downward trend is similar to that reported by other studies and might be related to use of latex gloves with lower allergen content (228--230). Natural rubber latex proteins responsible for latex allergy are attached to glove powder. When powdered latex gloves are worn, more latex protein reaches the skin. In addition, when powdered latex gloves are donned or removed, latex protein/powder particles become aerosolized and can be inhaled, contacting mucous membranes (231). As a result, allergic patients and DHCP can experience cutaneous, respiratory, and conjunctival symptoms related to latex protein exposure. DHCP can become sensitized to latex protein with repeated exposure (232--236). Work areas where only powder-free, low-allergen latex gloves are used demonstrate low or undetectable amounts of latex allergy-causing proteins (237--239) and fewer symptoms among HCP related to natural rubber latex allergy. Because of the role of glove powder in exposure to latex protein, NIOSH recommends that if latex gloves are chosen, HCP should be provided with reduced protein, powder-free gloves (32). Nonlatex (e.g., nitrile or vinyl) powder-free and low-protein gloves are also available (31,240). Although rare, potentially life-threatening anaphylactic reactions to latex can occur; dental practices should be appropriately equipped and have procedures in place to respond to such emergencies. DHCP and dental patients with latex allergy should not have direct contact with latex-containing materials and should be in a latex-safe environment with all latex-containing products removed from their vicinity (31). Dental patients with histories of latex allergy can be at risk from dental products (e.g., prophylaxis cups, rubber dams, orthodontic elastics, and medication vials) (241). Any latex-containing devices that cannot be removed from the treatment environment should be adequately covered or isolated. Persons might also be allergic to chemicals used in the manufacture of natural rubber latex and synthetic rubber gloves as well as metals, plastics, or other materials used in dental care. Taking thorough health histories for both patients and DHCP, followed by avoidance of contact with potential allergens can minimize the possibility of adverse reactions. Certain common predisposing conditions for latex allergy include previous history of allergies, a history of spina bifida, urogenital anomalies, or allergies to avocados, kiwis, nuts, or bananas. The following precautions should be considered to ensure safe treatment for patients who have possible or documented latex allergy:
Sterilization and Disinfection of Patient-Care ItemsPatient-care items (dental instruments, devices, and equipment) are categorized as critical, semicritical, or noncritical, depending on the potential risk for infection associated with their intended use (Table 4) (242). Critical items used to penetrate soft tissue or bone have the greatest risk of transmitting infection and should be sterilized by heat. Semicritical items touch mucous membranes or nonintact skin and have a lower risk of transmission; because the majority of semicritical items in dentistry are heat-tolerant, they also should be sterilized by using heat. If a semicritical item is heat-sensitive, it should, at a minimum, be processed with high-level disinfection (2). Noncritical patient-care items pose the least risk of transmission of infection, contacting only intact skin, which can serve as an effective barrier to microorganisms. In the majority of cases, cleaning, or if visibly soiled, cleaning followed by disinfection with an EPA-registered hospital disinfectant is adequate. When the item is visibly contaminated with blood or OPIM, an EPA-registered hospital disinfectant with a tuberculocidal claim (i.e., intermediate-level disinfectant) should be used (2,243,244). Cleaning or disinfection of certain noncritical patient-care items can be difficult or damage the surfaces; therefore, use of disposable barrier protection of these surfaces might be a preferred alternative. FDA-cleared sterilant/high-level disinfectants and EPA-registered disinfectants must have clear label claims for intended use, and manufacturer instructions for use must be followed (245). A more complete description of the regulatory framework in the United States by which liquid chemical germicides are evaluated and regulated is included (Appendix A). Three levels of disinfection, high, intermediate, and low, are used for patient-care devices that do not require sterility and two levels, intermediate and low, for environmental surfaces (242). The intended use of the patient-care item should determine the recommended level of disinfection. Dental practices should follow the product manufacturer's directions regarding concentrations and exposure time for disinfectant activity relative to the surface to be disinfected (245). A summary of sterilization and disinfection methods is included (Appendix C). Transporting and Processing Contaminated Critical and Semicritical Patient-Care Items DHCP can be exposed to microorganisms on contaminated instruments and devices through percutaneous injury, contact with nonintact skin on the hands, or contact with mucous membranes of the eyes, nose, or mouth. Contaminated instruments should be handled carefully to prevent exposure to sharp instruments that can cause a percutaneous injury. Instruments should be placed in an appropriate container at the point of use to prevent percutaneous injuries during transport to the instrument processing area (13). Instrument processing requires multiple steps to achieve sterilization or high-level disinfection. Sterilization is a complex process requiring specialized equipment, adequate space, qualified DHCP who are provided with ongoing training, and regular monitoring for quality assurance (247). Correct cleaning, packaging, sterilizer loading procedures, sterilization methods, or high-level disinfection methods should be followed to ensure that an instrument is adequately processed and safe for reuse on patients. Instrument Processing Area DHCP should process all instruments in a designated central processing area to more easily control quality and ensure safety (248). The central processing area should be divided into sections for 1) receiving, cleaning, and decontamination; 2) preparation and packaging; 3) sterilization; and 4) storage. Ideally, walls or partitions should separate the sections to control traffic flow and contain contaminants generated during processing. When physical separation of these sections cannot be achieved, adequate spatial separation might be satisfactory if the DHCP who process instruments are trained in work practices to prevent contamination of clean areas (248). Space should be adequate for the volume of work anticipated and the items to be stored (248). Receiving, Cleaning, and Decontamination Reusable instruments, supplies, and equipment should be received, sorted, cleaned, and decontaminated in one section of the processing area. Cleaning should precede all disinfection and sterilization processes; it should involve removal of debris as well as organic and inorganic contamination. Removal of debris and contamination is achieved either by scrubbing with a surfactant, detergent, and water, or by an automated process (e.g., ultrasonic cleaner or washer-disinfector) using chemical agents. If visible debris, whether inorganic or organic matter, is not removed, it will interfere with microbial inactivation and can compromise the disinfection or sterilization process (244,249--252). After cleaning, instruments should be rinsed with water to remove chemical or detergent residue. Splashing should be minimized during cleaning and rinsing (13). Before final disinfection or sterilization, instruments should be handled as though contaminated. Considerations in selecting cleaning methods and equipment include 1) efficacy of the method, process, and equipment; 2) compatibility with items to be cleaned; and 3) occupational health and exposure risks. Use of automated cleaning equipment (e.g., ultrasonic cleaner or washer-disinfector) does not require presoaking or scrubbing of instruments and can increase productivity, improve cleaning effectiveness, and decrease worker exposure to blood and body fluids. Thus, using automated equipment can be safer and more efficient than manually cleaning contaminated instruments (253). If manual cleaning is not performed immediately, placing instruments in a puncture-resistant container and soaking them with detergent, a disinfectant/detergent, or an enzymatic cleaner will prevent drying of patient material and make cleaning easier and less time-consuming. Use of a liquid chemical sterilant/high-level disinfectant (e.g., glutaraldehyde) as a holding solution is not recommended (244). Using work-practice controls (e.g., long-handled brush) to keep the scrubbing hand away from sharp instruments is recommended (14). To avoid injury from sharp instruments, DHCP should wear puncture-resistant, heavy-duty utility gloves when handling or manually cleaning contaminated instruments and devices (6). Employees should not reach into trays or containers holding sharp instruments that cannot be seen (e.g., sinks filled with soapy water in which sharp instruments have been placed). Work-practice controls should include use of a strainer-type basket to hold instruments and forceps to remove the items. Because splashing is likely to occur, a mask, protective eyewear or face shield, and gown or jacket should be worn (13). Preparation and Packaging In another section of the processing area, cleaned instruments and other dental supplies should be inspected, assembled into sets or trays, and wrapped, packaged, or placed into container systems for sterilization. Hinged instruments should be processed open and unlocked. An internal chemical indicator should be placed in every package. In addition, an external chemical indicator (e.g., chemical indicator tape) should be used when the internal indicator cannot be seen from outside the package. For unwrapped loads, at a minimum, an internal chemical indicator should be placed in the tray or cassette with items to be sterilized (254) (see Sterilization of Unwrapped Instruments). Dental practices should refer to the manufacturer's instructions regarding use and correct placement of chemical indicators (see Sterilization Monitoring). Critical and semicritical instruments that will be stored should be wrapped or placed in containers (e.g., cassettes or organizing trays) designed to maintain sterility during storage (2,247,255--257). Packaging materials (e.g., wraps or container systems) allow penetration of the sterilization agent and maintain sterility of the processed item after sterilization. Materials for maintaining sterility of instruments during transport and storage include wrapped perforated instrument cassettes, peel pouches of plastic or paper, and sterilization wraps (i.e., woven and nonwoven). Packaging materials should be designed for the type of sterilization process being used (256--259). Sterilization The sterilization section of the processing area should include the sterilizers and related supplies, with adequate space for loading, unloading, and cool down. The area can also include incubators for analyzing spore tests and enclosed storage for sterile items and disposable (single-use) items (260). Manufacturer and local building code specifications will determine placement and room ventilation requirements. Sterilization Procedures. Heat-tolerant dental instruments usually are sterilized by 1) steam under pressure (autoclaving), 2) dry heat, or 3) unsaturated chemical vapor. All sterilization should be performed by using medical sterilization equipment cleared by FDA. The sterilization times, temperatures, and other operating parameters recommended by the manufacturer of the equipment used, as well as instructions for correct use of containers, wraps, and chemical or biological indicators, should always be followed (243,247). Items to be sterilized should be arranged to permit free circulation of the sterilizing agent (e.g., steam, chemical vapor, or dry heat); manufacturer's instructions for loading the sterilizer should be followed (248,260). Instrument packs should be allowed to dry inside the sterilizer chamber before removing and handling. Packs should not be touched until they are cool and dry because hot packs act as wicks, absorbing moisture, and hence, bacteria from hands (247). The ability of equipment to attain physical parameters required to achieve sterilization should be monitored by mechanical, chemical, and biological indicators. Sterilizers vary in their types of indicators and their ability to provide readings on the mechanical or physical parameters of the sterilization process (e.g., time, temperature, and pressure). Consult with the sterilizer manufacturer regarding selection and use of indicators. Steam Sterilization. Among sterilization methods, steam sterilization, which is dependable and economical, is the most widely used for wrapped and unwrapped critical and semicritical items that are not sensitive to heat and moisture (260). Steam sterilization requires exposure of each item to direct steam contact at a required temperature and pressure for a specified time needed to kill microorganisms. Two basic types of steam sterilizers are the gravity displacement and the high-speed prevacuum sterilizer. The majority of tabletop sterilizers used in a dental practice are gravity displacement sterilizers, although prevacuum sterilizers are becoming more widely available. In gravity displacement sterilizers, steam is admitted through steam lines, a steam generator, or self-generation of steam within the chamber. Unsaturated air is forced out of the chamber through a vent in the chamber wall. Trapping of air is a concern when using saturated steam under gravity displacement; errors in packaging items or overloading the sterilizer chamber can result in cool air pockets and items not being sterilized. Prevacuum sterilizers are fitted with a pump to create a vacuum in the chamber and ensure air removal from the sterilizing chamber before the chamber is pressurized with steam. Relative to gravity displacement, this procedure allows faster and more positive steam penetration throughout the entire load. Prevacuum sterilizers should be tested periodically for adequate air removal, as recommended by the manufacturer. Air not removed from the chamber will interfere with steam contact. If a sterilizer fails the air removal test, it should not be used until inspected by sterilizer maintenance personnel and it passes the test (243,247). Manufacturer's instructions, with specific details regarding operation and user maintenance information, should be followed. Unsaturated Chemical-Vapor Sterilization. Unsaturated chemical-vapor sterilization involves heating a chemical solution of primarily alcohol with 0.23% formaldehyde in a closed pressurized chamber. Unsaturated chemical vapor sterilization of carbon steel instruments (e.g., dental burs) causes less corrosion than steam sterilization because of the low level of water present during the cycle. Instruments should be dry before sterilizing. State and local authorities should be consulted for hazardous waste disposal requirements for the sterilizing solution. Dry-Heat Sterilization. Dry heat is used to sterilize materials that might be damaged by moist heat (e.g., burs and certain orthodontic instruments). Although dry heat has the advantages of low operating cost and being noncorrosive, it is a prolonged process and the high temperatures required are not suitable for certain patient-care items and devices (261). Dry-heat sterilizers used in dentistry include static-air and forced-air types.
Sterilization of Unwrapped Instruments. An unwrapped cycle (sometimes called flash sterilization) is a method for sterilizing unwrapped patient-care items for immediate use. The time required for unwrapped sterilization cycles depends on the type of sterilizer and the type of item (i.e., porous or nonporous) to be sterilized (243). The unwrapped cycle in tabletop sterilizers is preprogrammed by the manufacturer to a specific time and temperature setting and can include a drying phase at the end to produce a dry instrument with much of the heat dissipated. If the drying phase requirements are unclear, the operation manual or manufacturer of the sterilizer should be consulted. If the unwrapped sterilization cycle in a steam sterilizer does not include a drying phase, or has only a minimal drying phase, items retrieved from the sterilizer will be hot and wet, making aseptic transport to the point of use more difficult. For dry-heat and chemical-vapor sterilizers, a drying phase is not required. Unwrapped sterilization should be used only under certain conditions: 1) thorough cleaning and drying of instruments precedes the unwrapped sterilization cycle; 2) mechanical monitors are checked and chemical indicators used for each cycle; 3) care is taken to avoid thermal injury to DHCP or patients; and 4) items are transported aseptically to the point of use to maintain sterility (134,258,262). Because all implantable devices should be quarantined after sterilization until the results of biological monitoring are known, unwrapped or flash sterilization of implantable items is not recommended (134). Critical instruments sterilized unwrapped should be transferred immediately by using aseptic technique, from the sterilizer to the actual point of use. Critical instruments should not be stored unwrapped (260). Semicritical instruments that are sterilized unwrapped on a tray or in a container system should be used immediately or within a short time. When sterile items are open to the air, they will eventually become contaminated. Storage, even temporary, of unwrapped semicritical instruments is discouraged because it permits exposure to dust, airborne organisms, and other unnecessary contamination before use on a patient (260). A carefully written protocol for minimizing the risk of contaminating unwrapped instruments should be prepared and followed (260). Other Sterilization Methods. Heat-sensitive critical and semicritical instruments and devices can be sterilized by immersing them in liquid chemical germicides registered by FDA as sterilants. When using a liquid chemical germicide for sterilization, certain poststerilization procedures are essential. Items need to be 1) rinsed with sterile water after removal to remove toxic or irritating residues; 2) handled using sterile gloves and dried with sterile towels; and 3) delivered to the point of use in an aseptic manner. If stored before use, the instrument should not be considered sterile and should be sterilized again just before use. In addition, the sterilization process with liquid chemical sterilants cannot be verified with biological indicators (263). Because of these limitations and because liquid chemical sterilants can require approximately 12 hours of complete immersion, they are almost never used to sterilize instruments. Rather, these chemicals are more often used for high-level disinfection (249). Shorter immersion times (12--90 minutes) are used to achieve high-level disinfection of semicritical instruments or items. These powerful, sporicidal chemicals (e.g., glutaraldehyde, peracetic acid, and hydrogen peroxide) are highly toxic (244,264,265). Manufacturer instructions (e.g., regarding dilution, immersion time, and temperature) and safety precautions for using chemical sterilants/high-level disinfectants must be followed precisely (15,245). These chemicals should not be used for applications other than those indicated in their label instructions. Misapplications include use as an environmental surface disinfectant or instrument-holding solution. When using appropriate precautions (e.g., closed containers to limit vapor release, chemically resistant gloves and aprons, goggles, and face shields), glutaraldehyde-based products can be used without tissue irritation or adverse health effects. However, dermatologic, eye irritation, respiratory effects, and skin sensitization have been reported (266--268). Because of their lack of chemical resistance to glutaraldehydes, medical gloves are not an effective barrier (200,269,270). Other factors might apply (e.g., room exhaust ventilation or 10 air exchanges/hour) to ensure DHCP safety (266,271). For all of these reasons, using heat-sensitive semicritical items that must be processed with liquid chemical germicides is discouraged; heat-tolerant or disposable alternatives are available for the majority of such items. Low-temperature sterilization with ethylene oxide gas (ETO) has been used extensively in larger health-care facilities. Its primary advantage is the ability to sterilize heat- and moisture-sensitive patient-care items with reduced deleterious effects. However, extended sterilization times of 10--48 hours and potential hazards to patients and DHCP requiring stringent health and safety requirements (272--274) make this method impractical for private-practice settings. Handpieces cannot be effectively sterilized with this method because of decreased penetration of ETO gas flow through a small lumen (250,275). Other types of low-temperature sterilization (e.g., hydrogen peroxide gas plasma) exist but are not yet practical for dental offices. Bead sterilizers have been used in dentistry to sterilize small metallic instruments (e.g., endodontic files). FDA has determined that a risk of infection exists with these devices because of their potential failure to sterilize dental instruments and has required their commercial distribution cease unless the manufacturer files a premarket approval application. If a bead sterilizer is employed, DHCP assume the risk of employing a dental device FDA has deemed neither safe nor effective (276). Sterilization Monitoring. Monitoring of sterilization procedures should include a combination of process parameters, including mechanical, chemical, and biological (247,248,277). These parameters evaluate both the sterilizing conditions and the procedure's effectiveness. Mechanical techniques for monitoring sterilization include assessing cycle time, temperature, and pressure by observing the gauges or displays on the sterilizer and noting these parameters for each load (243,248). Some tabletop sterilizers have recording devices that print out these parameters. Correct readings do not ensure sterilization, but incorrect readings can be the first indication of a problem with the sterilization cycle. Chemical indicators, internal and external, use sensitive chemicals to assess physical conditions (e.g., time and temperature) during the sterilization process. Although chemical indicators do not prove sterilization has been achieved, they allow detection of certain equipment malfunctions, and they can help identify procedural errors. External indicators applied to the outside of a package (e.g., chemical indicator tape or special markings) change color rapidly when a specific parameter is reached, and they verify that the package has been exposed to the sterilization process. Internal chemical indicators should be used inside each package to ensure the sterilizing agent has penetrated the packaging material and actually reached the instruments inside. A single-parameter internal chemical indicator provides information regarding only one sterilization parameter (e.g., time or temperature). Multiparameter internal chemical indicators are designed to react to >2 parameters (e.g., time and temperature; or time, temperature, and the presence of steam) and can provide a more reliable indication that sterilization conditions have been met (254). Multiparameter internal indicators are available only for steam sterilizers (i.e., autoclaves). Because chemical indicator test results are received when the sterilization cycle is complete, they can provide an early indication of a problem and where in the process the problem might exist. If either mechanical indicators or internal or external chemical indicators indicate inadequate processing, items in the load should not be used until reprocessed (134). Biological indicators (BIs) (i.e., spore tests) are the most accepted method for monitoring the sterilization process (278,279) because they assess it directly by killing known highly resistant microorganisms (e.g., Geobacillus or Bacillus species), rather than merely testing the physical and chemical conditions necessary for sterilization (243). Because spores used in BIs are more resistant and present in greater numbers than the common microbial contaminants found on patient-care equipment, an inactivated BI indicates other potential pathogens in the load have been killed (280). Correct functioning of sterilization cycles should be verified for each sterilizer by the periodic use (at least weekly) of BIs (2,9,134,243,278,279). Every load containing implantable devices should be monitored with such indicators (248), and the items quarantined until BI results are known. However, in an emergency, placing implantable items in quarantine until spore tests are known to be negative might be impossible. Manufacturer's directions should determine the placement and location of BI in the sterilizer. A control BI, from the same lot as the test indicator and not processed through the sterilizer, should be incubated with the test BI; the control BI should yield positive results for bacterial growth. In-office biological monitoring is available; mail-in sterilization monitoring services (e.g., from private companies or dental schools) can also be used to test both the BI and the control. Although some DHCP have expressed concern that delays caused by mailing specimens might cause false-negatives, studies have determined that mail delays have no substantial effect on final test results (281,282). Procedures to follow in the event of a positive spore test have been developed (243,247). If the mechanical (e.g., time, temperature, and pressure) and chemical (i.e., internal or external) indicators demonstrate that the sterilizer is functioning correctly, a single positive spore test probably does not indicate sterilizer malfunction. Items other than implantable devices do not necessarily need to be recalled; however the spore test should be repeated immediately after correctly loading the sterilizer and using the same cycle that produced the failure. The sterilizer should be removed from service, and all records reviewed of chemical and mechanical monitoring since the last negative BI test. Also, sterilizer operating procedures should be reviewed, including packaging, loading, and spore testing, with all persons who work with the sterilizer to determine whether operator error could be responsible (9,243,247). Overloading, failure to provide adequate package separation, and incorrect or excessive packaging material are all common reasons for a positive BI in the absence of mechanical failure of the sterilizer unit (260). A second monitored sterilizer in the office can be used, or a loaner from a sales or repair company obtained, to minimize office disruption while waiting for the repeat BI. If the repeat test is negative and chemical and mechanical monitoring indicate adequate processing, the sterilizer can be put back into service. If the repeat BI test is positive, and packaging, loading, and operating procedures have been confirmed as performing correctly, the sterilizer should remain out of service until it has been inspected, repaired, and rechallenged with BI tests in three consecutive empty chamber sterilization cycles (9,243). When possible, items from suspect loads dating back to the last negative BI should be recalled, rewrapped, and resterilized (9,283). A more conservative approach has been recommended (247) in which any positive spore test is assumed to represent sterilizer malfunction and requires that all materials processed in that sterilizer, dating from the sterilization cycle having the last negative biologic indicator to the next cycle indicating satisfactory biologic indicator results, should be considered nonsterile and retrieved, if possible, and reprocessed or held in quarantine until the results of the repeat BI are known. This approach is considered conservative because the margin of safety in steam sterilization is sufficient enough that infection risk, associated with items in a load indicating spore growth, is minimal, particularly if the item was properly cleaned and the temperature was achieved (e.g., as demonstrated by acceptable chemical indicator or temperature chart) (243). Published studies are not available that document disease transmission through a nonretrieved surgical instrument after a steam sterilization cycle with a positive biological indicator (243). This more conservative approach should always be used for sterilization methods other than steam (e.g., dry heat, unsaturated chemical vapor, ETO, or hydrogen peroxide gas plasma) (243). Results of biological monitoring should be recorded and sterilization monitoring records (i.e., mechanical, chemical, and biological) retained long enough to comply with state and local regulations. Such records are a component of an overall dental infection-control program (see Program Evaluation). Storage of Sterilized Items and Clean Dental Supplies The storage area should contain enclosed storage for sterile items and disposable (single-use) items (173). Storage practices for wrapped sterilized instruments can be either date- or event-related. Packages containing sterile supplies should be inspected before use to verify barrier integrity and dryness. Although some health-care facilities continue to date every sterilized package and use shelf-life practices, other facilities have switched to event-related practices (243). This approach recognizes that the product should remain sterile indefinitely, unless an event causes it to become contaminated (e.g., torn or wet packaging) (284). Even for event-related packaging, minimally, the date of sterilization should be placed on the package, and if multiple sterilizers are used in the facility, the sterilizer used should be indicated on the outside of the packaging material to facilitate the retrieval of processed items in the event of a sterilization failure (247). If packaging is compromised, the instruments should be recleaned, packaged in new wrap, and sterilized again. Clean supplies and instruments should be stored in closed or covered cabinets, if possible (285). Dental supplies and instruments should not be stored under sinks or in other locations where they might become wet. Environmental Infection ControlIn the dental operatory, environmental surfaces (i.e., a surface or equipment that does not contact patients directly) can become contaminated during patient care. Certain surfaces, especially ones touched frequently (e.g., light handles, unit switches, and drawer knobs) can serve as reservoirs of microbial contamination, although they have not been associated directly with transmission of infection to either DHCP or patients. Transfer of microorganisms from contaminated environmental surfaces to patients occurs primarily through DHCP hand contact (286,287). When these surfaces are touched, microbial agents can be transferred to instruments, other environmental surfaces, or to the nose, mouth, or eyes of workers or patients. Although hand hygiene is key to minimizing this transferal, barrier protection or cleaning and disinfecting of environmental surfaces also protects against health-care--associated infections. Environmental surfaces can be divided into clinical contact surfaces and housekeeping surfaces (249). Because housekeeping surfaces (e.g., floors, walls, and sinks) have limited risk of disease transmission, they can be decontaminated with less rigorous methods than those used on dental patient-care items and clinical contact surfaces (244). Strategies for cleaning and disinfecting surfaces in patient-care areas should consider the 1) potential for direct patient contact; 2) degree and frequency of hand contact; and 3) potential contamination of the surface with body substances or environmental sources of microorganisms (e.g., soil, dust, or water). Cleaning is the necessary first step of any disinfection process. Cleaning is a form of decontamination that renders the environmental surface safe by removing organic matter, salts, and visible soils, all of which interfere with microbial inactivation. The physical action of scrubbing with detergents and surfactants and rinsing with water removes substantial numbers of microorganisms. If a surface is not cleaned first, the success of the disinfection process can be compromised. Removal of all visible blood and inorganic and organic matter can be as critical as the germicidal activity of the disinfecting agent (249). When a surface cannot be cleaned adequately, it should be protected with barriers (2). Clinical Contact Surfaces Clinical contact surfaces can be directly contaminated from patient materials either by direct spray or spatter generated during dental procedures or by contact with DHCP's gloved hands. These surfaces can subsequently contaminate other instruments, devices, hands, or gloves. Examples of such surfaces include
Barrier protection of surfaces and equipment can prevent contamination of clinical contact surfaces, but is particularly effective for those that are difficult to clean. Barriers include clear plastic wrap, bags, sheets, tubing, and plastic-backed paper or other materials impervious to moisture (260,288). Because such coverings can become contaminated, they should be removed and discarded between patients, while DHCP are still gloved. After removing the barrier, examine the surface to make sure it did not become soiled inadvertently. The surface needs to be cleaned and disinfected only if contamination is evident. Otherwise, after removing gloves and performing hand hygiene, DHCP should place clean barriers on these surfaces before the next patient (1,2,288). If barriers are not used, surfaces should be cleaned and disinfected between patients by using an EPA-registered hospital disinfectant with an HIV, HBV claim (i.e., low-level disinfectant) or a tuberculocidal claim (i.e., intermediate-level disinfectant). Intermediate-level disinfectant should be used when the surface is visibly contaminated with blood or OPIM (2,244). Also, general cleaning and disinfection are recommended for clinical contact surfaces, dental unit surfaces, and countertops at the end of daily work activities and are required if surfaces have become contaminated since their last cleaning (13). To facilitate daily cleaning, treatment areas should be kept free of unnecessary equipment and supplies. Manufacturers of dental devices and equipment should provide information regarding material compatibility with liquid chemical germicides, whether equipment can be safely immersed for cleaning, and how it should be decontaminated if servicing is required (289). Because of the risks associated with exposure to chemical disinfectants and contaminated surfaces, DHCP who perform environmental cleaning and disinfection should wear gloves and other PPE to prevent occupational exposure to infectious agents and hazardous chemicals. Chemical- and puncture-resistant utility gloves offer more protection than patient examination gloves when using hazardous chemicals. Housekeeping Surfaces Evidence does not support that housekeeping surfaces (e.g., floors, walls, and sinks) pose a risk for disease transmission in dental health-care settings. Actual, physical removal of microorganisms and soil by wiping or scrubbing is probably as critical, if not more so, than any antimicrobial effect provided by the agent used (244,290). The majority of housekeeping surfaces need to be cleaned only with a detergent and water or an EPA-registered hospital disinfectant/detergent, depending on the nature of the surface and the type and degree of contamination. Schedules and methods vary according to the area (e.g., dental operatory, laboratory, bathrooms, or reception rooms), surface, and amount and type of contamination. Floors should be cleaned regularly, and spills should be cleaned up promptly. An EPA-registered hospital disinfectant/detergent designed for general housekeeping purposes should be used in patient-care areas if uncertainty exists regarding the nature of the soil on the surface (e.g., blood or body fluid contamination versus routine dust or dirt). Unless contamination is reasonably anticipated or apparent, cleaning or disinfecting walls, window drapes, and other vertical surfaces is unnecessary. However, when housekeeping surfaces are visibly contaminated by blood or OPIM, prompt removal and surface disinfection is appropriate infection-control practice and required by OSHA (13). Part of the cleaning strategy is to minimize contamination of cleaning solutions and cleaning tools (e.g., mop heads or cleaning cloths). Mops and cloths should be cleaned after use and allowed to dry before reuse, or single-use, disposable mop heads and cloths should be used to avoid spreading contamination. Cost, safety, product-surface compatibility, and acceptability by housekeepers can be key criteria for selecting a cleaning agent or an EPA-registered hospital disinfectant/detergent. PPE used during cleaning and housekeeping procedures followed should be appropriate to the task. In the cleaning process, another reservoir for microorganisms can be dilute solutions of detergents or disinfectants, especially if prepared in dirty containers, stored for long periods of time, or prepared incorrectly (244). Manufacturers' instructions for preparation and use should be followed. Making fresh cleaning solution each day, discarding any remaining solution, and allowing the container to dry will minimize bacterial contamination. Preferred cleaning methods produce minimal mists and aerosols or dispersion of dust in patient-care areas. Cleaning and Disinfection Strategies for Blood Spills The majority of blood contamination events in dentistry result from spatter during dental procedures using rotary or ultrasonic instrumentation. Although no evidence supports that HBV, HCV, or HIV have been transmitted from a housekeeping surface, prompt removal and surface disinfection of an area contaminated by either blood or OPIM are appropriate infection-control practices and required by OSHA (13,291). Strategies for decontaminating spills of blood and other body fluids differ by setting and volume of the spill (113,244). Blood spills on either clinical contact or housekeeping surfaces should be contained and managed as quickly as possible to reduce the risk of contact by patients and DHCP (244,292). The person assigned to clean the spill should wear gloves and other PPE as needed. Visible organic material should be removed with absorbent material (e.g., disposable paper towels discarded in a leak-proof, appropriately labeled container). Nonporous surfaces should be cleaned and then decontaminated with either an EPA-registered hospital disinfectant effective against HBV and HIV or an EPA-registered hospital disinfectant with a tuberculocidal claim (i.e., intermediate-level disinfectant). If sodium hypochlorite is chosen, an EPA-registered sodium hypochlorite product is preferred. However, if such products are unavailable, a 1:100 dilution of sodium hypochlorite (e.g., approximately ¼ cup of 5.25% household chlorine bleach to 1 gallon of water) is an inexpensive and effective disinfecting agent (113). Carpeting and Cloth Furnishings Carpeting is more difficult to clean than nonporous hard-surface flooring, and it cannot be reliably disinfected, especially after spills of blood and body substances. Studies have documented the presence of diverse microbial populations, primarily bacteria and fungi, in carpeting (293--295). Cloth furnishings pose similar contamination risks in areas of direct patient care and places where contaminated materials are managed (e.g., dental operatory, laboratory, or instrument processing areas). For these reasons, use of carpeted flooring and fabric-upholstered furnishings in these areas should be avoided. Nonregulated and Regulated Medical Waste Studies have compared microbial load and diversity of microorganisms in residential waste with waste from multiple health-care settings. General waste from hospitals or other health-care facilities (e.g., dental practices or clinical/research laboratories) is no more infective than residential waste (296,297). The majority of soiled items in dental offices are general medical waste and thus can be disposed of with ordinary waste. Examples include used gloves, masks, gowns, lightly soiled gauze or cotton rolls, and environmental barriers (e.g., plastic sheets or bags) used to cover equipment during treatment (298). Although any item that has had contact with blood, exudates, or secretions might be infective, treating all such waste as infective is neither necessary nor practical (244). Infectious waste that carries a substantial risk of causing infection during handling and disposal is regulated medical waste. A complete definition of regulated waste is included in OSHA's bloodborne pathogens standard (13). Regulated medical waste is only a limited subset of waste: 9%--15% of total waste in hospitals and 1%--2% of total waste in dental offices (298,299). Regulated medical waste requires special storage, handling, neutralization, and disposal and is covered by federal, state, and local rules and regulations (6,297,300,301). Examples of regulated waste found in dental-practice settings are solid waste soaked or saturated with blood or saliva (e.g., gauze saturated with blood after surgery), extracted teeth, surgically removed hard and soft tissues, and contaminated sharp items (e.g., needles, scalpel blades, and wires) (13). Regulated medical waste requires careful containment for treatment or disposal. A single leak-resistant biohazard bag is usually adequate for containment of nonsharp regulated medical waste, provided the bag is sturdy and the waste can be discarded without contaminating the bag's exterior. Exterior contamination or puncturing of the bag requires placement in a second biohazard bag. All bags should be securely closed for disposal. Puncture-resistant containers with a biohazard label, located at the point of use (i.e., sharps containers), are used as containment for scalpel blades, needles, syringes, and unused sterile sharps (13). Dental health-care facilities should dispose of medical waste regularly to avoid accumulation. Any facility generating regulated medical waste should have a plan for its management that complies with federal, state, and local regulations to ensure health and environmental safety. Discharging Blood or Other Body Fluids to Sanitary Sewers or Septic Tanks All containers with blood or saliva (e.g., suctioned fluids) can be inactivated in accordance with state-approved treatment technologies, or the contents can be carefully poured down a utility sink, drain, or toilet (6). Appropriate PPE (e.g., gloves, gown, mask, and protective eyewear) should be worn when performing this task (13). No evidence exists that bloodborne diseases have been transmitted from contact with raw or treated sewage. Multiple bloodborne pathogens, particularly viruses, are not stable in the environment for long periods (302), and the discharge of limited quantities of blood and other body fluids into the sanitary sewer is considered a safe method for disposing of these waste materials (6). State and local regulations vary and dictate whether blood or other body fluids require pretreatment or if they can be discharged into the sanitary sewer and in what volume. Dental Unit Waterlines, Biofilm, and Water QualityStudies have demonstrated that dental unit waterlines (i.e., narrow-bore plastic tubing that carries water to the high-speed handpiece, air/water syringe, and ultrasonic scaler) can become colonized with microorganisms, including bacteria, fungi, and protozoa (303--309). Protected by a polysaccharide slime layer known as a glycocalyx, these microorganisms colonize and replicate on the interior surfaces of the waterline tubing and form a biofilm, which serves as a reservoir that can amplify the number of free-floating (i.e., planktonic) microorganisms in water used for dental treatment. Although oral flora (303,310,311) and human pathogens (e.g., Pseudomonas aeruginosa [303,305,312,313], Legionella species [303,306,313], and nontuberculous Mycobacterium species [303,304]), have been isolated from dental water systems, the majority of organisms recovered from dental waterlines are common heterotrophic water bacteria (305,314,315). These exhibit limited pathogenic potential for immunocompetent persons. Clinical Implications Certain reports associate waterborne infections with dental water systems, and scientific evidence verifies the potential for transmission of waterborne infections and disease in hospital settings and in the community (306,312,316). Infection or colonization caused by Pseudomonas species or nontuberculous mycobacteria can occur among susceptible patients through direct contact with water (317--320) or after exposure to residual waterborne contamination of inadequately reprocessed medical instruments (321--323). Nontuberculous mycobacteria can also be transmitted to patients from tap water aerosols (324). Health-care--associated transmission of pathogenic agents (e.g., Legionella species) occurs primarily through inhalation of infectious aerosols generated from potable water sources or through use of tap water in respiratory therapy equipment (325--327). Disease outbreaks in the community have also been reported from diverse environmental aerosol-producing sources, including whirlpool spas (328), swimming pools (329), and a grocery store mist machine (330). Although the majority of these outbreaks are associated with species of Legionella and Pseudomonas (329), the fungus Cladosporium (331) has also been implicated. Researchers have not demonstrated a measurable risk of adverse health effects among DHCP or patients from exposure to dental water. Certain studies determined DHCP had altered nasal flora (332) or substantially greater titers of Legionella antibodies in comparisons with control populations; however, no cases of legionellosis were identified among exposed DHCP (333,334). Contaminated dental water might have been the source for localized Pseudomonas aeruginosa infections in two immunocompromised patients (312). Although transient carriage of P. aeruginosa was observed in 78 healthy patients treated with contaminated dental treatment water, no illness was reported among the group. In this same study, a retrospective review of dental records also failed to identify infections (312). Concentrations of bacterial endotoxin <1,000 endotoxin units/mL from gram-negative water bacteria have been detected in water from colonized dental units (335). No standards exist for an acceptable level of endotoxin in drinking water, but the maximum level permissible in United States Pharmacopeia (USP) sterile water for irrigation is only 0.25 endotoxin units/mL (336). Although the consequences of acute and chronic exposure to aerosolized endotoxin in dental health-care settings have not been investigated, endotoxin has been associated with exacerbation of asthma and onset of hypersensitivity pneumonitis in other occupational settings (329,337). Dental Unit Water Quality Research has demonstrated that microbial counts can reach <200,000 colony-forming units (CFU)/mL within 5 days after installation of new dental unit waterlines (305), and levels of microbial contamination <106 CFU/mL of dental unit water have been documented (309,338). These counts can occur because dental unit waterline factors (e.g., system design, flow rates, and materials) promote both bacterial growth and development of biofilm. Although no epidemiologic evidence indicates a public health problem, the presence of substantial numbers of pathogens in dental unit waterlines generates concern. Exposing patients or DHCP to water of uncertain microbiological quality, despite the lack of documented adverse health effects, is inconsistent with accepted infection-control principles. Thus in 1995, ADA addressed the dental water concern by asking manufacturers to provide equipment with the ability to deliver treatment water with <200 CFU/mL of unfiltered output from waterlines (339). This threshold was based on the quality assurance standard established for dialysate fluid, to ensure that fluid delivery systems in hemodialysis units have not been colonized by indigenous waterborne organisms (340). Standards also exist for safe drinking water quality as established by EPA, the American Public Health Association (APHA), and the American Water Works Association (AWWA); they have set limits for heterotrophic bacteria of <500 CFU/mL of drinking water (341,342). Thus, the number of bacteria in water used as a coolant/irrigant for nonsurgical dental procedures should be as low as reasonably achievable and, at a minimum, <500 CFU/mL, the regulatory standard for safe drinking water established by EPA and APHA/AWWA. Strategies To Improve Dental Unit Water Quality In 1993, CDC recommended that dental waterlines be flushed at the beginning of the clinic day to reduce the microbial load (2). However, studies have demonstrated this practice does not affect biofilm in the waterlines or reliably improve the quality of water used during dental treatment (315,338,343). Because the recommended value of <500 CFU/mL cannot be achieved by using this method, other strategies should be employed. Dental unit water that remains untreated or unfiltered is unlikely to meet drinking water standards (303--309). Commercial devices and procedures designed to improve the quality of water used in dental treatment are available (316); methods demonstrated to be effective include self-contained water systems combined with chemical treatment, in-line microfilters, and combinations of these treatments. Simply using source water containing <500 CFU/mL of bacteria (e.g., tap, distilled, or sterile water) in a self-contained water system will not eliminate bacterial contamination in treatment water if biofilms in the water system are not controlled. Removal or inactivation of dental waterline biofilms requires use of chemical germicides. Patient material (e.g., oral microorganisms, blood, and saliva) can enter the dental water system during patient treatment (311,344). Dental devices that are connected to the dental water system and that enter the patient's mouth (e.g., handpieces, ultrasonic scalers, or air/water syringes) should be operated to discharge water and air for a minimum of 20--30 seconds after each patient (2). This procedure is intended to physically flush out patient material that might have entered the turbine, air, or waterlines. The majority of recently manufactured dental units are engineered to prevent retraction of oral fluids, but some older dental units are equipped with antiretraction valves that require periodic maintenance. Users should consult the owner's manual or contact the manufacturer to determine whether testing or maintenance of antiretraction valves or other devices is required. Even with antiretraction valves, flushing devices for a minimum of 20--30 seconds after each patient is recommended. Maintenance and Monitoring of Dental Unit Water DHCP should be trained regarding water quality, biofilm formation, water treatment methods, and appropriate maintenance protocols for water delivery systems. Water treatment and monitoring products require strict adherence to maintenance protocols, and noncompliance with treatment regimens has been associated with persistence of microbial contamination in treated systems (345). Clinical monitoring of water quality can ensure that procedures are correctly performed and that devices are working in accordance with the manufacturer's previously validated protocol. Dentists should consult with the manufacturer of their dental unit or water delivery system to determine the best method for maintaining acceptable water quality (i.e., <500 CFU/mL) and the recommended frequency of monitoring. Monitoring of dental water quality can be performed by using commercial self-contained test kits or commercial water-testing laboratories. Because methods used to treat dental water systems target the entire biofilm, no rationale exists for routine testing for such specific organisms as Legionella or Pseudomonas, except when investigating a suspected waterborne disease outbreak (244). Delivery of Sterile Surgical Irrigation Sterile solutions (e.g., sterile saline or sterile water) should be used as a coolant/irrigation in the performance of oral surgical procedures where a greater opportunity exists for entry of microorganisms, exogenous and endogenous, into the vascular system and other normally sterile areas that support the oral cavity (e.g., bone or subcutaneous tissue) and increased potential exists for localized or systemic infection (see Oral Surgical Procedures). Conventional dental units cannot reliably deliver sterile water even when equipped with independent water reservoirs because the water-bearing pathway cannot be reliably sterilized. Delivery devices (e.g., bulb syringe or sterile, single-use disposable products) should be used to deliver sterile water (2,121). Oral surgery and implant handpieces, as well as ultrasonic scalers, are commercially available that bypass the dental unit to deliver sterile water or other solutions by using single-use disposable or sterilizable tubing (316). Boil-Water Advisories A boil-water advisory is a public health announcement that the public should boil tap water before drinking it. When issued, the public should assume the water is unsafe to drink. Advisories can be issued after 1) failure of or substantial interruption in water treatment processes that result in increased turbidity levels or particle counts and mechanical or equipment failure; 2) positive test results for pathogens (e.g., Cryptosporidium, Giardia, or Shigella) in water; 3) violations of the total coliform rule or the turbidity standard of the surface water treatment rule; 4) circumstances that compromise the distribution system (e.g., watermain break) coupled with an indication of a health hazard; or 5) a natural disaster (e.g., flood, hurricane, or earthquake) (346). In recent years, increased numbers of boil-water advisories have resulted from contamination of public drinking water systems with waterborne pathogens. Most notable was the outbreak of cryptosporidiosis in Milwaukee, Wisconsin, where the municipal water system was contaminated with the protozoan parasite Cryptosporidium parvum. An estimated 403,000 persons became ill (347,348). During a boil-water advisory, water should not be delivered to patients through the dental unit, ultrasonic scaler, or other dental equipment that uses the public water system. This restriction does not apply if the water source is isolated from the municipal water system (e.g., a separate water reservoir or other water treatment device cleared for marketing by FDA). Patients should rinse with bottled or distilled water until the boil-water advisory has been cancelled. During these advisory periods, tap water should not be used to dilute germicides or for hand hygiene unless the water has been brought to a rolling boil for >1 minute and cooled before use (346,349--351). For hand hygiene, antimicrobial products that do not require water (e.g., alcohol-based hand rubs) can be used until the boil-water notice is cancelled. If hands are visibly contaminated, bottled water and soap should be used for handwashing; if bottled water is not immediately available, an antiseptic towelette should be used (13,122). When the advisory is cancelled, the local water utility should provide guidance for flushing of waterlines to reduce residual microbial contamination. All incoming waterlines from the public water system inside the dental office (e.g., faucets, waterlines, and dental equipment) should be flushed. No consensus exists regarding the optimal duration for flushing procedures after cancellation of the advisory; recommendations range from 1 to 5 minutes (244,346,351,352). The length of time needed can vary with the type and length of the plumbing system leading to the office. After the incoming public water system lines are flushed, dental unit waterlines should be disinfected according to the manufacturer's instructions (346). Special ConsiderationsDental Handpieces and Other Devices Attached to Air and WaterlinesMultiple semicritical dental devices that touch mucous membranes are attached to the air or waterlines of the dental unit. Among these devices are high- and low-speed handpieces, prophylaxis angles, ultrasonic and sonic scaling tips, air abrasion devices, and air and water syringe tips. Although no epidemiologic evidence implicates these instruments in disease transmission (353), studies of high-speed handpieces using dye expulsion have confirmed the potential for retracting oral fluids into internal compartments of the device (354--358). This determination indicates that retained patient material can be expelled intraorally during subsequent uses. Studies using laboratory models also indicate the possibility for retention of viral DNA and viable virus inside both high-speed handpieces and prophylaxis angles (356,357,359). The potential for contamination of the internal surfaces of other devices (e.g., low-speed handpieces and ultrasonic scalers), has not been studied, but restricted physical access limits their cleaning. Accordingly, any dental device connected to the dental air/water system that enters the patient's mouth should be run to discharge water, air, or a combination for a minimum of 20--30 seconds after each patient (2). This procedure is intended to help physically flush out patient material that might have entered the turbine and air and waterlines (2,356,357). Heat methods can sterilize dental handpieces and other intraoral devices attached to air or waterlines (246,275,356, 357,360). For processing any dental device that can be removed from the dental unit air or waterlines, neither surface disinfection nor immersion in chemical germicides is an acceptable method. Ethylene oxide gas cannot adequately sterilize internal components of handpieces (250,275). In clinical evaluations of high-speed handpieces, cleaning and lubrication were the most critical factors in determining performance and durability (361--363). Manufacturer's instructions for cleaning, lubrication, and sterilization should be followed closely to ensure both the effectiveness of the process and the longevity of handpieces. Some components of dental instruments are permanently attached to dental unit waterlines and although they do not enter the patient's oral cavity, they are likely to become contaminated with oral fluids during treatment procedures. Such components (e.g., handles or dental unit attachments of saliva ejectors, high-speed air evacuators, and air/water syringes) should be covered with impervious barriers that are changed after each use. If the item becomes visibly contaminated during use, DHCP should clean and disinfect with an EPA-registered hospital disinfectant (intermediate-level) before use on the next patient. Saliva EjectorsBackflow from low-volume saliva ejectors occurs when the pressure in the patient's mouth is less than that in the evacuator. Studies have reported that backflow in low-volume suction lines can occur and microorganisms be present in the lines retracted into the patient's mouth when a seal around the saliva ejector is created (e.g., by a patient closing lips around the tip of the ejector, creating a partial vacuum) (364--366). This backflow can be a potential source of cross-contamination; occurrence is variable because the quality of the seal formed varies between patients. Furthermore, studies have demonstrated that gravity pulls fluid back toward the patient's mouth whenever a length of the suction tubing holding the tip is positioned above the patient's mouth, or during simultaneous use of other evacuation (high-volume) equipment (364--366). Although no adverse health effects associated with the saliva ejector have been reported, practitioners should be aware that in certain situations, backflow could occur when using a saliva ejector. Dental RadiologyWhen taking radiographs, the potential to cross-contaminate equipment and environmental surfaces with blood or saliva is high if aseptic technique is not practiced. Gloves should be worn when taking radiographs and handling contaminated film packets. Other PPE (e.g., mask, protective eyewear, and gowns) should be used if spattering of blood or other body fluids is likely (11,13,367). Heat-tolerant versions of intraoral radiograph accessories are available and these semicritical items (e.g., film-holding and positioning devices) should be heat-sterilized before patient use. After exposure of the radiograph and before glove removal, the film should be dried with disposable gauze or a paper towel to remove blood or excess saliva and placed in a container (e.g., disposable cup) for transport to the developing area. Alternatively, if FDA-cleared film barrier pouches are used, the film packets should be carefully removed from the pouch to avoid contamination of the outside film packet and placed in the clean container for transport to the developing area. Various methods have been recommended for aseptic transport of exposed films to the developing area, and for removing the outer film packet before exposing and developing the film. Other information regarding dental radiography infection control is available (260,367,368). However, care should be taken to avoid contamination of the developing equipment. Protective barriers should be used, or any surfaces that become contaminated should be cleaned and disinfected with an EPA-registered hospital disinfectant of low- (i.e., HIV and HBV claim) to intermediate-level (i.e., tuberculocidal claim) activity. Radiography equipment (e.g., radiograph tubehead and control panel) should be protected with surface barriers that are changed after each patient. If barriers are not used, equipment that has come into contact with DHCP's gloved hands or contaminated film packets should be cleaned and then disinfected after each patient use. Digital radiography sensors and other high-technology instruments (e.g., intraoral camera, electronic periodontal probe, occlusal analyzers, and lasers) come into contact with mucous membranes and are considered semicritical devices. They should be cleaned and ideally heat-sterilized or high-level disinfected between patients. However, these items vary by manufacturer or type of device in their ability to be sterilized or high-level disinfected. Semicritical items that cannot be reprocessed by heat sterilization or high-level disinfection should, at a minimum, be barrier protected by using an FDA-cleared barrier to reduce gross contamination during use. Use of a barrier does not always protect from contamination (369--374). One study determined that a brand of commercially available plastic barriers used to protect dental digital radiography sensors failed at a substantial rate (44%). This rate dropped to 6% when latex finger cots were used in conjunction with the plastic barrier (375). To minimize the potential for device-associated infections, after removing the barrier, the device should be cleaned and disinfected with an EPA-registered hospital disinfectant (intermediate-level) after each patient. Manufacturers should be consulted regarding appropriate barrier and disinfection/sterilization procedures for digital radiography sensors, other high-technology intraoral devices, and computer components. Aseptic Technique for Parenteral MedicationsSafe handling of parenteral medications and fluid infusion systems is required to prevent health-care--associated infections among patients undergoing conscious sedation. Parenteral medications can be packaged in single-dose ampules, vials or prefilled syringes, usually without bacteriostatic/preservative agents, and intended for use on a single patient. Multidose vials, used for more than one patient, can have a preservative, but both types of containers of medication should be handled with aseptic techniques to prevent contamination. Single-dose vials should be used for parenteral medications whenever possible (376,377). Single-dose vials might pose a risk for contamination if they are punctured repeatedly. The leftover contents of a single-dose vial should be discarded and never combined with medications for use on another patient (376,377). Medication from a single-dose syringe should not be administered to multiple patients, even if the needle on the syringe is changed (378). The overall risk for extrinsic contamination of multidose vials is probably minimal, although the consequences of contamination might result in life-threatening infection (379). If necessary to use a multidose vial, its access diaphragm should be cleansed with 70% alcohol before inserting a sterile device into the vial (380,381). A multidose vial should be discarded if sterility is compromised (380,381). Medication vials, syringes, or supplies should not be carried in uniform or clothing pockets. If trays are used to deliver medications to individual patients, they should be cleaned between patients. To further reduce the chance of contamination, all medication vials should be restricted to a centralized medication preparation area separate from the treatment area (382). All fluid infusion and administration sets (e.g., IV bags, tubing, and connections) are single-patient use because sterility cannot be guaranteed when an infusion or administration set is used on multiple patients. Aseptic technique should be used when preparing IV infusion and administration sets, and entry into or breaks in the tubing should be minimized (378). Single-Use or Disposable DevicesA single-use device, also called a disposable device, is designed to be used on one patient and then discarded, not reprocessed for use on another patient (e.g., cleaned, disinfected, or sterilized) (383). Single-use devices in dentistry are usually not heat-tolerant and cannot be reliably cleaned. Examples include syringe needles, prophylaxis cups and brushes, and plastic orthodontic brackets. Certain items (e.g., prophylaxis angles, saliva ejectors, high-volume evacuator tips, and air/water syringe tips) are commonly available in a disposable form and should be disposed of appropriately after each use. Single-use devices and items (e.g., cotton rolls, gauze, and irrigating syringes) for use during oral surgical procedures should be sterile at the time of use. Because of the physical construction of certain devices (e.g., burs, endodontic files, and broaches) cleaning can be difficult. In addition, deterioration can occur on the cutting surfaces of some carbide/diamond burs and endodontic files during processing (384) and after repeated processing cycles, leading to potential breakage during patient treatment (385--388). These factors, coupled with the knowledge that burs and endodontic instruments exhibit signs of wear during normal use, might make it practical to consider them as single-use devices. Preprocedural Mouth RinsesAntimicrobial mouth rinses used by patients before a dental procedure are intended to reduce the number of microorganisms the patient might release in the form of aerosols or spatter that subsequently can contaminate DHCP and equipment operatory surfaces. In addition, preprocedural rinsing can decrease the number of microorganisms introduced in the patient's bloodstream during invasive dental procedures (389,390). No scientific evidence indicates that preprocedural mouth rinsing prevents clinical infections among DHCP or patients, but studies have demonstrated that a preprocedural rinse with an antimicrobial product (e.g., chlorhexidine gluconate, essential oils, or povidone-iodine) can reduce the level of oral microorganisms in aerosols and spatter generated during routine dental procedures with rotary instruments (e.g., dental handpieces or ultrasonic scalers) (391--399). Preprocedural mouth rinses can be most beneficial before a procedure that requires using a prophylaxis cup or ultrasonic scaler because rubber dams cannot be used to minimize aerosol and spatter generation and, unless the provider has an assistant, high-volume evacuation is not commonly used (173). The science is unclear concerning the incidence and nature of bacteremias from oral procedures, the relationship of these bacteremias to disease, and the preventive benefit of antimicrobial rinses. In limited studies, no substantial benefit has been demonstrated for mouth rinsing in terms of reducing oral microorganisms in dental-induced bacteremias (400,401). However, the American Heart Association's recommendations regarding preventing bacterial endocarditis during dental procedures (402) provide limited support concerning preprocedural mouth rinsing with an antimicrobial as an adjunct for patients at risk for bacterial endocarditis. Insufficient data exist to recommend preprocedural mouth rinses to prevent clinical infections among patients or DHCP. Oral Surgical ProceduresThe oral cavity is colonized with numerous microorganisms. Oral surgical procedures present an opportunity for entry of microorganisms (i.e., exogenous and endogenous) into the vascular system and other normally sterile areas of the oral cavity (e.g., bone or subcutaneous tissue); therefore, an increased potential exists for localized or systemic infection. Oral surgical procedures involve the incision, excision, or reflection of tissue that exposes the normally sterile areas of the oral cavity. Examples include biopsy, periodontal surgery, apical surgery, implant surgery, and surgical extractions of teeth (e.g., removal of erupted or nonerupted tooth requiring elevation of mucoperiosteal flap, removal of bone or section of tooth, and suturing if needed) (see Hand Hygiene, PPE, Single Use or Disposable Devices, and Dental Unit Water Quality). Handling of Biopsy SpecimensTo protect persons handling and transporting biopsy specimens, each specimen must be placed in a sturdy, leakproof container with a secure lid for transportation (13). Care should be taken when collecting the specimen to avoid contaminating the outside of the container. If the outside of the container becomes visibly contaminated, it should be cleaned and disinfected or placed in an impervious bag (2,13). The container must be labeled with the biohazard symbol during storage, transport, shipment, and disposal (13,14). Handling of Extracted TeethDisposal Extracted teeth that are being discarded are subject to the containerization and labeling provisions outlined by OSHA's bloodborne pathogens standard (13). OSHA considers extracted teeth to be potentially infectious material that should be disposed in medical waste containers. Extracted teeth sent to a dental laboratory for shade or size comparisons should be cleaned, surface-disinfected with an EPA-registered hospital disinfectant with intermediate-level activity (i.e., tuberculocidal claim), and transported in a manner consistent with OSHA regulations. However, extracted teeth can be returned to patients on request, at which time provisions of the standard no longer apply (14). Extracted teeth containing dental amalgam should not be placed in a medical waste container that uses incineration for final disposal. Commercial metal-recycling companies also might accept extracted teeth with metal restorations, including amalgam. State and local regulations should be consulted regarding disposal of the amalgam. Educational Settings Extracted teeth are occasionally collected for use in preclinical educational training. These teeth should be cleaned of visible blood and gross debris and maintained in a hydrated state in a well-constructed closed container during transport. The container should be labeled with the biohazard symbol (13,14). Because these teeth will be autoclaved before clinical exercises or study, use of the most economical storage solution (e.g., water or saline) might be practical. Liquid chemical germicides can also be used but do not reliably disinfect both external surface and interior pulp tissue (403,404). Before being used in an educational setting, the teeth should be heat-sterilized to allow safe handling. Microbial growth can be eliminated by using an autoclave cycle for 40 minutes (405), but because preclinical educational exercises simulate clinical experiences, students enrolled in dental programs should still follow standard precautions. Autoclaving teeth for preclinical laboratory exercises does not appear to alter their physical properties sufficiently to compromise the learning experience (405,406). However, whether autoclave sterilization of extracted teeth affects dentinal structure to the point that the chemical and microchemical relationship between dental materials and the dentin would be affected for research purposes on dental materials is unknown (406). Use of teeth that do not contain amalgam is preferred in educational settings because they can be safely autoclaved (403,405). Extracted teeth containing amalgam restorations should not be heat-sterilized because of the potential health hazard from mercury vaporization and exposure. If extracted teeth containing amalgam restorations are to be used, immersion in 10% formalin solution for 2 weeks should be effective in disinfecting both the internal and external structures of the teeth (403). If using formalin, manufacturer MSDS should be reviewed for occupational safety and health concerns and to ensure compliance with OSHA regulations (15). Dental LaboratoryDental prostheses, appliances, and items used in their fabrication (e.g., impressions, occlusal rims, and bite registrations) are potential sources for cross-contamination and should be handled in a manner that prevents exposure of DHCP, patients, or the office environment to infectious agents. Effective communication and coordination between the laboratory and dental practice will ensure that appropriate cleaning and disinfection procedures are performed in the dental office or laboratory, materials are not damaged or distorted because of disinfectant overexposure, and effective disinfection procedures are not unnecessarily duplicated (407,408). When a laboratory case is sent off-site, DHCP should provide written information regarding the methods (e.g., type of disinfectant and exposure time) used to clean and disinfect the material (e.g., impression, stone model, or appliance) (2,407,409). Clinical materials that are not decontaminated are subject to OSHA and U.S. Department of Transportation regulations regarding transportation and shipping of infectious materials (13,410). Appliances and prostheses delivered to the patient should be free of contamination. Communication between the laboratory and the dental practice is also key at this stage to determine which one is responsible for the final disinfection process. If the dental laboratory staff provides the disinfection, an EPA-registered hospital disinfectant (low to intermediate) should be used, written documentation of the disinfection method provided, and the item placed in a tamper-evident container before returning it to the dental office. If such documentation is not provided, the dental office is responsible for final disinfection procedures. Dental prostheses or impressions brought into the laboratory can be contaminated with bacteria, viruses, and fungi (411,412). Dental prostheses, impressions, orthodontic appliances, and other prosthodontic materials (e.g., occlusal rims, temporary prostheses, bite registrations, or extracted teeth) should be thoroughly cleaned (i.e., blood and bioburden removed), disinfected with an EPA-registered hospital disinfectant with a tuberculocidal claim, and thoroughly rinsed before being handled in the in-office laboratory or sent to an off-site laboratory (2,244,249,407). The best time to clean and disinfect impressions, prostheses, or appliances is as soon as possible after removal from the patient's mouth before drying of blood or other bioburden can occur. Specific guidance regarding cleaning and disinfecting techniques for various materials is available (260,413--416). DHCP are advised to consult with manufacturers regarding the stability of specific materials during disinfection. In the laboratory, a separate receiving and disinfecting area should be established to reduce contamination in the production area. Bringing untreated items into the laboratory increases chances for cross infection (260). If no communication has been received regarding prior cleaning and disinfection of a material, the dental laboratory staff should perform cleaning and disinfection procedures before handling. If during manipulation of a material or appliance a previously undetected area of blood or bioburden becomes apparent, cleaning and disinfection procedures should be repeated. Transfer of oral microorganisms into and onto impressions has been documented (417--419). Movement of these organisms onto dental casts has also been demonstrated (420). Certain microbes have been demonstrated to remain viable within gypsum cast materials for <7 days (421). Incorrect handling of contaminated impressions, prostheses, or appliances, therefore, offers an opportunity for transmission of microorganisms (260). Whether in the office or laboratory, PPE should be worn until disinfection is completed (1,2,7,10,13). If laboratory items (e.g., burs, polishing points, rag wheels, or laboratory knives) are used on contaminated or potentially contaminated appliances, prostheses, or other material, they should be heat-sterilized, disinfected between patients, or discarded (i.e., disposable items should be used) (260,407). Heat-tolerant items used in the mouth (e.g., metal impression tray or face bow fork) should be heat-sterilized before being used on another patient (2,407). Items that do not normally contact the patient, prosthetic device, or appliance but frequently become contaminated and cannot withstand heat-sterilization (e.g., articulators, case pans, or lathes) should be cleaned and disinfected between patients and according to the manufacturer's instructions. Pressure pots and water baths are particularly susceptible to contamination with microorganisms and should be cleaned and disinfected between patients (422). In the majority of instances, these items can be cleaned and disinfected with an EPA-registered hospital disinfectant. Environmental surfaces should be barrier-protected or cleaned and disinfected in the same manner as in the dental treatment area. Unless waste generated in the dental laboratory (e.g., disposable trays or impression materials) falls under the category of regulated medical waste, it can be discarded with general waste. Personnel should dispose of sharp items (e.g., burs, disposable blades, and orthodontic wires) in puncture-resistant containers. Laser/Electrosurgery Plumes or Surgical SmokeDuring surgical procedures that use a laser or electrosurgical unit, the thermal destruction of tissue creates a smoke byproduct. Laser plumes or surgical smoke represent another potential risk for DHCP (423--425). Lasers transfer electromagnetic energy into tissues, resulting in the release of a heated plume that includes particles, gases (e.g., hydrogen cyanide, benzene, and formaldehyde), tissue debris, viruses, and offensive odors. One concern is that aerosolized infectious material in the laser plume might reach the nasal mucosa of the laser operator and adjacent DHCP. Although certain viruses (e.g., varicella-zoster virus and herpes simplex virus) appear not to aerosolize efficiently (426,427), other viruses and various bacteria (e.g., human papilloma virus, HIV, coagulase-negative Staphylococcus, Corynebacterium species, and Neisseria species) have been detected in laser plumes (428--434). However, the presence of an infectious agent in a laser plume might not be sufficient to cause disease from airborne exposure, especially if the agent's normal mode of transmission is not airborne. No evidence indicates that HIV or HBV have been transmitted through aerosolization and inhalation (435). Although continuing studies are needed to evaluate the risk for DHCP of laser plumes and electrosurgery smoke, following NIOSH recommendations (425) and practices developed by the Association of periOperative Registered Nurses (AORN) might be practical (436). These practices include using 1) standard precautions (e.g., high-filtration surgical masks and possibly full face shields) (437); 2) central room suction units with in-line filters to collect particulate matter from minimal plumes; and 3) dedicated mechanical smoke exhaust systems with a high-efficiency filter to remove substantial amounts of laser plume particles. Local smoke evacuation systems have been recommended by consensus organizations, and these systems can improve the quality of the operating field. Employers should be aware of this emerging problem and advise employees of the potential hazards of laser smoke (438). However, this concern remains unresolved in dental practice and no recommendation is provided here. M. tuberculosisPatients infected with M. tuberculosis occasionally seek urgent dental treatment at outpatient dental settings. Understanding the pathogenesis of the development of TB will help DHCP determine how to manage such patients. M. tuberculosis is a bacterium carried in airborne infective droplet nuclei that can be generated when persons with pulmonary or laryngeal TB sneeze, cough, speak, or sing (439). These small particles (1--5 µm) can stay suspended in the air for hours (440). Infection occurs when a susceptible person inhales droplet nuclei containing M. tuberculosis, which then travel to the alveoli of the lungs. Usually within 2--12 weeks after initial infection with M. tuberculosis, immune response prevents further spread of the TB bacteria, although they can remain alive in the lungs for years, a condition termed latent TB infection. Persons with latent TB infection usually exhibit a reactive tuberculin skin test (TST), have no symptoms of active disease, and are not infectious. However, they can develop active disease later in life if they do not receive treatment for their latent infection. Approximately 5% of persons who have been recently infected and not treated for latent TB infection will progress from infection to active disease during the first 1--2 years after infection; another 5% will develop active disease later in life. Thus, approximately 90% of U.S. persons with latent TB infection do not progress to active TB disease. Although both latent TB infection and active TB disease are described as TB, only the person with active disease is contagious and presents a risk of transmission. Symptoms of active TB disease include a productive cough, night sweats, fatigue, malaise, fever, and unexplained weight loss. Certain immunocompromising medical conditions (e.g., HIV) increase the risk that TB infection will progress to active disease at a faster rate (441). Overall, the risk borne by DHCP for exposure to a patient with active TB disease is probably low (20,21). Only one report exists of TB transmission in a dental office (442), and TST conversions among DHCP are also low (443,444). However, in certain cases, DHCP or the community served by the dental facility might be at relatively high risk for exposure to TB. Surgical masks do not prevent inhalation of M. tuberculosis droplet nuclei, and therefore, standard precautions are not sufficient to prevent transmission of this organism. Recommendations for expanded precautions to prevent transmission of M. tuberculosis and other organisms that can be spread by airborne, droplet, or contact routes have been detailed in other guidelines (5,11,20). TB transmission is controlled through a hierarchy of measures, including administrative controls, environmental controls, and personal respiratory protection. The main administrative goals of a TB infection-control program are early detection of a person with active TB disease and prompt isolation from susceptible persons to reduce the risk of transmission. Although DHCP are not responsible for diagnosis and treatment of TB, they should be trained to recognize signs and symptoms to help with prompt detection. Because potential for transmission of M. tuberculosis exists in outpatient settings, dental practices should develop a TB control program appropriate for their level of risk (20,21).
Creutzfeldt-Jakob Disease and Other Prion DiseasesCreutzfeldt-Jakob disease (CJD) belongs to a group of rapidly progressive, invariably fatal, degenerative neurological disorders, transmissible spongiform encephalopathies (TSEs) that affect both humans and animals and are thought to be caused by infection with an unusual pathogen called a prion. Prions are isoforms of a normal protein, capable of self-propagation although they lack nucleic acid. Prion diseases have an incubation period of years and are usually fatal within 1 year of diagnosis. Among humans, TSEs include CJD, Gerstmann-Straussler-Scheinker syndrome, fatal familial insomnia, kuru, and variant CJD (vCJD). Occurring in sporadic, familial, and acquired (i.e., iatrogenic) forms, CJD has an annual incidence in the United States and other countries of approximately 1 case/million population (445--448). In approximately 85% of affected patients, CJD occurs as a sporadic disease with no recognizable pattern of transmission. A smaller proportion of patients (5%--15%) experience familial CJD because of inherited mutations of the prion protein gene (448). vCJD is distinguishable clinically and neuropathologically from classic CJD, and strong epidemiologic and laboratory evidence indicates a causal relationship with bovine spongiform encephalopathy (BSE), a progressive neurological disorder of cattle commonly known as mad cow disease (449--451). vCJD, was reported first in the United Kingdom in 1996 (449) and subsequently in other European countries (452). Only one case of vCJD has been reported in the United States, in an immigrant from the United Kingdom (453). Compared with CJD patients, those with vCJD are younger (28 years versus 68 years median age at death), and have a longer duration of illness (13 months versus 4.5 months). Also, vCJD patients characteristically exhibit sensory and psychiatric symptoms that are uncommon with CJD. Another difference includes the ease with which the presence of prions is consistently demonstrated in lymphoreticular tissues (e.g., tonsil) in vCJD patients by immunohistochemistry (454). CJD and vCJD are transmissible diseases, but not through the air or casual contact. All known cases of iatrogenic CJD have resulted from exposure to infected central nervous tissue (e.g., brain and dura mater), pituitary, or eye tissue. Studies in experimental animals have determined that other tissues have low or no detectable infectivity (243,455,456). Limited experimental studies have demonstrated that scrapie (a TSE in sheep) can be transmitted to healthy hamsters and mice by exposing oral tissues to infectious homogenate (457,458). These animal models and experimental designs might not be directly applicable to human transmission and clinical dentistry, but they indicate a theoretical risk of transmitting prion diseases through perioral exposures. According to published reports, iatrogenic transmission of CJD has occurred in humans under three circumstances: after use of contaminated electroencephalography depth electrodes and neurosurgical equipment (459); after use of extracted pituitary hormones (460,461); and after implant of contaminated corneal (462) and dura mater grafts (463,464) from humans. The equipment-related cases occurred before the routine implementation of sterilization procedures used in health-care facilities. Case-control studies have found no evidence that dental procedures increase the risk of iatrogenic transmission of TSEs among humans. In these studies, CJD transmission was not associated with dental procedures (e.g., root canals or extractions), with convincing evidence of prion detection in human blood, saliva, or oral tissues, or with DHCP becoming occupationally infected with CJD (465--467). In 2000, prions were not found in the dental pulps of eight patients with neuropathologically confirmed sporadic CJD by using electrophoresis and a Western blot technique (468). Prions exhibit unusual resistance to conventional chemical and physical decontamination procedures. Considering this resistance and the invariably fatal outcome of CJD, procedures for disinfecting and sterilizing instruments potentially contaminated with the CJD prion have been controversial for years. Scientific data indicate the risk, if any, of sporadic CJD transmission during dental and oral surgical procedures is low to nil. Until additional information exists regarding the transmissibility of CJD or vCJD, special precautions in addition to standard precautions might be indicated when treating known CJD or vCJD patients; the following list of precautions is provided for consideration without recommendation (243,249,277,469):
Potential infectivity of oral tissues in CJD or vCJD patients is an unresolved concern. CDC maintains an active surveillance program on CJD. Additional information and resources are available at http://www.cdc.gov/ncidod/diseases/cjd/cjd.htm. Program EvaluationThe goal of a dental infection-control program is to provide a safe working environment that will reduce the risk of health-care--associated infections among patients and occupational exposures among DHCP. Medical errors are caused by faulty systems, processes, and conditions that lead persons to make mistakes or fail to prevent errors being made by others (470). Effective program evaluation is a systematic way to ensure procedures are useful, feasible, ethical, and accurate. Program evaluation is an essential organizational practice; however, such evaluation is not practiced consistently across program areas, nor is it sufficiently well-integrated into the day-to-day management of the majority of programs (471). A successful infection-control program depends on developing standard operating procedures, evaluating practices, routinely documenting adverse outcomes (e.g., occupational exposures to blood) and work-related illnesses in DHCP, and monitoring health-care--associated infections in patients. Strategies and tools to evaluate the infection-control program can include periodic observational assessments, checklists to document procedures, and routine review of occupational exposures to bloodborne pathogens. Evaluation offers an opportunity to improve the effectiveness of both the infection-control program and dental-practice protocols. If deficiencies or problems in the implementation of infection-control procedures are identified, further evaluation is needed to eliminate the problems. Examples of infection-control program evaluation activities are provided (Table 5). Infection-Control Research ConsiderationsAlthough the number of published studies concerning dental infection control has increased in recent years, questions regarding infection-control practices and their effectiveness remain unanswered. Multiple concerns were identified by the working group for this report, as well as by others during the public comment period (Box. This list is not exhaustive and does not represent a CDC research agenda, but rather is an effort to identify certain concerns, stimulate discussion, and provide direction for determining future action by clinical, basic science, and epidemiologic investigators, as well as health and professional organizations, clinicians, and policy makers. RecommendationsEach recommendation is categorized on the basis of existing scientific data, theoretical rationale, and applicability. Rankings are based on the system used by CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) to categorize recommendations: Category IA. Strongly recommended for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies. Category IB. Strongly recommended for implementation and supported by experimental, clinical, or epidemiologic studies and a strong theoretical rationale. Category IC. Required for implementation as mandated by federal or state regulation or standard. When IC is used, a second rating can be included to provide the basis of existing scientific data, theoretical rationale, and applicability. Because of state differences, the reader should not assume that the absence of a IC implies the absence of state regulations. Category II. Suggested for implementation and supported by suggestive clinical or epidemiologic studies or a theoretical rationale. Unresolved issue. No recommendation. Insufficient evidence or no consensus regarding efficacy exists. I. Personnel Health Elements of an Infection-Control Program
II. Preventing Transmission of Bloodborne Pathogens
III. Hand Hygiene
IV. PPE
V. Contact Dermatitis and Latex Hypersensitivity
VI. Sterilization and Disinfection of Patient-Care Items
VII. Environmental Infection Control
VIII. Dental Unit Waterlines, Biofilm, and Water Quality
IX. Special Considerations
Infection-Control Internet ResourcesAdvisory Committee on Immunization Practices American Dental Association American Institute of Architects Academy of Architecture for Health
American Society of Heating, Refrigeration, Air-conditioning Engineers
Association for Professionals in Infection Control and Epidemiology, Inc.
CDC, Division of Healthcare Quality Promotion
CDC, Division of Oral Health, Infection Control
CDC, Morbidity and Mortality Weekly Report
CDC, NIOSH CDC Recommends, Prevention Guidelines System
EPA, Antimicrobial Chemicals
Immunization Action Coalition
Infectious Diseases Society of America
OSHA, Dentistry, Bloodborne Pathogens
Organization for Safety and Asepsis Procedures
Society for Healthcare Epidemiology of America, Inc., Position Papers AcknowledgementThe Division of Oral Health thanks the working group as well as CDC and other federal and external reviewers for their efforts in developing and reviewing drafts of this report and acknowledges that all opinions of the reviewers might not be reflected in all of the recommendations. References1. CDC. Recommended infection-control practices for dentistry. MMWR 1986;35:237--42. 2. CDC. Recommended infection-control practices for dentistry, 1993. MMWR 1993;42(No. RR-8). 3. US Census Bureau. Statistical Abstract of the United States: 2001. Washington, DC: US Census Bureau, 2001. Available at http://www. census.gov/prod/www/statistical-abstract-02.html. 4. Health Resources and Services Administration, Bureau of Health Professions. United States health workforce personnel factbook. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, 2000. 5. Bolyard EA, Tablan OC, Williams WW, Pearson ML, Shapiro CN, Deitchman SD, Hospital Infection Control Practices Advisory Committee. Guideline for infection control in health care personnel, 1998. Am J Infect Control 1998;26:289--354. 6. Greene VW. Microbiological contamination control in hospitals. 1. Perspectives. Hospitals 1969;43:78--88. 9. Garner JS, Favero MS. CDC guideline for handwashing and hospital environmental control, 1985. Infect Control 1986;7:231--43. 10. CDC. Recommendations for prevention of HIV transmission in health-care settings. MMWR 1987;36(suppl No. 2S). 11. Garner JS, Hospital Infection Control Practices Advisory Committee. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol 1996;17:53--80. 12. Chiarello LA, Bartley J. Prevention of blood exposure in healthcare personnel. Seminars in Infection Control 2001;1:30--43. 13. US Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries; final rule. Federal Register 2001;66:5317--25. As amended from and includes 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; final rule. Federal Register 1991;56:64174--82. Available at http://www.osha.gov/SLTC/dentistry/index.html. 14. US Department of Labor, Occupational Safety and Health Administration. OSHA instruction: enforcement procedures for the occupational exposure to bloodborne pathogens. Washington, DC: US Department of Labor, Occupational Safety and Health Administration, 2001; directive no. CPL 2-2.69. 15. US Department of Labor, Occupational Safety and Health Administration. 29 CFR 1910.1200. Hazard communication. Federal Register 1994;59:17479. 16. Gershon RR, Karkashian CD, Grosch JW, et al. Hospital safety climate and its relationship with safe work practices and workplace exposure incidents. Am J Infect Control 2000;28:211--21. 18. Association for Professionals in Infection Control and Epidemiology. APIC position paper: immunization. Am J Infect Control 1999;27:52--3. 21. Cleveland JL, Gooch BF, Bolyard EA, Simone PM, Mullan RJ, Marianos DW. TB infection control recommendations from the CDC, 1994: considerations for dentistry. J Am Dent Assoc 1995;126:593--9. 22. Herwaldt LA, Pottinger JM, Carter CD, Barr BA, Miller ED. Exposure workups. Infect Control Hosp Epidemiol 1997;18:850--71. 23. Nash KD. How infection control procedures are affecting dental practice today. J Am Dent Assoc 1992;123:67--73. 24. Berky ZT, Luciano WJ, James WD. Latex glove allergy: a survey of the US Army Dental Corps. JAMA 1992;268:2695--7. 25. Bubak ME, Reed CE, Fransway AF, et al. Allergic reactions to latex among health-care workers. Mayo Clin Proc 1992;67:1075--9. 26. Fisher AA. Allergic contact reactions in health personnel. J Allergy Clin Immunol 1992;90:729--38. 27. Smart ER, Macleod RI, Lawrence CM. Allergic reactions to rubber gloves in dental patients: report of three cases. Br Dent J 1992;172:445--7. 28. Yassin MS, Lierl MB, Fischer TJ, O'Brien K, Cross J, Steinmetz C. Latex allergy in hospital employees. Ann Allergy 1994;72:245--9. 29. Zaza S, Reeder JM, Charles LE, Jarvis WR. Latex sensitivity among perioperative nurses. AORN J 1994;60:806--12. 30. Hunt LW, Fransway AF, Reed CE, et al. An epidemic of occupational allergy to latex involving health care workers. J Occup Environ Med 1995;37:1204--9. 31. American Dental Association Council on Scientific Affairs. The dental team and latex hypersensitivity. J Am Dent Assoc 1999;130:257--64. 32. CDC. National Institute for Occupational Safety and Health. NIOSH Alert: preventing allergic reactions to natural rubber latex in the workplace. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1997. 33. Terezhalmy GT, Molinari JA. Personal protective equipment and barrier techniques. In: Cottone JA, Terezhalmy GT, Molinari JA, eds. Practical infection control in dentisty. 2nd ed. Baltimore, MD: Williams & Wilkins, 1996:136--145. 34. US Department of Health and Human Services, Office of the Secretary, Office for Civil Rights. 45 CFR Parts 160 and 164. Standards for privacy of individually identifiable health information; final rule. Federal Register 2000;65:82462--829. 35. Occupational Safety and Health Administration. Access to medical and exposure records. Washington, DC: US Department of Labor, Occupational Safety and Health Administration, 2001. OSHA publication no. 3110. 36. Mast EE, Alter MJ. Prevention of hepatitis B virus infection among health-care workers. In: Ellis RW, ed. Hepatitis B vaccines in clinical practice. New York, NY: Marcel Dekker, 1993:295--307. 37. Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev 2000;13:385--407. 38. Werner BG, Grady GF. Accidental hepatitis-B-surface-antigen-positive inoculations: use of e antigen to estimate infectivity. Ann Intern Med 1982;97:367--9. 39. Bond WW, Petersen NJ, Favero MS. Viral hepatitis B: aspects of environmental control. Health Lab Sci 1977;14:235--52. 40. Garibaldi RA, Hatch FE, Bisno AL, Hatch MH, Gregg MB. Nonparenteral serum hepatitis: report of an outbreak. JAMA 1972; 220:963--6. 41. Rosenberg JL, Jones DP, Lipitz LR, Kirsner JB. Viral hepatitis: an occupational hazard to surgeons. JAMA 1973;223:395--400. 42. Callender ME, White YS, Williams R. Hepatitis B virus infection in medical and health care personnel. Br Med J 1982;284:324--6. 43. Chaudhuri AK, Follett EA. Hepatitis B virus infection in medical and health care personnel [Letter]. Br Med J 1982;284:1408. 44. Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week [Letter]. Lancet 1981;1:550--1. 45. Francis DP, Favero MS, Maynard JE. Transmission of hepatitis B virus [Review]. Semin Liver Dis 1981;1:27--32. 46. Favero MS, Maynard JE, Petersen NJ, et al. Hepatitis-B antigen on environmental surfaces [Letter]. Lancet 1973;2:1455. 47. Lauer JL, VanDrunen NA, Washburn JW, Balfour HH Jr. Transmission of hepatitis B virus in clinical laboratory areas. J Infect Dis 1979;140:513--6. 48. Hennekens CH. Hemodialysis-associated hepatitis: an outbreak among hospital personnel. JAMA 1973;225:407--8. 49. Garibaldi RA, Forrest JN, Bryan JA, Hanson BF, Dismukes WE. Hemodialysis-associated hepatitis. JAMA 1973;225:384--9. 50. Snydman DR, Bryan JA, Macon EJ, Gregg MB. Hemodialysis-associated hepatitis: a report of an epidemic with further evidence on mechanisms of transmission. Am J Epidemiol 1976;104:563--70. 51. Shapiro CN. Occupational risk of infection with hepatitis B and hepatitis C virus. Surg Clin North Am 1995;75:1047--56. 52. Cleveland JL, Siew C, Lockwood SA, Gruninger SE, Gooch BF, Shapiro CN. Hepatitis B vaccination and infection among U.S. dentists, 1983--1992. J Am Dent Assoc 1996;127:1385--90. 54. Chamberland ME. HIV transmission from health care worker to patient: what is the risk [Letter]? Ann Intern Med 1992;116:871--3. 55. Robert LM, Chamberland ME, Cleveland JL, et al. Investigation of patients of health care workers infected with HIV: the Centers for Disease Control and Prevention database. Ann Intern Med 1995;122:653--7. 56. CDC. Investigations of persons treated by HIV-infected health-care workers---United States. MMWR 1993;42:329--331, 337. 57. Siew C, Chang SB, Gruninger SE, Verrusio AC, Neidle EA. Self-reported percutaneous injuries in dentists: implications for HBV, HIV, transmission risk. J Am Dent Assoc 1992;123:36--44. 58. Ahtone J, Goodman RA. Hepatitis B and dental personnel: transmission to patients and prevention issues. J Am Dent Assoc 1983;106:219--22. 59. Hadler SC, Sorley DL, Acree KH, et al. An outbreak of hepatitis B in a dental practice. Ann Intern Med 1981;95:133--8. 61. Levin ML, Maddrey WC, Wands JR, Mendeloff AL. Hepatitis B transmission by dentists. JAMA 1974;228:1139--40. 62. Rimland D, Parkin WE, Miller GB Jr, Schrack WD. Hepatitis B outbreak traced to an oral surgeon. N Engl J Med 1977;296:953--8. 63. Goodwin D, Fannin SL, McCracken BB. An oral surgeon-related hepatitis-B outbreak. California Morbidity 1976;14:1. 64. Reingold AL, Kane MA, Murphy BL, Checko P, Francis DP, Maynard JE. Transmission of hepatitis B by an oral surgeon. J Infect Dis 1982; 145:262--8. 65. Goodman RA, Ahtone JL, Finton RJ. Hepatitis B transmission from dental personnel to patients: unfinished business. Ann Intern Med 1982;96:119. 66. Shaw FE Jr, Barrett CL, Hamm R, et al. Lethal outbreak of hepatitis B in a dental practice. JAMA 1986;255:3260--4. 68. US Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; final rule. Federal Register 1991;56:64004--182. 70. Polish LB, Gallagher M, Fields HA, Hadler SC. Delta hepatitis: molecular biology and clinical and epidemiological features. Clin Microbiol Rev 1993;6:211--29. 71. Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997;1:559--68. 72. Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers: Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995;23:273--7. 73. Lanphear BP, Linnemann CC Jr, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745--50. 74. Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992;16: 1109--14. 75. Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993;25:270--1. 76. Ippolito G, Puro V, De Carli G. The risk of occupational human immunodeficiency virus in health care workers: Italian Multicenter Study, The Italian Study Group on Occupational Risk of HIV Infection. Arch Intern Med 1993;153:1451--8. 77. Beltrami EM, Kozak A, Williams IT, et al. Transmission of HIV and hepatitis C virus from a nursing home patient to a health care worker. Am J Infec Control. 2003; 31:168--75. 78. Cooper BW, Krusell A, Tilton RC, Goodwin R, Levitz RE. Seroprevalence of antibodies to hepatitis C virus in high-risk hospital personnel. Infect Control Hosp Epidemiol 1992;13:82--5. 79. Panlilio AL, Shapiro CN, Schable CA, et al. Serosurvey of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among hospital-based surgeons. Serosurvey Study Group. J Am Coll Surg 1995;180:16--24. 80. Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993;21:196--200. 81. Shapiro CN, Tokars JI, Chamberland ME, American Academy of Orthopaedic Surgeons Serosurvey Study Committee. Use of the hepatitis-B vaccine and infection with hepatitis B and C among orthopaedic surgeons. J Bone Joint Surg Am 1996;78:1791--800. 82. Gerberding JL. Incidence and prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and cytomegalovirus among health care personnel at risk for blood exposure: final report from a longitudinal study. J Infect Dis 1994;170:1410--7. 83. Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991;338:1539--42. 84. Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996;100:41--5. 85. Cleveland JL, Gooch BF, Shearer BG, Lyerla RL. Risk and prevention of hepatitis C virus infection: implications for dentistry. J Am Dent Assoc 1999;130:641--7. 86. Gruninger SE, Siew C, Azzolin KL, Meyer DM. Update of hepatitis C infection among dental professionals [Abstract 1825]. J Dent Res 2001;80:264. 87. Esteban JI, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med 1996;334:555--60. 88. Duckworth GJ, Heptonstall J, Aitken C. Transmission of hepatitis C virus from a surgeon to a patient: the Incident Control Team. Commun Dis Public Health 1999;2:188--92. 89. Ross RS, Viazov S, Gross T, Hofmann F, Seipp HM, Roggendorf M. Brief report: transmission of hepatitis C virus from a patient to an anesthesiology assistant to five patients. N Engl J Med 2000;343:1851--4. 90. Cody SH, Nainan OV, Garfein RS, et al. Hepatitis C virus transmission from an anesthesiologist to a patient. Arch Intern Med 2002; 162:345--50. 91. Do AN, Ciesielski CA, Metler RP, Hammett TA, Li J, Fleming PL. Occupationally acquired human immunodeficiency virus (HIV) infection: national case surveillance data during 20 years of the HIV epidemic in the United States. Infect Control Hosp Epidemiol 2003;24:86--96. 92. Ciesielski C, Marianos D, Ou CY, et al. Transmission of human immunodeficiency virus in a dental practice. Ann Intern Med 1992; 116:798--805. 94. Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med 1997;102(5B):9--15. 95. Cardo DM, Culver DH, Ciesielski CA, et al, Centers for Disease Control and Prevention Needlestick Surveillance Group. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med 1997;337:1485--90. 96. Beltrami EM. The risk and prevention of occupational human immunodeficiency virus infection. Seminars in Infection Control 2001;1:2--18. 97. CDC. National Institute for Occupational Safety and Health. NIOSH alert: Preventing needlestick injuries in health care settings. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1999. 98. Klein RS, Phelan JA, Freeman K, et al. Low occupational risk of human immunodeficiency virus infection among dental professionals. N Engl J Med 1988;318:86--90. 99. Gruninger SE, Siew C, Chang SB, et al. Human immunodeficiency virus type I: infection among dentists. J Am Dent Assoc 1992;123:59--64. 100. Siew C, Gruninger SE, Miaw CL, Neidle EA. Percutaneous injuries in practicing dentists: a propective study using a 20-day diary. J Am Dent Assoc 1995;126:1227--34. 101. Cleveland JL, Lockwood SA, Gooch BF, et al. Percutaneous injuries in dentistry: an observational study. J Am Dent Assoc 1995;126:745--51. 102. Ramos-Gomez F, Ellison J, Greenspan D, Bird W, Lowe S, Gerberding JL. Accidental exposures to blood and body fluids among health care workers in dental teaching clinics: a prospective study. J Am Dent Assoc 1997;128:1253--61. 103. Cleveland JL, Gooch BF, Lockwood SA. Occupational blood exposure in dentistry: a decade in review. Infect Control Hosp Epidemiol 1997; 18:717--21. 104. Gooch BF, Siew C, Cleveland JL, Gruninger SE, Lockwood SA, Joy ED. Occupational blood exposure and HIV infection among oral and maxillofacial surgeons. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:128--34. 105. Gooch BF, Cardo DM, Marcus R, et al. Percutaneous exposures to HIV--infected blood among dental workers enrolled in the CDC needlestick study. J Am Dent Assoc 1995;126:1237--42. 106. Younai FS, Murphy DC, Kotelchuck D. Occupational exposures to blood in a dental teaching environment: results of a ten-year surveillance study. J Dent Educ 2001;65:436--8. 107. Carlton JE, Dodson TB, Cleveland JL, Lockwood SA. Percutaneous injuries during oral and maxillofacial surgery procedures. J Oral Maxillofac Surg 1997;55:553--6. 108. Harte J, Davis R, Plamondon T, Richardson B. The influence of dental unit design on percutaneous injury. J Am Dent Assoc 1998; 129:1725--31. 109. US Department of Labor, Occupational Health and Safety Administration. 29 CFR Part 1910. Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries, final rule. Federal Register 2001;66:5325. 112. Mendelson MH, Lin-Chen BY, Solomon R, Bailey E, Kogan G, Goldbold J. Evaluation of a safety resheathable winged steel needle for prevention of percutaneous injuries associated with intravascular-access procedures among healthcare workers. Infect Control Hosp Epidemiol 2003;24:105--12. 115. CDC. National Institute for Occupational Safety and Health. Selecting, evaluating, and using sharps disposal containers. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1998. DHHS publication no. (NIOSH) 97-111. 120. Steere AC, Mallison GF. Handwashing practices for the prevention of nosocomial infections. Ann Intern Med 1975;83:683--90. 121. Garner JS. CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in November 1985). Revised. Infect Control 1986;7:193--200. 122. Larson EL. APIC guideline for hand washing and hand antisepsis in health-care settings. Am J Infect Control 1995;23:251--69. 124. Casewell M, Phillips I. Hands as route of transmission for Klebsiella species. Br Med J 1977;2:1315--7. 125. Larson EL, Early E, Cloonan P, Sugrue S, Parides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infections. Behav Med 2000;26:14--22. 126. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet 2000;356:1307--12. 127. Price PB. New studies in surgical bacteriology and surgical technique. JAMA 1938;111:1993--6. 128. Dewar NE, Gravens DL. Effectiveness of septisol antiseptic foam as a surgical scrub agent. Appl Microbiol 1973;26:544--9. 129. Lowbury EJ, Lilly HA. Disinfection of the hands of surgeons and nurses. Br Med J 1960;1445--50. 130. Rotter M. Hand washing and hand disinfection. In: Mayhall CG, ed. Hospital epidemiology and infection control. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 1999:1339--55. 131. Widmer AF. Replace hand washing with use of a waterless alcohol hand rub? Clin Infect Dis 2000;31:136--43. 132. Larson EL, Butz AM, Gullette DL, Laughon BA. Alcohol for surgical scrubbing? Infect Control Hosp Epidemiol 1990;11:139--43. 133. Faoagali J, Fong J, George N, Mahoney P, O'Rouke V. Comparison of the immediate, residual, and cumulative antibacterial effects of Novaderm R,* Novascrub R,* Betadine Surgical Scrub, Hibiclens, and liquid soap. Am J Infect Control 1995;23:337--43. 134. Association of Perioperative Registered Nurses. Recommended practices for sterilization in the practice setting. In: Fogg D, Parker N, Shevlin D, eds. 2002 standards, recommended practices, and guidelines. Denver, CO: AORN, 2002:333--42. 135. US Department Of Health and Human Services, Food and Drug Administration. Tentative final monograph for healthcare antiseptic drug products; proposed rule. Federal Register 1994;59:31441--52. 136. Larson E. A causal link between handwashing and risk of infection? Examination of the evidence. Infection Control.1988;9:28--36. 137. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:250--78. 138. Doebbeling BN, Pfaller MA, Houston AK, Wenzel RP. Removal of nosocomial pathogens from the contaminated glove. Ann Intern Med 1988;109:394--8. 139. Kabara JJ, Brady MB. Contamination of bar soaps under "in-use" conditions. J Environ Pathol Toxicol Oncol 1984;5:1--14. 140. Ojajärvi J. The importance of soap selection for routine hand hygiene in hospital. J Hyg (Lond) 1981;86:275--83. 141. Larson E, Leyden JJ, McGinley KJ, Grove GL, Talbot GH. Physiologic and microbiologic changes in skin related to frequent handwashing. Infect Control 1986;7:59--63. 142. Larson E. Handwashing: it's essential---even when you use gloves. Am J Nurs 1989;89:934--9. 143. Field EA, McGowan P, Pearce PK, Martin MV. Rings and watches: should they be removed prior to operative dental procedures? J Dent 1996;24:65--9. 144. Hobson DW, Woller W, Anderson L, Guthery E. Development and evaluation of a new alcohol-based surgical hand scrub formulation with persistent antimicrobial characteristics and brushless application. Am J Infect Control 1998;26:507--12. 145. Mulberry G, Snyder AT, Heilman J, Pyrek J, Stahl J. Evaluation of a waterless, scrubless chlorhexidine gluconate/ethanol surgical scrub for antimicrobial efficacy. Am J Infect Control 2001;29:377--82. 146. Association of Perioperative Registered Nurses. Recommended practices for surgical hand scrubs. In: Fogg D, Parker N, eds. 2003 standards, recommended practices, and guidelines. Denver, CO: AORN, Inc., 2003:277--80. 147. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93--9. 148. Zimakoff J, Kjelsberg AB, Larson SO, Holstein B. A multicenter questionnaire investigation of attitudes toward hand hygiene, assessed by the staff in fifteen hospitals in Denmark and Norway. Am J Infec Control 1992;20:58--64. 149. Grohskopf LA, Roth VR, Feikin DR, et al. Serratia liquefaciens bloodstream infections from contamination of epoetin alfa at a hemodialysis center. N Engl J Med 2001;344:1491--7. 150. Archibald LK, Corl A, Shah B, et al. Serratia marcescens outbreak associated with extrinsic contamination of 1% chlorxylenol soap. Infect Control Hosp Epidemiol 1997;18:704--9. 151. Larson EL, Norton Hughes CA, Pyrak JD, Sparks SM, Cagatay EU, Bartkus JM. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control 1998;26:513--21. 152. Ojajärvi J, Mäkelä P, Rantasalo I. Failure of hand disinfection with frequent hand washing: a need for prolonged field studies. J Hyg (Lond) 1977;79:107--19. 153. Berndt U, Wigger-Alberti W, Gabard B, Elsner P. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis 2000;42:77--80. 154. McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control 2000;28:302--10. 155. Larson E, Anderson JK, Baxendale L, Bobo L. Effects of a protective foam on scrubbing and gloving. Am J Infect Control 1993;21:297--301. 156. McGinley KJ, Larson EL, Leyden JJ. Composition and density of microflora in the subungual space of the hand. J Clin Microbiol 1988;26:950--3. 157 Pottinger J, Burns S, Manske C. Bacterial carriage by artificial versus natural nails. Am J Infect Control 1989;17:340--4. 158. McNeil SA, Foster CL, Hedderwick SA, Kauffman CA. Effect of hand cleansing with antimicrobial soap or alcohol-based gel on microbial colonization of artificial fingernails worn by health care workers. Clin Infect Dis 2001;32:367--72. 159. Rubin DM. Prosthetic fingernails in the OR: a research study. AORN J 1988;47:944--5. 160. Hedderwick SA, McNeil SA, Lyons MJ, Kauffman CA. Pathogenic organisms associated with artificial fingernails worn by healthcare workers. Infect Control Hosp Epidemiol 2000;21:505--9. 161. Passaro DJ, Waring L, Armstrong R, et al. Postoperative Serratia marcescens wound infections traced to an out-of-hospital source. J Infect Dis 1997;175:992--5. 162. Foca M, Jakob K, Whittier S, et al. Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N Engl J Med 2000; 343:695--700. 163. Parry MF, Grant B, Yukna M, et al. Candida osteomyelitis and diskitis after spinal surgery: an outbreak that implicates artificial nail use. Clin Infect Dis 2001;32:352--7. 164. Moolenaar RL, Crutcher M, San Joaquin VH, et al. A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: did staff fingernails play a role in disease transmission? Infect Control Hosp Epidemiol 2000;21:80--5. 165. Baumgardner CA, Maragos CS, Walz J, Larson E. Effects of nail polish on microbial growth of fingernails: dispelling sacred cows. AORN J 1993;58:84--8. 166. Wynd CA, Samstag DE, Lapp AM. Bacterial carriage on the fingernails of OR nurses. AORN J 1994;60:796, 799--805. 167. Lowbury EJ. Aseptic methods in the operating suite. Lancet 1968;1:705--9. 168. Hoffman PN, Cooke EM, McCarville MR, Emmerson AM. Micro-organisms isolated from skin under wedding rings worn by hospital staff. Br Med J 1985;290:206--7. 169. Jacobson G, Thiele JE, McCune JH, Farrell LD. Handwashing: ring-wearing and number of microorganisms. Nurs Res 1985;34:186--8. 170. Trick WE, Vernon MO, Hayes RA, et al. Impact of ring wearing on hand contamination and comparison of hand hygiene agents in a hospital. Clin Infect Dis 2003; 36:1383--90. 171. Salisbury DM, Hutfilz P, Treen LM, Bollin GE, Gautam S. The effect of rings on microbial load of health care workers' hands. Am J Infec Control 1997;25:24--7. 172. Cochran MA, Miller CH, Sheldrake MS. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J Am Dent Assoc 1989;119:141--4. 173. Miller CH, Palenik DJ. Aseptic techniques [Chapter 10]. In: Miller CH, Palenik DJ, eds. Infection control and management of hazardous materials for the dental team. 2nd ed. St. Louis, MO: Mosby, 1998. 174. CDC. National Institute for Occupational Safety and Health. TB respiratory protection program in health care facilities: administrator's guide. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1999. DHHS publication no. (NIOSH) 99-143. 175. US Department of Labor, Occupational Safety and Health Administration. OSHA 29 CFR 1910.139. Respiratory protection for M. tuberculosis. Federal Register 1998;49:442--9. 176. CDC. National Institute for Occupational Safety and Health. NIOSH guide to the selection and use of particulate respirators certified under 42 CFR 84. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1996. DHHS publication no. (NIOSH) 96-101. 177. DeGroot-Kosolcharoen J, Jones JM. Permeability of latex and vinyl gloves to water and blood. Am J Infect Control 1989;17:196--201. 178. Korniewicz DM, Laughon BE, Butz A, Larson E. Integrity of vinyl and latex procedure gloves. Nurs Res 1989;38:144--6. 179. Olsen RJ, Lynch P, Coyle MB, Cummings J, Bokete T, Stamm WE. Examination gloves as barriers to hand contamination in clinical practice. JAMA 1993;270:350--3. 180. Murray CA, Burke FJ, McHugh S. An assessment of the incidence of punctures in latex and non-latex dental examination gloves in routine clinical practice. Br Dent J 2001;190:377--80. 181. Burke FJ, Baggett FJ, Lomax AM. Assessment of the risk of glove puncture during oral surgery procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:18--21. 182. Burke FJ, Wilson NH. The incidence of undiagnosed punctures in non-sterile gloves. Br Dent J 1990;168:67--71. 183. Nikawa H, Hamada T, Tamamoto M, Abekura H. Perforation and proteinaceous contamination of dental gloves during prosthodontic treatments. Int J Prosthodont 1994;7:559--66. 184. Nikawa H, Hamada T, Tamamoto M, Abekura H, Murata H. Perforation of dental gloves during prosthodontic treatments as assessed by the conductivity and water inflation tests. Int J Prosthodont 1996;9:362--6. 185. Avery CM, Hjort A, Walsh S, Johnson PA. Glove perforation during surgical extraction of wisdom teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:23--5. 186. Otis LL, Cottone JA. Prevalence of perforations in disposable latex gloves during routine dental treatment. J Am Dent Assoc 1989; 118:321--4. 187. Kotilainen HR, Brinker JP, Avato JL, Gantz NM. Latex and vinyl examination gloves. Quality control procedures and implications for health care workers. Arch Intern Med 1989;149:2749--53. 188. Food and Drug Administration. Glove powder report. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 1997. Available at http://www.fda.gov/cdrh/glvpwd.html. 189. Morgan DJ, Adams D. Permeability studies on protective gloves used in dental practice. Br Dent J 1989;166:11--3. 190. Albin MS, Bunegin L, Duke ES, Ritter RR, Page CP. Anatomy of a defective barrier: sequential glove leak detection in a surgical and dental environment. Crit Care Med 1992;20:170--84. 191. Merchant VA, Molinari JA, Pickett T. Microbial penetration of gloves following usage in routine dental procedures. Am J Dent 1992;5:95--6. 192. Pitten FA, Herdemann G, Kramer A. The integrity of latex gloves in clinical dental practice. Infection 2000;28:388--92. 193. Jamal A, Wilkinson S. The mechanical and microbiological integrity of surgical gloves. ANZ J Surg 2003;73:140--3. 194. Korniewicz DM, El-Masri MM, Broyles JM, Martin CD, O'Connell KP. A laboratory-based study to assess the performance of surgical gloves. AORN J 2003;77:772--9. 195. Schwimmer A, Massoumi M, Barr CE. Efficacy of double gloving to prevent inner glove perforation during outpatient oral surgical procedures. J Am Dent Assoc 1994;125:196--8. 196. Patton LL, Campbell TL, Evers SP. Prevalence of glove perforations during double-gloving for dental procedures. Gen Dent 1995;43:22--6. 197. Gerberding JL, Littell C, Tarkington A, Brown A, Schecter WP. Risk of exposure of surgical personnel to patients' blood during surgery at San Francisco General Hospital. N Engl J Med 1990;322:1788--93. 198. Klein RC, Party E, Gershey EL. Virus penetration of examination gloves. Biotechniques 1990;9:196--9. 199. Mellstrom GA, Lindberg M, Boman A. Permeation and destructive effects of disinfectants on protective gloves. Contact Dermatitis 1992;26:163--70. 200. Jordan SL, Stowers MF, Trawick EG, Theis AB. Glutaraldehyde permeation: choosing the proper glove. Am J Infect Control 1996;24:67--9. 201. Cappuccio WR, Lees PS, Breysse PN, Margolick JB. Evaluation of integrity of gloves used in a flow cytometry laboratory. Infect Control Hosp Epidemiol 1997;18:423--5. 202. Monticello MV, Gaber DJ. Glove resistance to permeation by a 7.5% hydrogen peroxide sterilizing and disinfecting solution. Am J Infec Control 1999;27:364--6. 203. Baumann MA, Rath B, Fischer JH, Iffland R. The permeability of dental procedure and examination gloves by an alcohol based disinfectant. Dent Mater 2000;16:139--44. 204. Ready MA, Schuster GS, Wilson JT, Hanes CM. Effects of dental medicaments on examination glove permeability. J Prosthet Dent 1989;61:499--503. 205. Richards JM, Sydiskis RJ, Davidson WM, Josell SD, Lavine DS. Permeability of latex gloves after contact with dental materials. Am J Orthod Dentofacial Orthop 1993;104:224--9. 206. Andersson T, Bruze M, Bjorkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis 1999;41:254--9. 207. Reitz CD, Clark NP. The setting of vinyl polysiloxane and condensation silicone putties when mixed with gloved hands. J Am Dent Assoc 1988;116:371--5. 208. Kahn RL, Donovan TE, Chee WW. Interaction of gloves and rubber dam with a poly (vinyl siloxane) impression material: a screening test. Int J Prosthodont 1989;2:342--6. 209. Matis BA, Valadez D, Valadez E. The effect of the use of dental gloves on mixing vinyl polysiloxane putties. J Prosthodont 1997;6:189--92. 210. Wright JG, McGeer AJ, Chyatte D, Ransohoff DF. Mechanisms of glove tears and sharp injuries among surgical personnel. JAMA 1991;266:1668--71. 211. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg 1988;75:966--8. 212. Adams D, Bagg J, Limaye M, Parsons K, Absi EG. A clinical evaluation of glove washing and re-use in dental practice. J Hosp Infect 1992;20:153--62. 213. Martin MV, Dunn HM, Field EA, et al. A physical and microbiological evaluation of the re-use of non-sterile gloves. Br Dent J 1988;165:321--4. 214. US Department of Health and Human Services, Food and Drug Administration. 21 CFR Part 800. Medical devices; patient examination and surgeon's gloves. Adulteration, final rule. Federal Register 1990;55:51254--8. 215. Giglio JA, Roland RW, Laskin DM, Grenevicki L. The use of sterile versus nonsterile gloves during out-patient exodontia. Quintessence Int 1993;24:543--5. 216. Cheung LK, Chow LK, Tsang MH, Tung LK. An evaluation of complications following dental extractions using either sterile or clean gloves. Int J Oral Maxillofac Surg 2001;30:550--4. 217. Gani JS, Anseline PF, Bissett RL. Efficacy of double versus single gloving in protecting the operating team. Aust N Z J Surg 1990;60:171--5. 218. Short LJ, Bell DM. Risk of occupational infection with blood-borne pathogens in operating and delivery room settings. Am J Infect Control 1993;21:343--50. 219. Tokars JI, Culver DH, Mendelson MH, et al. Skin and mucous membrane contacts with blood during surgical procedures: risk and prevention. Infect Control Hosp Epidemiol 1995;16:703--11. 220. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection (Cochrane Review). The Cochrane Library 2003;(Issue 2):1--32. 221. Webb JM, Pentlow BD. Double gloving and surgical technique. Ann R Coll Surg Engl 1993;75:291--2. 222. Watts D, Tassler PL, Dellon AL. The effect of double gloving on cutaneous sensibility, skin compliance and suture identification. Contemp Surg 1994;44:289--92. 223. Wilson SJ, Sellu D, Uy A, Jaffer MA. Subjective effects of double gloves on surgical performance. Ann R Coll Surg Engl 1996;78:20--2. 224. Food and Drug Administration. Guidance for industry and FDA: medical glove guidance manual [Draft guidance]. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 1999. Available at http://www.fda.gov/cdrh/dsma/135.html#_Toc458914315. 225. Dillard SF, Hefflin B, Kaczmarek RG, Petsonk EL, Gross TP. Health effects associated with medical glove use. AORN J 2002;76:88--96. 226. Hamann CP, Turjanmaa K, Rietschel R, et al. Natural rubber latex hypersensitivity: incidence and prevalence of type I allergy in the dental professional. J Am Dent Assoc 1998;129:43--54. 227. Siew C, Hamann C, Gruninger SE, Rodgers P, Sullivan KM. 2003.Type I Latex Allergic Reactions among Dental Professionals, 1996--2001. Journal of Dental Research, 82 (Special Issue): #1718. 228. Saary MJ, Kanani A, Alghadeer H, Holness DL, Tarlo SM. Changes in rates of natural rubber latex sensitivity among dental school students and staff members after changes in latex gloves. J Allergy Clin Immunol 2002;109:131--5. 229. Hunt LW, Kelkar P, Reed CE, Yunginger JW. Management of occupational allergy to natural rubber latex in a medical center: the importance of quantitative latex allergen measurement and objective follow-up. J Allergy Clin Immunol 2002; 110(suppl 2):S96--106. 230. Turjanmaa K, Kanto M, Kautiainen H, Reunala T, Palosuo T. Long-term outcome of 160 adult patients with natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S70--4. 231. Heilman DK, Jones RT, Swanson MC, Yunginger JW. A prospective, controlled study showing that rubber gloves are the major contributor to latex aeroallergen levels in the operating room. J Allergy Clin Immunol 1996;98:325--30. 232. Baur X, Jager D. Airborne antigens from latex gloves. Lancet 1990; 335:912. 233. Turjanmaa K, Reunala T, Alenius H, Brummer-Korvenkontio H, Palosuo T. Allergens in latex surgical gloves and glove powder. Lancet 1990;336:1588. 234. Baur X, Chen Z, Allmers H. Can a threshold limit value for natural rubber latex airborne allergens be defined? J Allergy Clin Immunol 1998;101:24--7. 235. Trape M, Schenck P, Warren A. Latex gloves use and symptoms in health care workers 1 year after implementaion of a policy restricting the use of powdered gloves. Am J Infec Control 2000;28:352--8. 236. Allmers H, Brehler R, Chen Z, Raulf-Heimsoth M, Fels H, Baur X. Reduction of latex aeroallergens and latex-specific IgE antibodies in sensitized workers after removal of powdered natural rubber latex gloves in a hospital. Allergy Clin Immunol 1998;102:841--6. 237. Tarlo SM, Sussman G, Contala A, Swanson MC. Control of airborne latex by use of powder-free latex gloves. J Allergy Clin Immunol 1994;93:985--9. 238. Swanson MC, Bubak ME, Hunt LW, Yunginger JW, Warner MA, Reed CE. Quantification of occupational latex aeroallergens in a medical center. J Allergy Clin Immunol 1994;94:445--551. 239. Hermesch CB, Spackman GK, Dodge WW, Salazar A. Effect of powder-free latex examination glove use on airborne powder levels in a dental school clinic. J Dent Educ 1999;63:814--20. 240. Miller CH. Infection control strategies for the dental office [Chapter 29]. In: Ciancio SG, ed. ADA guide to dental therapeutics. 2nd ed. Chicago, IL: ADA Publishing, 2000:543--58. 241. Primeau MN, Adkinson NF Jr, Hamilton RG. Natural rubber pharmaceutical vial closures release latex allergens that produce skin reactions. J Allergy Clin Immunol 2001;107:958--62. 242. Spaulding EH. Chemical disinfection of medical and surgical materials [Chapter 32]. In: Lawrence CA, Block SS, eds. Disinfection, sterilization and preservation. Philadelphia, PA: Lea & Febiger, 1968: 517--31. 243. CDC. Guideline for disinfection and sterilization in healthcare facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR (in press). 245. US Environmental Protection Agency. 40 CFR Parts 152, 156, and 158. Exemption of certain pesticide substances from federal insecticide, fungicide, and rodenticide act requirements. Amended 1996. Federal Register 1996;61:8876--9. 246. Food and Drug Administration. Dental handpiece sterilization [Letter]. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 1992. 247. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Steam sterilization and sterility assurance in health care facilities. ANSI/AAMI ST46-2002. Arlington, VA: Association for the Advancement of Medical Instrumentation, 2002. 248. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Steam sterilization and sterility assurance using table-top sterilizers in office-based, ambulatory-care medical, surgical, and dental facilities. ANSI/AAMI ST40-1998. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1998. 249. Favero MS, Bond WW. Chemical disinfection of medical and surgical material [Chapter 43]. In: Block SS, ed. Disinfection, sterilization and preservation. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:881--917. 250. Parker HH 4th, Johnson RB. Effectiveness of ethylene oxide for sterilization of dental handpieces. J Dent 1995;23:113--5. 251. Alfa MJ, Olson N, Degagne P, Hizon R. New low temperature sterilization technologies: microbicidal activity and clinical efficacy [Chapter 9]. In: Rutala WA, ed. Disinfection, sterilization, and antisepsis in health-care. Champlain, NY: Polyscience Publications, 1998:67--78. 252. Rutala WA, Weber DJ. Clinical effectiveness of low-temperature sterilization technologies. Infect Control Hosp Epidemiol 1998; 19:798--804. 253. Miller CH, Tan CM, Beiswanger MA, Gaines DJ, Setcos JC, Palenik CJ. Cleaning dental instruments: measuring the effectiveness of an instrument washer/disinfector. Am J Dent 2000;13:39--43. 254. Association for the Advancement of Medical Instrumentation. Chemical indicators---guidance for the selection, use, and interpretation of results. AAMI Technical Information Report No. 25. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1999. 255. Ninemeier J. Central service technical manual. 5th ed. Chicago, IL: International Association of Healthcare Central Service Materiel Management, 1998. 256. Rutala WA, Weber DJ. Choosing a sterilization wrap for surgical packs. Infection Control Today 2000;4:64,70. 257. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Good hospital practice: steam sterilization and sterility assurance. ANSI/AAMI ST46-1993. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1993. 258. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Flash sterilization: steam sterilization of patient care items for immediate use. ANSI/AAMI ST37-1996. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1996. 259. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Ethylene oxide sterilization in health care facilities: safety and effectiveness. ANSI/AAMI ST41-1999. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1999. 260. Miller CH, Palenik CJ. Sterilization, disinfection, and asepsis in dentistry [Chapter 53]. In: Block SS, ed. 5th ed. Disinfection, sterilization, and preservation. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:1049--68. 261. Joslyn LJ. Sterilization by heat [Chapter 36]. In: Block SS, ed. 5th ed. Disinfection, sterilization, and preservation. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:695--728. 262. Rutala WA, Weber DJ, Chappell KJ. Patient injury from flash-sterilized instruments. Infect Control Hosp Epidemiol 1999;20:458. 263. Bond WW. Biological indicators for a liquid chemical sterilizer: a solution to the instrument reprocessing problem? Infect Control Hosp Epidemiol 1993;14:309--12. 264. Stingeni L, Lapomarda V, Lisi P. Occupational hand dermatitis in hospital environments. Contact Dermatitis 1995;33:172--6. 265. Ashdown BC, Stricof DD, May ML, Sherman SJ, Carmody RF. Hydrogen peroxide poisoning causing brain infarction: neuroimaging findings. Am J Roentgenol 1998;170:1653--5. 266. Ballantyne B. Toxicology of glutaraldehyde: review of studies and human health effects. Danbury, CT: Union Carbide, 1995. 267. CDC. National Institute for Occupational Safety and Health. Glutaraldehyde: occupational hazards in hospitals. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 2001. DHHS publication no. (NIOSH) 2001-115. 269. Lehman PA, Franz TJ, Guin JD. Penetration of glutaraldehyde through glove material: tactylon versus natural rubber latex. Contact Dermatitis 1994;30:176--7. 270. Hamann CP, Rodgers PA, Sullivan K. Allergic contact dermatitis in dental professionals: effective diagnosis and treatment. J Am Dent Assoc 2003;134:185--94. 271. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Safe use and handling of glutaraldehyde-based products in health care facilities. ANSI/AAMI ST58-1996. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1996. 272. Fisher AA. Ethylene oxide dermatitis. Cutis 1984;34:20, 22, 24. 273. Jay WM, Swift TR, Hull DS. Possible relationship of ethylene oxide exposure to cataract formation. Am J Ophthalmol 1982;93:727--32. 274. US Department of Labor, Occupational Safety and Health Administration. Review of the ethylene oxide standard. Federal Register 2000;65:35127--8. 275. Pratt LH, Smith DG, Thornton RH, Simmons JB, Depta BB, Johnson RB. The effectiveness of two sterilization methods when different precleaning techniques are employed. J Dent 1999;27:247--8. 276. US Department of Health and Human Services, Food and Drug Administration. 21 CFR Part 872.6730. Dental devices; endodontic dry heat sterilizer; final rule. Federal Register 1997;62:2903. 277. Favero MS. Current issues in hospital hygiene and sterilization technology. J Infect Control (Asia Pacific Edition) 1998;1:8--10. 278. Greene WW. Control of sterilization process [Chapter 22]. In: Russell AD, Hugo WB, Ayliffe GA, eds. Principles and practice of disinfection, preservation, and sterilization. Oxford, England: Blackwell Scientific Publications, 1992:605--24. 279. Favero MS. Developing indicators for sterilization [Chapter 13]. In: Rutala W, ed. Disinfection, sterilization, and antisepsis in health care. Washington, DC: Association for Professionals in Infection Control and Epidemiology, Inc., 1998:119--32. 280. Maki DG, Hassemer CA. Flash sterilization: carefully measured haste. Infect Control 1987;8:307--10. 281. Andres MT, Tejerina JM, Fierro JF. Reliability of biologic indicators in a mail-return sterilization-monitoring service: a review of 3 years. Quintessence Int 1995;26:865--70. 282. Miller CH, Sheldrake MA. The ability of biological indicators to detect sterilization failures. Am J Dent 1994;7:95--7. 283. Association of Operating Room Nurses. AORN standards and recommended practices for perioperative nursing. Denver, CO: AORN, 1987. 284. Mayworm D. Sterile shelf life and expiration dating. J Hosp Supply Process Distrib 1984;2:32--5. 285. Cardo DM, Sehulster LM. Central sterile supply [Chapter 65]. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 1999:1023--30. 286. Maki DG, Alvarado CJ, Hassemer CA, Zilz MA. Relation of the inanimate hospital environment to endemic nosocomial infection. N Engl J Med 1982;307:1562--6. 287. Danforth D, Nicolle LE, Hume K, Alfieri N, Sims H. Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent. J Hosp Infect 1987;10:229--35. 288. Crawford JJ. Clinical asepsis in dentistry. Mesquite, TX: Oral Medicine Press, 1987. 289. Food and Drug Administration. Design control guidance for medical device manufacturers. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 1997. 290. Fauerbach LL, Janelle JW. Practical applications in infection control [Chapter 45]. In: Block SS, ed. 5th ed. Disinfection, sterilization, and preservation. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:935--44. 291. Martin LS, McDougal JS, Loskoski SL. Disinfection and inactivation of the human T lymphotrophic virus type III/lymphadenopathy-associated virus. J Infect Dis 1985;152:400--3. 292. Bloomfield SF, Smith-Burchnell CA, Dalgleish AG. Evaluation of hypochlorite-releasing disinfectants against the human immunodeficiency virus (HIV). J Hosp Infect 1990;15:273--8. 293. Gerson SL, Parker P, Jacobs MR, Creger R, Lazarus HM. Aspergillosis due to carpet contamination. Infect Control Hosp Epidemiol 1994;15:221--3. 294. Suzuki A, Namba Y, Matsuura M, Horisawa A. Bacterial contamination of floors and other surfaces in operating rooms: a five--year survey. J Hyg (Lond) 1984;93:559--66. 295. Skoutelis AT, Westenfelder GO, Beckerdite M, Phair JP. Hospital carpeting and epidemiology of Clostridium difficile. Am J Infect Control 1994;22:212--7. 296. Rutala WA, Odette RL, Samsa GP. Management of infectious waste by US hospitals. JAMA 1989;262:1635--40. 298. Palenik CJ. Managing regulated waste in dental environments. J Contemp Dent Pract 2003;4:76. 299. Rutala WA, Mayhall CG. Medical waste. Infect Control Hosp Edidemiol 1992;13:38--48. 300. Greene R, State and Territorial Association on Alternate Treatment Technologies. Technical assistance manual: state regulatory oversight of
medical waste treatment technologies.
2nd ed. Washington, DC: US Environmental Protection Agency, 1994. Available at
301. US Environmental Protection Agency. 40 CFR Part 60. Standards of performance for new stationary sources and emission guidelines for existing sources: hospital/medical/infectious waste incinerators; final rule. Federal Register 1997;62:48347--91. 302. Slade JS, Pike EB, Eglin RP, Colbourne JS, Kurtz JB. The survival of human immunodeficiency virus in water, sewage, and sea water. Water Sci Tech 1989;21:55--9. 303. Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol 2000;66:3363--7. 304. Schulze-Robbecke R, Feldmann C, Fischeder R, Janning B, Exner M, Wahl G. Dental units: an environmental study of sources of potentially pathogenic mycobacteria. Tuber Lung Dis 1995;76:318--23. 305. Barbeau J, Tanguay R, Faucher E, et al. Multiparametric analysis of waterline contamination in dental units. Appl Environ Microbiol 1996;62:3954--9. 306. Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol 1995;61:1208--13. 307. Kelstrup J, Funder-Nielsen T, Theilade J. Microbial aggregate contamination of water lines in dental equipment and its control. Acta Pathol Microbiol Scand [B] 1977;85:177--83. 308. Challacombe SJ, Fernandes LL. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J Am Dent Assoc 1995;126:603--8. 309. Mayo JA, Oertling KM, Andrieu SC. Bacterial biofilm: a source of contamination in dental air-water syringes. Clin Prev Dent 1990;12:13--20. 310. Scheid RC, Kim CK, Bright JS, Whitely MS, Rosen S. Reduction of microbes in handpieces by flushing before use. J Am Dent Assoc 1982;105:658--60. 311. Bagga BS, Murphy RA, Anderson AW, Punwani I. Contamination of dental unit cooling water with oral microorganisms and its prevention. J Am Dent Assoc 1984;109:712--6. 312. Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J 1987;163:152--4. 313. Pankhurst CL, Philpott-Howard JN, Hewitt JH, Casewell MW. The efficacy of chlorination and filtration in the control and eradication of Legionella from dental chair water systems. J Hosp Infect 1990;16:9--18. 314. Mills SE, Lauderdale PW, Mayhew RB. Reduction of microbial contamination in dental units with povidone-iodine 10%. J Am Dent Assoc 1986;113:280--4. 315. Williams JF, Johnston AM, Johnson B, Huntington MK, Mackenzie CD. Microbial contamination of dental unit waterlines: prevalence, intensity and microbiological characteristics. J Am Dent Assoc 1993;124:59--65. 316. Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. J Am Dent Assoc 2000;131:1427--41. 317. Jones F, Bartlett CL. Infections associated with whirlpools and spas. Soc Appl Bacteriol Symp Ser 1985;14:61S--6S. 318. Hollyoak V, Allison D, Summers J. Pseudomonas aeruginosa wound infection associated with a nursing home's whirlpool bath. Commun Dis Rep CDR Rev 1995;5:R100--2. 319. Begg N, O'Mahony M, Penny P, Richardson EA, Basavaraj DS. Mycobacterium chelonei associated with a hospital hydrotherapy pool. Community Med 1986;8:348--50. 320. Laussucq S, Baltch AL, Smith RP, et al. Nosocomial Mycobacterium fortuitum colonization from a contaminated ice machine. Am Rev Respir Dis 1988;138:891--4. 321. Struelens MJ, Rost F, Deplano A, et al. Pseudomonas aeruginosa and Enterobacteriaceae bacteremia after biliary endoscopy: an outbreak investigation using DNA macrorestriction analysis. Am J Med 1993;95: 489--98. 322. Kuritsky JN, Bullen MG, Broome CV, Silcox VA, Good RC, Wallace RJ Jr. Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med 1983;98:938--9. 323. Bolan G, Reingold AL, Carson LA, et al. Infections with Mycobacterium chelonei in patients receiving dialysis and using processed hemodialyzers. J Infect Dis 1985;152:1013--9. 324. Lessing MP, Walker MM. Fatal pulmonary infection due to Mycobacterium fortuitum. J Clin Pathol 1993;46:271--2. 325. Arnow PM, Chou T, Weil D, Shapiro EN, Kretzschmar C. Nosocomial Legionnaires' disease caused by aerosolized tap water from respiratory devices. J Infect Dis 1982;146:460--7. 326. Breiman RF, Fields BS, Sanden GN, Volmer L, Meier A, Spika JS. Association of shower use with Legionnaires' disease: possible role of amoebae. JAMA 1990;263:2924--6. 327. Garbe PL, Davis BJ, Weisfeld JS, et al. Nosocomial Legionnaires' disease: epidemiologic demonstration of cooling towers as a source. JAMA 1985;254:521--4. 328. Fallon RJ, Rowbotham TJ. Microbiological investigations into an outbreak of Pontiac fever due to Legionella micdadei associated with use of a whirlpool. J Clin Pathol 1990;43:479--83. 329. Rose CS, Martyny JW, Newman LS, et al. "Lifeguard lung": endemic granulomatous pneumonitis in an indoor swimming pool. Am J Public Health 1998;88:1795--1800. 331. Jacobs RL, Thorner RE, Holcomb JR, Schwietz LA, Jacobs FO. Hypersensitivity pneumonitis caused by Cladosporium in an enclosed hot-tub area. Ann Intern Med 1986;105:204--6. 332. Clark A. Bacterial colonization of dental units and the nasal flora of dental personnel. Proc Roy Soc Med 1974;67:1269--70. 333. Fotos PG, Westfall HN, Snyder IS, Miller RW, Mutchler BM. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res 1985;64:1382--5. 334. Reinthaler FF, Mascher F, Stunzner D. Serological examinations for antibodies against Legionella species in dental personnel. J Dent Res 1988;67:942--3. 335. Putnins EE, Di Giovanni D, Bhullar AS. Dental unit waterline contamination and its possible implications during periodontal surgery. J Periodontol 2001;72:393--400. 336. United States Pharmacopeial Convention. Sterile water for irrigation. In: United States Pharmacopeial Convention. United States pharmacopeia and national formulary. USP 24--NF 19. Rockville, MD: United States Pharmacopeial Convention, 1997:1753. 337. Milton DK, Wypij D, Kriebel D, Walters MD, Hammond SK, Evans JS. Endotoxin exposure-response in a fiberglass manufacturing facility. Am J Ind Med 1996;29:3--13. 338. Santiago JI. Microbial contamination of dental unit waterlines: short and long term effects of flushing. Gen Dent 1994;42:528--35. 339. Shearer BG. Biofilm and the dental office. J Am Dent Assoc 1996; 127:181--9. 340. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Hemodialysis systems. ANSI/AAMI RD5-1992. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1993. 341. US Environmental Protection Agency. National primary drinking water regulations, 1999: list of contaminants. Washington DC: US Environmental Protection Agency, 1999. Available at http://www.epa.gov/safewater/mcl.html. 342. American Public Health Association, American Water Works Association, Water Environment Foundation. In: Eaton AD, Clesceri LS, Greenberg AE, eds. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association, 1999. 343. Williams HN, Johnson A, Kelley JI, et al. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int 1995;26:331--7. 344. Scheid RC, Rosen S, Beck FM. Reduction of CFUs in high-speed handpiece water lines over time. Clin Prev Dent 1990;12:9--12. 345. Williams HN, Kelley J, Folineo D, Williams GC, Hawley CL, Sibiski J. Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. J Am Dent Assoc 1994;125: 1205--11. 346. CDC, Working Group on Waterborne Cryptosporidiosis. Cryptosporidium and water: a public health handbook. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC, 1997. 347. MacKenzie WR, Hoxie NJ, Proctor ME, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med 1994;331:161--7. 348. Kaminski JC. Cryptosporidium and the public water supply. N Engl J Med 1994;331:1529--30. 351. Office of Water, US Environmental Protection Agency. Lead and copper rule: summary of revisions. EPA 815--R--99--020. Washington DC: US Environmental Protection Agency, 2000. 352. US Environmental Protection Agency. 65 CFR Parts 141 and 142. National primary drinking water regulations for lead and copper, final rule. Federal Register 2000;1949--2015. 353. Gooch B, Marianos D, Ciesielski C, et al. Lack of evidence for patient-to-patient transmission of HIV in a dental practice. J Am Dent Assoc 1993;124:38--44. 354. Crawford JJ, Broderius C. Control of cross-infection risks in the dental operatory: preventon of water retraction by bur cooling spray systems. J Am Dent Assoc 1988;116:685--7. 355. Mills SE, Kuehne JC, Bradley DV Jr. Bacteriological analysis of high-speed handpiece turbines. J Am Dent Assoc 1993;124:59--62. 356. Lewis DL, Arens M, Appleton SS, et al. Cross-contamination potential with dental equipment. Lancet 1992;340:1252--4. 357. Lewis DL, Boe RK. Cross-infection risks associated with current procedures for using high-speed dental handpieces. J Clin Microbiol 1992;30:401--6. 358. Checchi L, Montebugnoli L, Samaritani S. Contamination of the turbine air chamber: a risk of cross infection. J Clin Periodontol 1998;25:607--11. 359. Epstein JB, Rea G, Sibau L, Sherlock CH, Le ND. Assessing viral retention and elimination in rotary dental instruments. J Am Dent Assoc 1995;126:87--92. 360. Kolstad RA. How well does the chemiclave sterilize handpieces? J Am Dent Assoc 1998;129:985--91. 361. Kuehne JS, Cohen ME, Monroe SB. Performance and durability of autoclavable high-speed dental handpieces. NDRI-PR 92-03. Bethesda, MD: Naval Dental Research Institute, 1992. 362. Andersen HK, Fiehn NE, Larsen T. Effect of steam sterilization inside the turbine chambers of dental turbines. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:184--8. 363. Leonard DL, Charlton DG. Performance of high-speed dental handpieces subjected to simulated clinical use and sterilization. J Am Dent Assoc 1999;130:1301--11. 364. Barbeau J, ten Bokum L, Gauthier C, Prevost AP. Cross-contamination potential of saliva ejectors used in dentistry. J Hosp Infect 1998; 40:303--11. 365. Mann GL, Campbell TL, Crawford JJ. Backflow in low-volume suction lines: the impact of pressure changes. J Am Dent Assoc 1996;127:611--5. 366. Watson CM, Whitehouse RL. Possibility of cross-contamination between dental patients by means of the saliva ejector. J Am Dent Assoc 1993;124:77--80. 367. Glass BJ, Terezhalmy GT. Infection control in dental radiology [Chapter 15]. In: Cottone JA, Terezhalamy GT, Molinari JA, eds. Practical infection control in dentisty. 2nd ed. Baltimore. MD: Williams & Wilkins, 1996:229--38. 368. Haring JI, Jansen L. Infection control and the dental radiographer. In: Haring JI, Jansen L, eds. Dental radiography: principles and techniques. Philadelphia, PA: WB Saunders Co., 2000:194--204. 369. Hignett M, Claman P. High rates of perforation are found in endovaginal ultrasound probe covers before and after oocyte retrieval for in vitro fertilization-embryo transfer. J Assist Reprod Genet 1995;12:606--9. 370. Fritz S, Hust MH, Ochs C, Gratwohl I, Staiger M, Braun B. Use of a latex cover sheath for transesophageal echocardiography (TEE) instead of regular disinfection of the echoscope? Clin Cardiol 1993;16:737--40. 371. Milki AA, Fisch JD. Vaginal ultrasound probe cover leakage: implications for patient care. Fertil Steril 1998;69:409--11. 372. Storment JM, Monga M, Blanco JD. Ineffectiveness of latex condoms in preventing contamination of the transvaginal ultrasound transducer head. South Med J 1997;90:206--8. 373. Amis S, Ruddy M, Kibbler CC, Economides DL, MacLean AB. Assessment of condoms as probe covers for transvaginal sonography. J Clin Ultrasound 2000;28:295--8. 374. Rooks VJ, Yancey MK, Elg SA, Brueske L. Comparison of probe sheaths for endovaginal sonography. Obstet Gynecol 1996;87:27--9. 375. Hokett SD, Honey JR, Ruiz F, Baisden MK, Hoen MM. Assessing the effectiveness of direct digital radiography barrier sheaths and finger cots. J Am Dent Assoc 2000;131:463--7. 376. ASHP Council on Professional Affairs. ASHP guidelines on quality assurance for pharmacy-prepared sterile products. Am J Health Syst Pharm 2000;57:1150--69. 377. Green KA, Mustachi B, Schoer K, Moro D, Blend R, McGeer A. Gadolinium-based MR contrast media: potential for growth of microbial contaminants when single vials are used for multiple patients. Am J Roentgenol 1995;165:669--71. 378. American Society of Anesthesiologists. Recommendations for infection control for the practice of anesthesiology. 2nd ed. Park Ridge, IL: American Society of Anesthesiologists,1999. 379. Henry B, Plante-Jenkins C, Ostrowska K. An outbreak of Serratia marcescens associated with the anesthetic agent propofol. Am J Infect Control 2001;29:312--5. 380. Plott RT, Wagner RF Jr, Tyring SK. Iatrogenic contamination of multidose vials in simulated use. A reassessment of current patient injection technique. Arch Dermatol 1990;126:1441--4. 381. Arrington ME, Gabbert KC, Mazgaj PW, Wolf MT. Multidose vial contamination in anesthesia. AANA J 1990;58:462--6. 383. Food and Drug Administration. Labeling recommendations for single-use devices reprocessed by third parties and hospitals; final guidance for industry and FDA. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 2001. 384. Villasenor A, Hill SD, Seale NS. Comparison of two ultrasonic cleaning units for deterioration of cutting edges and debris removal on dental burs. Pediatr Dent 1992;14:326--30. 385. Rapisarda E, Bonaccorso A, Tripi TR, Condorelli GG. Effect of sterilization on the cutting efficiency of rotary nickel-titanium endodontic files. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:343--7. 386. Filho IB, Esberard RM, Leonardo R, del Rio CE. Microscopic evaluation of three endodontic files pre- and postinstrumentation. J Endodontics 1998;24:461--4. 387. Silvaggio J, Hicks ML. Effect of heat sterilization on the torsional properties of rotary nickel-titanium endodontic files. J Endodontics 1997;23:731--4. 388. Kazemi RB, Stenman E, Spangberg LS. The endodontic file is a disposable instrument. J Endodontics 1995;21:451--5. 389. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA 1990;264:2919--22. 390. Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically compromised patient. Periodontology 2000 1996;10:107--38. 391. Litsky BY, Mascis JD, Litsky W. Use of an antimicrobial mouthwash to minimize the bacterial aerosol contamination generated by the high-speed drill. Oral Surg Oral Med Oral Pathol 1970;29:25--30. 392. Mohammed CI, Monserrate V. Preoperative oral rinsing as a means of reducing air contamination during use of air turbine handpieces. Oral Surg Oral Med Oral Pathol 1970;29:291--4. 393. Wyler D, Miller RL, Micik RE. Efficacy of self-administered preoperative oral hygiene procedures in reducing the concentration of bacteria in aerosols generated during dental procedures. J Dent Res 1971;50:509. 394. Muir KF, Ross PW, MacPhee IT, Holbrook WP, Kowolik MJ. Reduction of microbial contamination from ultrasonic scalers. Br Dent J 1978;145:76--8. 395. Fine DH, Mendieta C, Barnett ML, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. J Periodontol 1992;63:821--4. 396. Fine DH, Furgang D, Korik I, Olshan A, Barnett ML, Vincent JW. Reduction of viable bacteria in dental aerosols by preprocedural rinsing with an antiseptic mouthrinse. Am J Dent 1993;6:219--21. 397. Fine DH, Yip J, Furgang D, Barnett ML, Olshan AM, Vincent J. Reducing bacteria in dental aerosols: pre-procedural use of an antiseptic mouth rinse. J Am Dent Assoc 1993;124:56--8. 398. Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc 1995;126:1634--9. 399. Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. Gen Dent 2001;49:648--52. 400. Brown AR, Papasian CJ, Shultz P, Theisen FC, Shultz RE. Bacteremia and intraoral suture removal: can an antimicrobial rinse help? J Am Dent Assoc 1998;129:1455--61. 401. Lockhart PB. An analysis of bacteremias during dental extractions. A double-blind, placebo-controlled study of chlorhexidine. Arch Intern Med 1996;156:513--20. 402. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA 1997;277:1794--1801. 403. Tate WH, White RR. Disinfection of human teeth for educational purposes. J Dent Educ 1991;55:583--5. 404. Pantera EA Jr, Zambon JJ, Shih-Levine M. Indirect immunofluorescence for the detection of Bacteroides species in human dental pulp. J Endodontics 1988;14:218--23. 405. Pantera EA Jr, Schuster GS. Sterilization of extracted human teeth. J Dent Educ 1990;54:283--5. 406. Parsell DE, Stewart BM, Barker JR, Nick TG, Karns L, Johnson RB. The effect of steam sterilization on the physical properties and perceived cutting characteristics of extracted teeth. J Dent Educ 1998; 62;260--3. 407. American Dental Association's Council on Scientific Affairs and Council on Dental Practice. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc 1996;127:672--80. 408. Dental Laboratory Relationship Working Group, Organization for Safety and Asepsis Procedures (OSAP). Laboratory asepsis position paper. Annapolis, MD: OSAP Foundation, 1998. Available at http://www.osap.org/issues/pages/position/LAB.pdf. 409. Kugel G, Perry RD, Ferrari M, Lalicata P. Disinfection and communication practices: a survey of U. S. dental laboratories. J Am Dent Assoc 2000;131:786--92. 410. US Department of Transportation. 49 CFR 173.196 infectious substances (etiologic agents) 173.197 regulated medical waste. Available at http://www.access.gpo.gov/nara/cfr/waisidx_02/49cfr173_02.html. 411. Chau VB, Saunders TR, Pimsler M, Elfring DR. In-depth disinfection of acrylic resins. J Prosthet Dent 1995;74:309--13. 412. Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent 1990;64:235--7. 413. Giblin J, Podesta R, White J. Dimensional stability of impression materials immersed in an iodophor disinfectant. Int J Prosthodont 1990;3:72--7. 414. Plummer KD, Wakefield CW. Practical infection control in dental laboratories. Gen Dent 1994;42:545--8. 415. Merchant VA. Infection control in the dental laboratory equipment [Chapter 16]. In: Cottone JA, Terezhalamy GT, Molinari JA, eds. Practical infection control in dentisty. 2nd ed. Baltimore, MD: Williams & Wilkins, 1996:239--54. 416. Molinari J. Dental. In: Association for Professionals in Infection Control and Epidemiology, Inc. (APIC). APIC text of infection control and epidemiology. Washington, DC: Association for Professionals in Infection Control and Epidemiology, Inc, 2002. 417. Sofou A, Larsen T, Fiehn NE, Owall B. Contamination level of alginate impressions arriving at a dental laboratory. Clin Oral Invest 2002;6:161--5. 418. McNeill MR, Coulter WA, Hussey DL. Disinfection of irreversible hydrocolloid impressions: a comparative study. Int J Prosthodont 1992;5:563--7. 419. Gerhardt DE, Sydiskis RJ. Impression materials and virus. J Am Dent Assoc 1991;122:51--4. 420. Leung RL, Schonfeld SE. Gypsum casts as a potential source of microbial cross-contamination. J Prosthet Dent 1983;49:210--1. 421. Huizing KL, Palenik CJ, Setcos JC, Sheldrake MA, Miller, CH. Method of evaluating the antimicrobial abilities of disinfectant-containing gypsum products. QDT Yearbook 1994;17:172--6. 422. Verran J, Kossar S, McCord JF. Microbiological study of selected risk areas in dental technology laboratories. J Dent 1996;24:77--80. 423. CDC. National Institute for Occupational Safety and Health. NIOSH Health Hazard Evaluation and Technical Assistance Report. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1988. HETA 85-136-1932. 424. CDC. National Institute for Occupational Safety and Health. NIOSH Health Hazard Evaluation and Technical Assistance Report. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1990. HETA 88-101-2008. 425. CDC. National Institute for Occupational Safety and Health. Control of smoke from laser/electric surgical procedures. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, CDC, National Institute for Occupational Safety and Health, 1996. DHHS publication no. (NIOSH) 96-128. 426. Taravella MJ, Weinberg A, Blackburn P, May M. Do intact viral particles survive excimer laser ablation? Arch Ophthalmol 1997;115:1028--30. 427. Hagen KB, Kettering JD, Aprecio RM, Beltran F, Maloney RK. Lack of virus transmission by the excimer laser plume. Am J Ophthalmol 1997;124:206--11. 428. Kunachak S, Sithisarn P, Kulapaditharom B. Are laryngeal papilloma virus-infected cells viable in the plume derived from a continuous mode carbon dioxide laser, and are they infectious? A preliminary report on one laser mode. J Laryng Otol 1996;110:1031--3. 429. Hughes PS, Hughes AP. Absence of human papillomavirus DNA in the plume of erbium: YAG laser-treated warts. J Am Acad Dermatol 1998;38:426--8. 430. Garden JM, O'Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA 1988;259: 1199--1202. 431. Sawchuk WS, Weber PJ, Lowry DR, Dzubow LM. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol 1989;21:41--9. 432. Baggish MS, Poiesz BJ, Joret D, Williamson P, Rafai A. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med 1991;11:197--203. 433. Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med 1998;23:172--4. 434. McKinley IB Jr, Ludlow MO. Hazards of laser smoke during endodontic therapy. J Endodontics 1994;20:558--9. 435. Favero MS, Bolyard EA. Microbiologic considerations. Disinfection and sterilization strategies and the potential for airborne transmission of bloodborne pathogens. Surg Clin North Am 1995;75:1071--89. 436. Association of Operating Room Nurses. Recommended practices for laser safety in the practice setting. In: Fogg D, ed. Standards, recommended practices and guidelines. Denver, CO: AORN, 2003. 437. Streifel AJ. Recognizing IAQ risk and implementing an IAQ program. In: Hansen W, ed. A guide to managing indoor air quality in health care organizations. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations Publishers, 1997. 438. US Department of Labor, Occupational Safety and Health Administration. Safety and health topics: laser/electrosurgery plume. Washington DC:
US Department of Labor, Occupational Safety and Health Administration, 2003. Available at
439. American Thoracic Society, CDC. Diagnostic standards and classification of tuberculosis in adults and children. Am J Resp Crit Care 2000;161:1376--95. 440. Wells WF. Aerodynamics of droplet nuclei [Chapter 3]. In: Wells WF, ed. Airborne contagion and air hygiene: an ecological study of droplet infections. Cambridge, MA: Harvard University Press, 1955. 442. Smith WH, Davies D, Mason KD, Onions JP. Intraoral and pulmonary tuberculosis following dental treatment. Lancet 1982;1:842--4. 444. Mikitka D, Mills SE, Dazey SE, Gabriel ME. Tuberculosis infection in US Air Force dentists. Am J Dent 1995;8:33--6. 446. CDC. Surveillance for Creutzfeldt-Jakob disease---United States. MMWR 1996;45:665--8. 447. Johnson RT, Gibbs CJ Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med 1998;339: 1994--2004. 448. CDC. New variant CJD: fact sheet. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC, 2003. Available at http://www.cdc.gov/ncidod/diseases/cjd/cjd_fact_sheet.htm. 449. Will RG, Ironside JW, Zeidler M, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 1996;347:921--5. 450. Bruce ME, Will RG, Ironside JW, et al. Transmission to mice indicate that `new variant' CJD is caused by the BSE agent. Nature 1997;389: 498--501. 451. Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of `new variant' CJD. Nature 1996;383:685--90. 452. World Health Organization. Bovine spongiform encephalopathy (BSE). Fact Sheet No. 113. Geneva, Switzerland: World Health Organization, 2002. Available at http://www.who.int/mediacentre/factsheets/fs113/en/. 454. Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy specimens. Lancet 1999;353:183--9. 455. Brown P, Gibbs CJ Jr, Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 1994;35:513--29. 456. Brown P. Environmental causes of human spongiform encephalopathy [Chapter 8]. In: Baker HF, Baker HF, eds. Prion diseases. Totowa, NJ: Humana Press Inc, 1996:139--54. 457. Carp RI. Transmission of scrapie by oral route: effect of gingival scarification. Lancet 1982;1:170--1. 458. Ingrosso L, Pisani F, Pocchiari M. Transmission of the 263K scrapie strain by the dental route. J Gen Virol 1999;80:3043--7. 459. Bernoulli C, Siegfried J, Baumgartner G, et al. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet 1977;1:478--9. 460. Brown P, Gajdusek DC, Gibbs CJ Jr, Asher DM. Potential epidemic of Creutzfeldt-Jakob disease from human growth hormone therapy. N Engl J Med 1985;313:728--31. 462. Duffy P, Wolf J, Collins G, DeVoe AG, Streeten B, Cowen D. Possible person-to-person transmission of Creutzfeldt-Jakob disease. N Engl J Med 1974;290:692--3. 464. Thadani V, Penar PL, Partington J, et al. Creutzfeldt-Jakob disease probably acquired from a cadaveric dura mater graft. Case report. J Neurosurg 1988;69:766--9. 465. Kondo K, Kuroiwa Y. A case control study of Creutzfeldt-Jakob disease: association with physical injuries. Ann Neurol 1982;11:377--81. 466. Van Duijn CM, Delasnerie-Laupretre N, Masullo C, et al, and European Union (EU) Collaborative Study Group of Creutzfeldt-Jacob disease (CJD). Case-control study of risk factors of Creutzfeldt-Jakob disease in Europe during 1993--95. Lancet 1998;351:1081--5. 467. Collins S, Law MG, Fletcher A, Boyd A, Kaldor J, Masters CL. Surgical treatment and risk of sporadic Creutzfeldt-Jakob disease: a case-control study. Lancet 1999;353:693--7. 468. Blanquet-Grossard F, Sazdovitch V, Jean A, et al. Prion protein is not detectable in dental pulp from patients with Creutzfeldt-Jakob disease. J Dent Res 2000;79:700. 469. World Health Organization. Infection control guidelines for transmissible spongiform encephalopathies: report of a WHO consultation,
Geneva, Switzerland, 23--26 March 1999. Geneva, Switzerland: World Health Organization, 2000. Available at
470. Institute of Medicine, Committee on Quality of Health Care in America. Kohn LT, Corrigan JM, Donadlson MS, eds. To err is human: building a safe health system. Washington, DC: National Academy Press, 1999. 471. CDC. Framework for program evaluation in public health. MMWR 1999;48(No. RR-11).
Advisory Group Joseph A. Bartoloni, D.M.D., United States Air Force Dental Investigation Service, Great Lakes, Illinois; Nancy Bjerke, M.A., M.Ed., M.P.H., Infection Control Associates, San Antonio, Texas; Walter W. Bond, M.S., RCSA Inc., Lawrenceville, Georgia; Eve Cuny, M.S., University of Pacific School of Dentistry, San Francisco, California; Kathy J. Eklund, M.H.P., Forsyth Institute, Boston, Massachusetts; Curt Hamann, M.D., Smart Practice, Phoenix, Arizona; Jennifer A. Harte, D.D.S., United States Air Force Dental Investigation Service, Great Lakes, Illinois; Chris Miller, Ph.D., Indiana University School of Dentistry, Bloomington, Indiana; Shannon E. Mills, D.D.S., Air Force Medical Operations Agency, Bolling AFB, Washington, District of Columbia; John Molinari, Ph.D., University of Detroit Mercy School of Dentistry, Detroit, Michigan; William A. Rutala, Ph.D., University of North Carolina School of Medicine, Chapel Hill, North Carolina; Brian Shearer, Ph.D., Bayer, Inc., Chicago, Illinois.
CDC Consultants Matthew Arduino, Dr.P.H., Elizabeth Bolyard, M.P.H., Denise Cardo, M.D., Joe Carpenter, Linda Chiarello, M.P.H., Lynne Sehulster, Ph.D., Division of Healthcare Quality Promotion, National Center for Infectious Diseases (NCID), Atlanta, Georgia; Miriam J. Alter, Ph.D., Division of Viral Hepatitis, NCID; Larry Schonberger, Ph.D., Ermias Belay, M.D., Division of Viral and Ricketsial Diseases, NCID, Atlanta, Georgia; Susan Y. Chu, Ph.D., National Immunization Program, Atlanta, Georgia; Paul A. Jensen, National Center for HIV, STD and TB Prevention, Atlanta, Georgia; Janice Huy, National Institute of Occupational Safety and Health (NIOSH), Cincinnati, Ohio; Lee Petsonk, NIOSH, Morgantown, West Virginia; Jennifer L. Cleveland, D.D.S., Amy S. Collins, M.P.H., Barbara F. Gooch, D.M.D., William G. Kohn, D.D.S., Dolores M. Malvitz, Dr.P.H., Division of Oral Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta Georgia.
Other Federal Consultants Susan Runner, D.D.S., Food and Drug Administration, Rockville, Maryland; Elise Handelman, Occupational Safety and Health Administration, Washington, District of Columbia; Jeffrey Kempter, M.S., David Liem, Ph.D., Michelle Wingfield, Environmental Protection Agency, Washington, District of Columbia.
Outside Consultants Martin S. Favero, Ph.D., Advanced Sterilization Products, Johnson and Johnson, Irvine, California; Pamela Rodgers, Ph.D., SmartHealth Inc., Phoenix, Arizona; Daniel M. Meyer, D.D.S., American Dental Association, Chicago, Illinois; Deborah Greenspan, BDS, DSC University of California San Francisco School of Dentistry, San Francisco, California; Helene Bednarsh, Boston Department of Public Health, Boston, Massachusetts; Steve Peake, Barnstead-Harvey Corp, Dubuque, Iowa; Chakwan Siew, Ph.D., American Dental Association, Chicago, Illinois. Table 1 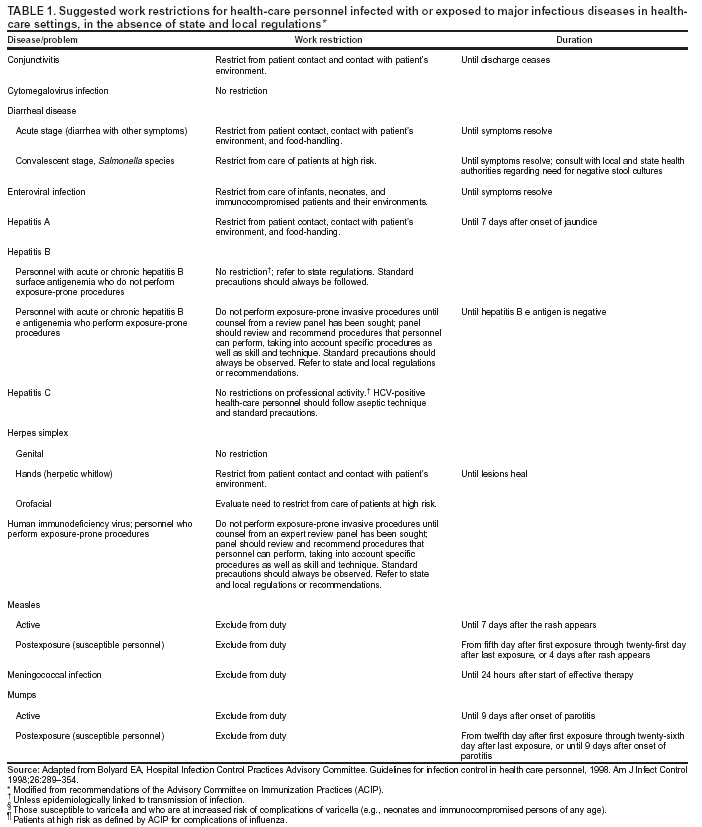 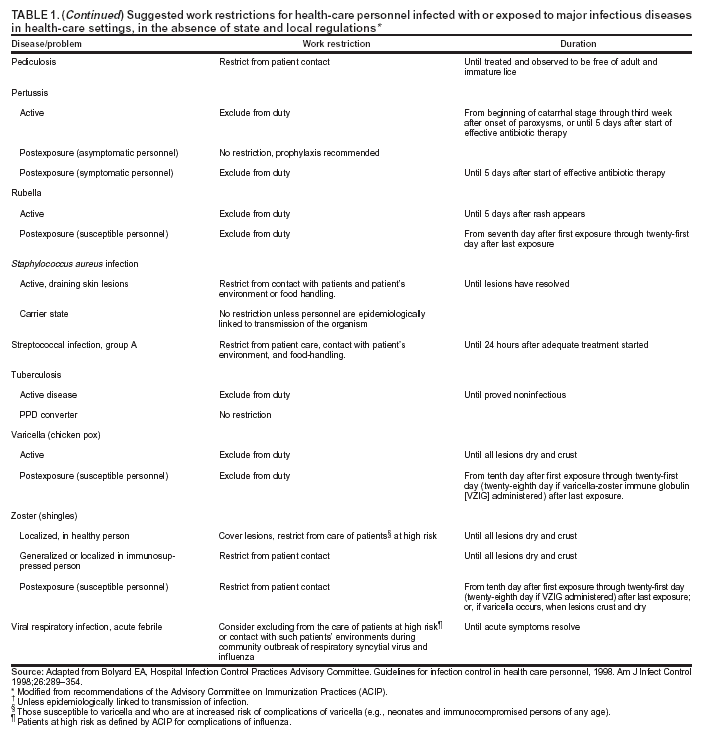 Return to top. Table 2 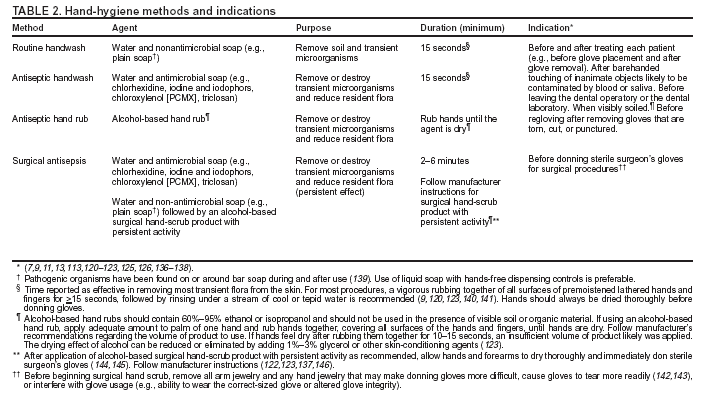 Return to top. Table 3 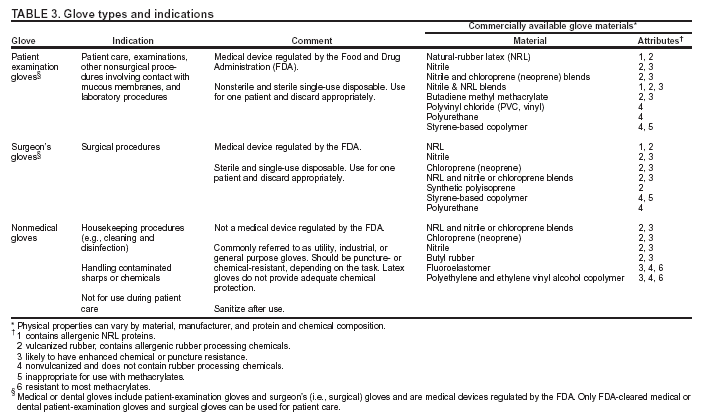 Return to top. Table 4 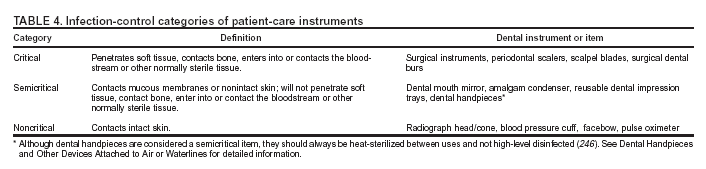 Return to top. Table 5 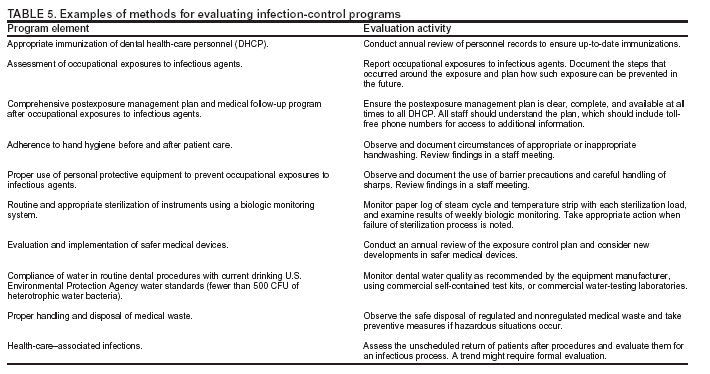 Return to top. Box 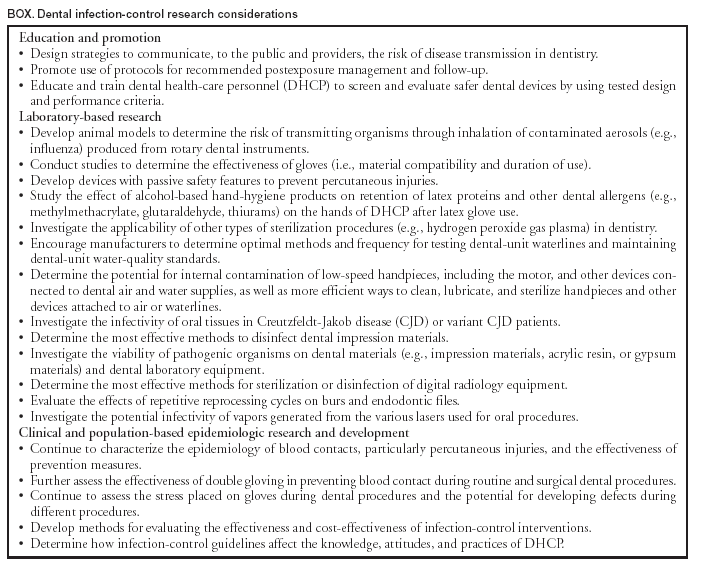 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/9/2003 |
|||||||||
This page last reviewed 12/9/2003
|