 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommendations for Using Smallpox Vaccine in a Pre-Event Vaccination ProgramSupplemental Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC)Prepared by The material in this report originated in the National Immunization Program, Walter A. Orenstein, M.D., Director, and the Epidemiology and Surveillance Division, Melinda Wharton, M.D., Director; and the National Center for Infectious Diseases, James M. Hughes, M.D., Director, and the Bioterrorism Preparedness and Response Program, Charles Schable, M.D., Acting Director. Summary This report supplements the 2001 statement by the Advisory Committee on Immunization Practices (ACIP) (CDC. Vaccinia [smallpox] vaccine: recommendations of the Advisory Committee on Immunization Practices [ACIP], 2001. MMWR 2001;50[No. RR-10]:1--25). This supplemental report provides recommendations for using smallpox vaccine in the pre-event vaccination program in the United States. To facilitate preparedness and response, smallpox vaccination is recommended for persons designated by public health authorities to conduct investigation and follow-up of initial smallpox cases that might necessitate direct patient contact. ACIP recommends that each state and territory establish and maintain >1 smallpox response team. ACIP and the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommend that each acute-care hospital identify health-care workers who can be vaccinated and trained to provide direct medical care for the first smallpox patients requiring hospital admission and to evaluate and manage patients who are suspected as having smallpox. When feasible, the first-stage vaccination program should include previously vaccinated health-care personnel to decrease the potential for adverse events. Additionally, persons administering smallpox vaccine in this pre-event vaccination program should be vaccinated. Smallpox vaccine is administered by using the multiple-puncture technique with a bifurcated needle, packaged with the vaccine and diluent. According to the product labeling, 2--3 punctures are recommended for primary vaccination and 15 punctures for revaccination. A trace of blood should appear at the vaccination site after 15--20 seconds; if no trace of blood is visible, an additional 3 insertions should be made by using the same bifurcated needle without reinserting the needle into the vaccine vial. If no evidence of vaccine take is apparent after 7 days, the person can be vaccinated again. Optimal infection-control practices and appropriate site care should prevent transmission of vaccinia virus from vaccinated health-care workers to patients. Health-care personnel providing direct patient care should keep their vaccination sites covered with gauze in combination with a semipermeable membrane dressing to absorb exudates and to provide a barrier for containment of vaccinia virus to minimize the risk of transmission; the dressing should also be covered by a layer of clothing. Dressings used to cover the site should be changed frequently to prevent accumulation of exudates and consequent maceration. The most critical measure in preventing contact transmission is consistent hand hygiene. Hospitals should designate staff to assess dressings for all vaccinated health-care workers. When feasible, staff responsible for dressing changes for smallpox health-care teams should be vaccinated; all persons handling dressings should observe contact precautions. Administrative leave is not required routinely for newly vaccinated health-care personnel, unless they are physically unable to work as a result of systemic signs and symptoms of illness; have extensive skin lesions that cannot be adequately covered; or if they are unable to adhere to the recommended infection-control precautions. Persons outside the patient-care setting can keep their vaccination sites covered with a porous dressing; hand hygiene remains key to preventing inadvertent inoculation. FDA has recommended that recipients of smallpox vaccine be deferred from donating blood for 21 days or until the scab has separated. Contacts of vaccinees, who have inadvertently contracted vaccinia, also should be deferred from donating blood for 14 days after complete resolution of their complication. In the pre-event vaccination program, smallpox vaccination is contraindicated for persons with a history or presence of eczema or atopic dermatitis; who have other acute, chronic, or exfoliative skin conditions; who have conditions associated with immunosuppression; are aged <1 year; who have a serious allergy to any component of the vaccine; or who are pregnant or breastfeeding. ACIP does not recommend smallpox vaccination for children and adolescents aged <18 years during the pre-event vaccination program. Pre-event vaccination also is contraindicated among persons with household contacts who have a history or presence of eczema or atopic dermatitis; who have other acute, chronic, or exfoliative skin conditions; who have conditions associated with immunosuppression; or who are pregnant. For purposes of screening for contraindications for pre-event vaccination, household contacts include persons with prolonged intimate contact (e.g., sexual contacts) with the potential vaccinee and others who might have direct contact with the vaccination site. Persons with inflammatory eye disease might be at increased risk for inadvertent inoculation as a result of touching or rubbing the eye. Therefore, deferring vaccination is prudent for persons with inflammatory eye diseases requiring steroid treatment until the condition resolves and the course of therapy is complete. Eczema vaccinatum, a serious form of disseminated vaccinia infection, can occur among persons with atopic dermatitis and other dermatologic conditions. Potential vaccinees should be queried regarding the diagnosis of atopic dermatitis or eczema in themselves or any member of their household, or regarding the presence of chronic or recurrent rashes consistent with these diagnoses. Persons reporting such a rash in themselves or household members should not be vaccinated, unless a health-care provider determines that the rash is not eczema or atopic dermatitis. Before vaccination, women of childbearing age should be asked if they are pregnant or intend to become pregnant during the next 4 weeks; women who respond positively should not be vaccinated. Any woman who thinks she might be pregnant or who wants additional assurance that she is not pregnant should perform a urine pregnancy test on the day scheduled for vaccination. If a pregnant woman is inadvertently vaccinated or if she becomes pregnant within 4 weeks after smallpox vaccination, she should be counseled regarding concerns for the fetus. Vaccination during pregnancy should not ordinarily be a reason to terminate pregnancy. CDC has established a pregnancy registry to prospectively follow the outcome of such pregnancies and facilitate the investigation of any adverse pregnancy outcome among pregnant women who were inadvertently vaccinated. For enrollment in the registry, contact CDC at 404-639-8253. Smallpox vaccine should not be administered to persons with human immunodeficiency virus infection (HIV) or acquired immunodeficiency syndrome (AIDS) as part of a pre-event program because of their increased risk for progressive vaccinia. HIV testing is recommended for persons who have any history of a risk factor for HIV infection or for anyone who is concerned that he or she might have HIV infection. HIV testing should be available in a confidential or anonymous setting, in accordance with local laws and regulations, with results communicated to the potential vaccinee before the planned date of vaccination. Smallpox vaccine can be administered simultaneously with any inactivated vaccine. With the exception of varicella vaccine, smallpox vaccine can be administered simultaneously with other live-virus vaccines. To avoid confusion in ascertaining which vaccine might have caused postvaccination skin lesions or other adverse events, varicella vaccine and smallpox vaccine should be administered >4 weeks apart. Health-care workers scheduled to receive an annual purified protein derivative (PPD) skin test for tuberculosis screening should not receive the skin test until >1 month after smallpox vaccination. Persons with progressive vaccinia, eczema vaccinatum, and severe generalized vaccinia or inadvertent inoculation might benefit from therapy with VIG or cidofovir, although the latter has not been approved by FDA for this indication. Suspected cases of these illnesses or other severe adverse events after smallpox vaccination should be reported immediately to state health departments. VIG and cidofovir are available from CDC under Investigational New Drug protocols. Clinically severe adverse events after smallpox vaccination should be reported to the Vaccine Adverse Event Reporting System. Reports can be made online at https://secure.vaers.org/VaersDataEntryintro.htm, or by postage-paid form, which is available by calling 800-822-7967 (toll-free). ACIP will review these recommendations periodically as new information becomes available related to smallpox disease, smallpox vaccines, the risk of smallpox attack, smallpox vaccine adverse events, and the experience gained as recent recommendations are implemented. Revised recommendations will be developed as needed. IntroductionIn June 2001, the Advisory Committee on Immunization Practices (ACIP) made recommendations for using smallpox (vaccinia) vaccine to protect persons working with orthopoxviruses and to prepare for and respond to a possible terrorist attack involving smallpox (1). Because of the terrorist attacks in 2001, CDC asked ACIP to review its previous recommendations for smallpox vaccination. These supplemental recommendations update the 2001 recommendations for vaccination of persons designated to respond to or care for a suspected or confirmed case of smallpox. In addition, they clarify and expand the primary strategy for control and containment of smallpox in the event of an outbreak (see Box for clinical summary). Recommendations remain unchanged for vaccination of laboratory workers who directly handle recombinant vaccinia viruses derived from nonhighly attenuated vaccinia strains or other orthopoxviruses that infect humans (e.g., monkeypox, cowpox, vaccinia, and variola) (1). The following recommendations were developed after formation of a joint working group of ACIP and the National Vaccine Advisory Committee (NVAC) in April 2002. That working group was joined in September 2002 by the Healthcare Infection Control Practices Advisory Committee (HICPAC). A series of public meetings and forums also were held to review available data related to smallpox, smallpox vaccine, smallpox-control strategies, and other concerns related to smallpox vaccination. Smallpox Transmission and ControlSmallpox is transmitted from an infected person to another person. Patients are most infectious during the first 7--10 days after rash onset; transmission can occur during the prodromal period, immediately before rash onset, when lesions in the mouth ulcerate, releasing virus into oral secretions. Infection is transmitted by large-droplet nuclei and occasionally by direct contact or contact with fomites (e.g., clothes or bedding). Airborne transmission has occurred rarely (2). Epidemiologic studies have demonstrated that smallpox has a lower rate of transmission than certain other diseases (e.g., measles, pertussis, and influenza) (2,3). The greatest risk for infection occurs among household members and close contacts of persons with smallpox, especially those with prolonged face-to-face exposure. Isolation of infected patients and vaccination and close monitoring of contacts of patients at greatest risk for infection have been demonstrated to interrupt transmission of smallpox (4,5). During the smallpox era, inadequate infection-control practices sometimes resulted in transmission in hospitals (6,7); a review of importations into Europe during 1950--1971 determined that >50% of the spread cases were associated with hospitals, with approximately 20% of all spread cases related to infections among health-care workers (6). In a review of European smallpox outbreaks, the communicability of smallpox decreased by approximately one half when hospital-based transmission was excluded (8). The primary strategy to control a smallpox outbreak and interrupt disease transmission is surveillance and containment, which includes isolation of smallpox cases and vaccination of persons at risk for contracting smallpox. This strategy involves identification of infected persons through intensive surveillance, isolation of smallpox patients to prevent further transmission, vaccination of household contacts and other close contacts of infected persons (i.e., primary contacts), and vaccination of close contacts of the primary contact (i.e., a secondary contact who would be exposed if disease developed in the primary contact). This strategy was instrumental in the eradication of smallpox as a naturally occurring disease, including in areas that had low vaccination coverage (4). During the smallpox eradication era, depending on the size of the smallpox outbreak and the resources that were available for rapid and thorough contact tracing, surveillance and containment activities in areas with identified smallpox cases were sometimes supplemented with vaccination of other persons in the area where the outbreak occurred. This was done to expand the ring of immune persons within an outbreak area and to further reduce the chance of secondary transmission from smallpox patients before they could be identified and isolated. Regardless of the geographic distribution, number of cases, or number of concurrent outbreaks, surveillance and containment activities remained the primary disease-control strategy (4). Critical ConsiderationsMultiple factors and assumptions were used in developing these supplemental recommendations, as follows:
Smallpox Vaccines and VIG AvailabilityThe only smallpox vaccine licensed in the United States is Dryvax® (manufactured by Wyeth Laboratories, Inc., Marietta, Pennsylvania), which is a dried calf-lymph--type vaccine. Dryvax is a lyophilized preparation of live vaccinia virus grown on the skin of calves (Wyeth Laboratories. Dryvax [Package insert]. Marietta, PA: Wyeth Laboratories, 1994). On October 25, 2002, the Food and Drug Administration (FDA) approved a labeling supplement and a manufacturing supplement to Wyeth's biologics license application for Dryvax. The manufacturing supplement provides for a new kit that includes lyophilized vaccine in a 100-dose vial, a new supply of diluent (one prefilled diluent syringe), one transfer needle, and 100 individually wrapped bifurcated needles. With approval of this supplement, Dryvax can again be distributed and used as a licensed product. Licensed lots must meet lot-release specifications, which include recent testing to demonstrate that the vaccine retains its potency.* As of December 16, 2002, two lots that included a total of 2.7 million doses of Dryvax had full approval for use as a licensed product. Additional lots of Dryvax are expected to be released by FDA under the license. Licensed Dryvax vaccine for civilian use will only be available through CDC. Licensed vaccine will be used for vaccinating laboratory or health-care workers who directly handle cultures, animals, or contaminated materials containing nonhighly attenuated vaccinia or recombinant vaccinia viruses, or other orthopoxviruses that infect humans (1). Requests for smallpox vaccine for vaccinating laboratory workers involved in vaccinia or orthopoxvirus research activities should be directed to CDC Drug Services State health departments are developing plans for vaccinating smallpox public health and health-care teams and are responsible for making vaccine requests to CDC for vaccination of these teams. CDC's National Pharmaceutical Stockpile (NPS) has protocols for rapid, simultaneous delivery of smallpox vaccine to every state and U.S. territory within 12--24 hours. State and local terrorism-response plans should provide for rapid distribution of vaccine within their jurisdictions. VIG is available from CDC only under Investigational New Drug (IND) protocols (i.e., protocols for products that are not yet licensed). As of January 31, 2003, enough VIG was available under an IND protocol to treat approximately 4,000 serious adverse events, which is enough VIG doses to treat the expected number of adverse reactions resulting from vaccination of 40 million persons, on the basis of previously observed rates of adverse reactions (10). SurveillanceCases of febrile rash illnesses for which smallpox is considered in the differential diagnosis should be immediately reported to local or state health departments. After evaluation by the health departments, if smallpox laboratory diagnostics are considered necessary, CDC's Rash Illness Evaluation Team should be consulted at 770-488-7100. Because smallpox was officially certified as eradicated in 1980 and no longer occurs naturally, an initial case of smallpox must be laboratory-confirmed, which is available only at CDC. Clinical consultation and a preliminary laboratory diagnosis can be completed within 8--24 hours. To assist medical and public health personnel in evaluating the likelihood of smallpox among patients with febrile rash illnesses, CDC has developed a rash illness assessment algorithm.† Surveillance activities, including notification procedures and laboratory confirmation of cases, will change if smallpox disease is confirmed in >1 patient.§ Preoutbreak Vaccination of Selected Groups To Enhance Smallpox Response ReadinessSmallpox Response TeamsSmallpox vaccination is recommended for persons designated by appropriate terrorism and public health authorities to conduct investigations and follow-up of initial smallpox cases that might necessitate direct patient contact. Additionally, persons responsible for administering smallpox vaccine in the pre-event vaccination program should be vaccinated (see Vaccinating Persons Administering Smallpox Vaccine in the Pre-Event Vaccination Program). To enhance public health preparedness and response for smallpox control, specific teams at the federal, state, and local levels should be established to facilitate diagnostic evaluation of initial suspected cases of smallpox and to initiate control measures. These smallpox response teams might include persons designated as medical team leaders, public health advisors, medical epidemiologists, disease investigators, diagnostic laboratory scientists, nurses, personnel who could administer smallpox vaccines, security or law enforcement personnel, and other medical personnel to assist in evaluating suspected smallpox cases. ACIP recommends that each state and territory establish and maintain >1 smallpox response team. Considerations for additional teams should include population and geographic concerns and should be developed in accordance with federal, state, and local terrorism-response plans. Smallpox Health-Care TeamsACIP and HICPAC recommend that in the first stage of the pre-event smallpox vaccination program, each acute-care hospital identify groups of health-care workers to be vaccinated and trained to provide direct medical care for the first smallpox patients requiring hospital admission and to evaluate and manage patients who are examined at emergency departments with suspected smallpox. This team should provide care 24 hours/day for the first >2 days after patients with smallpox have been identified, until additional health-care personnel are vaccinated. Nonvaccinated workers should be restricted from entering the rooms of smallpox patients or, under emergency conditions, should wear personal protective equipment. ACIP and HICPAC recommend that smallpox health-care teams include
ACIP and HICPAC anticipate that the size and composition of smallpox health-care teams will vary according to the institutions and their patient populations, but each hospital should ideally have enough vaccinated personnel from each occupational category to ensure continuity of care. When feasible, the first-stage vaccination program should include previously vaccinated health-care personnel to further decrease the potential for adverse events, because adverse events occur less commonly among previously vaccinated persons. Clinical laboratory workers are not recommended for inclusion in the initial phase of pre-event smallpox vaccination because the quantity of smallpox virus likely to be in clinical specimens of blood and body fluids is low. Consistent adherence to the Standard Precautions and biosafety protocols for protection of laboratory workers will prevent exposure to smallpox virus in clinical specimens (11--14). Vaccination MethodThe skin over the insertion of the deltoid muscle or the posterior aspect of the arm over the triceps muscle is the preferred site for smallpox vaccination. Skin preparation for vaccination is not required unless the area is grossly contaminated, in which case soap and water should be used to clean the site. If alcohol or another chemical antiseptic is used, the skin must be allowed to dry thoroughly to prevent inactivation of the vaccine virus by the antiseptic. The multiple-puncture technique uses a presterilized bifurcated needle that is inserted vertically into the vaccine vial, causing a small droplet of vaccine (approximately 0.0025 mL) to adhere between the prongs of the needle. The droplet contains the recommended dosage of vaccine, and its presence within the prongs of the bifurcated needle should be confirmed visually. Holding the bifurcated needle perpendicular to the skin, punctures are made rapidly, with strokes vigorous enough to allow a trace of blood to appear after 15--20 seconds (4). According to the product labeling, 2--3 punctures are recommended for primary vaccination and 15 punctures for revaccination. If no trace of blood is visible after vaccination, an additional three insertions should be made by using the same bifurcated needle without reinserting the needle into the vaccine vial. If no evidence of vaccine take is apparent after 7 days, the person can be vaccinated again. Any remaining vaccine should be wiped off the skin with dry sterile gauze and the gauze disposed of in a biohazard waste container. Vaccinating Persons Administering Smallpox Vaccine in the Pre-Event Vaccination ProgramHistorically, vaccinators were administering smallpox vaccine as part of a disease control or eradication program and were revaccinated frequently. No data exist regarding the risks for inadvertent inoculation of vaccinia among susceptible vaccinators, but they are assumed to have a certain level of risk. The risk might be analogous to that observed among laboratory workers handling nonhighly attenuated vaccinia strains; ACIP recommends that these workers be vaccinated (1). Prior vaccination probably confers substantial protection, but local reactions can occur among revaccinees; thus, protection from clinically significant inadvertent inoculation cannot be considered absolute (15). ACIP and HICPAC recommend that persons administering smallpox vaccine in the pre-event vaccination program be vaccinated to minimize clinical effects of inadvertent inoculation, if inadvertent inoculation occurs. Ideally, vaccinators should have a confirmed vaccine take before vaccinating others, but administering vaccine to vaccinators immediately before beginning work in vaccination clinics is acceptable. Vaccination of this group will also contribute to preparedness for smallpox response. If a smallpox release occurs, experienced vaccinators could immediately be deployed for terrorism response. Preventing Contact Transmission of Vaccinia VirusAfter primary smallpox vaccination, vaccinia virus can be isolated from the vaccination site, beginning with development of a papule (i.e., 2--5 days after vaccination) until the scab separates from the skin lesion (i.e., 14--21 days after vaccination), with maximal shedding at 4--14 days after vaccination. Viral shedding might be of shorter duration among revaccinees (16,17). During the interval in which vaccinia virus is shed, inadvertent inoculation can occur from the vaccination site to another area of the body, most commonly the face, eyelid, nose, lips, genitalia, or anus. In addition, transmission could occur to another nonimmune person, leading to self-limited infections or to more serious complications, particularly among persons with medical contraindications to vaccination. The risk for mortality from eczema vaccinatum might be higher among infected contacts than among vaccinees (10,18,19). Data from the smallpox eradication era indicate that primary vaccinees were the major source of vaccinia infection among contacts, presumably because they had a larger or longer duration of viral shedding than did revaccinees (16,18). Transmission usually required close interaction, occurred most often in the home, and often involved children (18). Nosocomial transmission of vaccinia from either patients or health-care workers to patients has rarely been described; in the majority of instances, the source of vaccinia was a patient suffering from an adverse event after vaccination. The majority of these cases involved direct person-to-person transmission, though for certain persons, the mode of spread was not determined (18,20--22). These data indicate that secondary transmission of vaccinia virus occurs infrequently, especially from adults, and requires close contact. However, today, both the risk for transmission and the risk that a serious adverse event might result if transmission occurs might be greater than during the smallpox era. At the time of the earlier studies, the majority of health-care workers would have been vaccinated previously and therefore were less likely to transmit vaccinia; moreover, the majority of patients were vaccinated also and were less likely to be susceptible to vaccinia. The number of health-care workers who had been vaccinated during these earlier study periods is unknown, but vaccination of health-care workers was routinely recommended. The number of hospitalized patients at risk for serious complications of vaccinia infection is higher now and includes those persons with compromised immune systems from human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS), chemotherapy, or other immunosuppressive medications, organ transplantation, or similar conditions. More patients with indwelling invasive devices requiring frequent manipulation (e.g., intravenous lines, arterial lines, dialysis, ostomies, or central venous lines) are being cared for on hospital wards. Infection-control practices have improved also, and health-care workers are more cognizant of infection-control practices than in earlier years. Additionally, new approaches to vaccination site care (i.e., semipermeable dressings) provide an effective barrier for containment of vaccinia virus (16,23). After considering the data and the caveats noted previously, ACIP and HICPAC concluded that optimal infection-control practices should essentially eliminate the risk of vaccinated health-care workers transmitting vaccinia to patients, and that placing health-care workers on administrative leave could create staffing shortages that might pose a risk to patients (24,25). Consequently, ACIP and HICPAC recommend that, after smallpox vaccination, health-care personnel providing direct patient care should keep their vaccination sites covered with gauze or a similar absorbent material in combination with a semipermeable dressing to absorb exudates that develop and to provide a barrier for containment of vaccinia virus to minimize the risk of transmission (16,23). Alternatively, products combining an absorbent base with an overlying semipermeable layer can be used to cover the site. Semipermeable dressings provide an effective barrier to vaccinia virus, but use of a semipermeable dressing alone is associated with maceration of the vaccination site and increased irritation and itching at the site (23), thereby causing touching, scratching, and possible contamination of the hands. The vaccination site should be covered with gauze, a semipermeable dressing, and a layer of clothing during direct patient care until the scab separates. Dressings used to cover the site should be changed frequently (e.g., every 3--5 days or more frequently if exudates accumulate) to prevent buildup of exudates and consequent maceration. The most critical measure in preventing contact transmission is consistent hand hygiene with antimicrobial soap and water or an approved alcohol-based hand-rub (i.e., one that contains >60% alcohol) after any contact with the vaccination site or with materials that have come into contact with the site and before patient contact (26). In addition, care should be taken to prevent contact with the site or contaminated materials from the site. Hospitals should include a vaccination site-care component in their smallpox vaccination programs in which designated staff assess dressings for all vaccinated health-care workers daily (whether the workers are involved in direct patient care or in other duties), determine if dressings need changing (e.g., when accumulation of purulent material is visible or the integrity of the dressing has been disrupted), and change the dressing, if indicated. These designated staff should assess the vaccination site for local reactions and for vaccine take; reinforce education of vaccinees regarding the need for meticulous hand hygiene; and record and report serious adverse events after vaccination (see Reporting and Managing Adverse Events). When feasible, staff responsible for dressing changes for teams should be vaccinated, but having nonvaccinated staff change dressings is acceptable. All persons handling bandages should observe contact precautions. Persons outside the patient-care setting (e.g., members of public health response teams not involved in patient care, or health-care workers who are not at work) can keep their vaccination sites covered with a porous dressing (e.g., gauze); hand hygiene remains critical in preventing inadvertent inoculation. In nonpatient-care settings in which transmission of vaccinia is a concern because of close personal contact with children or other persons, the vaccination site should be covered with gauze or a similar absorbent material and covered with clothing. Hypoallergenic tape should be used for persons who experience tape hypersensitivity. The vaccination site should be kept dry, although normal showering or bathing can continue. A waterproof dressing might decrease the risk for autoinoculation while washing; if the site is uncovered, care should be taken to avoid touching it. After showering, if the vaccination site is wet, it should be blotted dry with gauze, which is then discarded. If a towel is used to dry the site, the towel should not be used to dry the rest of the body. Alternatively, the site can be allowed to air dry before replacing the bandage. No salves, creams, or ointments should be placed on the site. Contaminated bandages and, if possible, the vaccination site scab, after it has fallen off, should be placed in sealed plastic bags before disposal in the trash to further decrease the potential for inadvertent transmission of the live virus contained in the materials. Clothing, towels, and other cloth materials that have had contact with the site can be decontaminated with routine laundering in hot water (27,28). Administrative Leave for Vaccinated Health-Care WorkersAdministrative leave is not required routinely for newly vaccinated health-care personnel unless they 1) are physically unable to work because of systemic signs and symptoms of illness; 2) have extensive skin lesions that cannot be covered adequately; or 3) are unable to adhere to the recommended infection-control precautions. The close contact required for transmission of vaccinia to household contacts is unlikely to occur in the health-care setting. Vaccination and Blood DonationFDA has recommended that vaccinees be deferred from donating blood for 21 days or until the scab has separated. Contacts of vaccinees who have inadvertently contracted vaccinia also should be deferred from donating blood for 14 days after complete resolution of their complication.** If a substantial number of persons are vaccinated within a brief period, the resulting donor deferrals could impact blood availability. Blood supply shortages can be serious. Blood and platelet donors can help sustain blood supplies by donating immediately before being vaccinated and donating again after they are eligible. Because the donor deferral period needs to be documented carefully, all vaccinees should save the written record of their vaccination. Saving this record also will help to determine vaccination status and donor eligibility in the event of a smallpox outbreak. Contraindications for Use of Smallpox Vaccine in the Pre-Event Vaccination ProgramThe conditions discussed in this section are contraindications in the pre-event vaccination program. No absolute contraindications exist to defer vaccination for persons with high-risk exposure to smallpox; persons at greatest risk for experiencing serious vaccination complications are also at greatest risk for death if they become infected with the smallpox virus. If a relative contraindication to vaccination exists in the setting of a terrorism threat or exposure, the risk of experiencing serious vaccination complications must be weighed against the risk of experiencing a potentially fatal smallpox infection (1). In the pre-event vaccination program, smallpox vaccination is contraindicated (Table) for persons
According to the package insert (Wyeth Laboratories. Dryvax [Package insert]. Marietta, PA: Wyeth Laboratories, 1994), the vaccine might contain trace amounts of polymyxin B, streptomycin, tetracycline, and neomycin, and the diluent contains glycerin and phenol. Atopic dermatitis, irrespective of disease severity or activity, is a risk factor for developing eczema vaccinatum after smallpox vaccination among either vaccinees or their close contacts (10,29--33), but no data exist to predict the absolute risk for this population. Because the majority of primary-care providers do not distinguish between eczema and atopic dermatitis, including when describing chronic exfoliative skin conditions among infants (34,35), ACIP recommends that smallpox vaccine not be administered to persons with a history of eczema or atopic dermatitis, irrespective of disease severity or activity. Persons with other active acute, chronic, or exfoliative conditions (e.g., burns, impetigo, varicella zoster, herpes, severe acne, severe diaper dermatitis with extensive areas of denuded skin, or psoriasis) are at higher risk for clinically severe inadvertent inoculation and should not be vaccinated until the condition resolves. Additionally, persons with Darier disease (keratosis follicularis) can develop eczema vaccinatum and therefore should not be vaccinated (32,36). Replication of vaccinia virus can be enhanced among persons with cellular or humoral immunodeficiencies and among those with immunosuppression (e.g., HIV/AIDS, leukemia, lymphoma, generalized malignancy, solid organ transplantation, or therapy with alkylating agents, antimetabolites, radiation, or high-dose corticosteroids [i.e., >2 mg/kg body weight or 20 mg/day of prednisone for >2 weeks]). Persons who are taking or have taken high-dose corticosteroids should not be vaccinated within 1 month of completing corticosteroid therapy, and persons treated with other immunosuppressive drugs within the previous 3 months should not be vaccinated (37). Persons with immunosuppression also include hematopoietic stem cell transplant recipients who are <24 months posttransplant, and hematopoietic stem cell transplant recipients who are >24 months posttransplant, but have graft-versus-host disease or disease relapse. Patients with severe clinical manifestations of certain autoimmune diseases (e.g., systemic lupus erythematosis) might have a degree of immunocompromise as a component of the disease (38). Although no data exist to indicate that a person is at risk from live-virus vaccines because of severe autoimmune disease in the absence of immunosuppressive therapy, persons with immunodeficiency as a clinical component of their autoimmune disease should not receive smallpox vaccine during the pre-event vaccination program. According to product labeling, smallpox vaccine is not recommended for use among breastfeeding women (Wyeth Laboratories. Dryvax [Package insert]. Marietta, PA: Wyeth Laboratories, 1994); whether vaccine virus or antibodies are excreted in human milk is unknown. ACIP does not recommend smallpox vaccination of children and adolescents aged <18 years in the pre-event vaccination program, and smallpox vaccine is contraindicated for infants aged <1 year. Pre-event vaccination is also contraindicated among persons with household contacts who have a history or presence of eczema or atopic dermatitis, irrespective of disease severity or activity; who have other acute, chronic, or exfoliative skin conditions; who have conditions associated with immunosuppression (see previous discussion); or who are pregnant. For purposes of screening for contraindications for pre-event vaccination, household contacts include persons with prolonged intimate contact with the potential vaccinee (e.g., sexual contacts) and others who might have direct contact with the vaccination site. The presence of an adolescent or child (including an infant) in the household is not a contraindication to vaccination of adult members of the household; the risk for serious complications from transmission from an adult to a child is limited. Nonetheless, ACIP recognizes that programs might defer vaccination of household contacts of infants aged <1 year because of data indicating a higher risk for adverse events among primary vaccinees in this age group, compared with that among older children (31). The presence of a breastfeeding woman or a person with a vaccine component allergy in the household is also not a contraindication to vaccination of other household members (Table). Precautions for Smallpox VaccinationPersons with inflammatory eye diseases can be at increased risk for inadvertent inoculation as a result of touching or rubbing the eye. Therefore, deferring vaccination of persons with inflammatory eye diseases requiring steroid treatment is prudent until the condition resolves and the course of therapy is complete. Screening for Atopic Dermatitis as a Contraindication for VaccinationTo assist providers in identifying persons who should defer smallpox vaccination, ACIP recommends using two screening questions (Figure). Although sensitive, this approach to screening might preclude vaccination of persons who could otherwise be safely vaccinated. Certain organizations (e.g., the military or CDC) might elect to develop more precise screening tools for persons among whom the dermatologic risk factor or diagnosis is uncertain. These secondary screening tools should weigh the person's risk of developing an adverse event with the requirement of occupational readiness through safe smallpox vaccination. Screening for Pregnancy as a Contraindication for VaccinationFetal vaccinia is a rare, but serious, complication of smallpox vaccination during pregnancy or immediately before conception. Infection, which can spread to the fetus if viremia occurs after vaccination, is manifested by typical skin lesions, organ involvement, and fetal or early neonatal death (39). During 1932--1972, of 20 affected pregnancies, 18 occurred when the pregnant woman was vaccinated, and two occurred among pregnant contacts; 13 occurred among primary vaccinees, and three among those being revaccinated. Seven occurred during the first trimester, and 13 in the second trimester. Only one of 20 pregnancies was maintained until term, and of 21 affected births (one birth was of twins), three infants survived (39). A cohort study of pregnant women vaccinated during a mass campaign in Sweden in 1963 demonstrated a higher than expected rate of fetal loss (40); however, pathology was not performed to evaluate causation, and vaccinees might have been at higher risk for adverse outcomes of pregnancy. Smallpox vaccination of pregnant women has not been associated with an increased risk for congenital malformations (41). Because of the limited risk but severe consequences of fetal infection, smallpox vaccine should not be administered in a pre-event setting to pregnant women or to women who are trying to become pregnant. Before vaccination, women of childbearing age should be asked if they are pregnant or intend to become pregnant in the next 4 weeks; women who respond positively should not be vaccinated. To further reduce the risk for inadvertently vaccinating a woman who is pregnant, at the time of prescreening women of childbearing age should be educated regarding what is known about fetal vaccinia. Women should be counseled to avoid becoming pregnant until >4 weeks after vaccination, and abstinence or highly effective contraceptive measures should be recommended to reduce the risk of pregnancy before or within 4 weeks after vaccination. Any woman who believes she might be pregnant or who wants additional assurance that she is not pregnant should perform a urine pregnancy test by using her first-morning--void urine on the day scheduled for vaccination. Such tests could be made available at the prescreening and vaccination sites to avoid cost or other barriers to testing. However, women should be informed that a negative urine pregnancy test cannot exclude a very early pregnancy, and therefore, they and their health-care providers should not base a decision regarding their pregnancy status solely on a urine pregnancy test result (42). If a pregnant woman is inadvertently vaccinated or if she becomes pregnant within 4 weeks after smallpox vaccination, she should be counseled regarding concern for the fetus. Smallpox vaccination during pregnancy should not ordinarily be a reason to terminate pregnancy. To expand understanding of the risk for fetal vaccinia and to document whether other adverse pregnancy outcomes might be associated with vaccination, CDC has established a pregnancy registry to prospectively follow the outcome of such pregnancies and facilitate the investigation of any adverse pregnancy outcome among pregnant women who were inadvertently vaccinated. For enrollment in the registry, contact CDC at 404-639-8253. Screening for HIV Infection as a Contraindication for VaccinationPersons with HIV infection or AIDS might have an increased risk for severe adverse reactions resulting from live-virus vaccines. Because the HIV epidemic began after the cessation of routine smallpox vaccination, data are limited regarding the risks from vaccination among HIV-infected persons. A single case report has been published of a U.S. military recruit who developed disseminated vaccinia after smallpox vaccination and who was successfully treated with VIG, but later died from complications of AIDS (43). Although the exact number of HIV-infected persons who were vaccinated in the military program is unclear, 732 recruits who were in the service during 1981--1985, when vaccinations were administered, tested HIV-positive during 1985--1988, for an estimated frequency of serious adverse events among HIV-positive persons of 1/732, or 0.137% (95% confidence interval [CI] = 0.084%--0.22%); if only half were HIV-positive at the time of vaccination, the frequency increases to 1/366, or 0.273% (95% CI = 0.17%--0.44%) (Col. Deborah L. Birx, M.D., Walter Reed Army Institute of Medicine, personal communication, September 2002). Because the immunologic status of an HIV-infected person is probably the key to the risk from vaccination and the immunologic status of the recruits at the time of vaccination was unknown, these estimated rates might not apply to other groups of HIV-infected persons. An estimated 850,000--950,000 HIV-infected persons are living in the United States (prevalence: 0.3%), and of these, an estimated 180,000--280,000 are unaware that they are infected (44). Estimates of the number of HIV-infected health-care workers range from approximately 21,000 to 48,000 (CDC, Division of Health Care Quality Promotion, unpublished data, 2002), and the proportion of these infected health-care workers who remain undiagnosed is unknown. Risk assessment followed by counseling and testing is useful in identifying persons with HIV infection. However, substantial numbers of HIV-infected persons might not recognize or acknowledge their risk during risk-assessment screening (45). Smallpox vaccine should not be administered to persons with HIV infection or AIDS as part of a pre-event program because of their increased risk for progressive vaccinia (vaccinia necrosum). Before vaccination, potential vaccinees should be educated regarding the risk for severe vaccinial complications among persons with HIV infection or other immunosuppressive conditions; persons who think they might have one of these conditions should not be vaccinated. ACIP does not recommend mandatory HIV testing before smallpox vaccination, but recommends that HIV testing should be readily available to all persons considering smallpox vaccination. HIV testing is recommended for persons who have any history of a risk factor for HIV infection and who are unsure of their HIV infection status. Because known risk factors cannot be identified for certain persons with HIV infection, anyone who is concerned that they could have HIV infection also should be tested. HIV testing should be available in a confidential or anonymous setting, as allowed by local laws and regulations, with results communicated to the potential vaccinee before the planned date of vaccination. Persons with a positive test result should be advised not to be vaccinated. Information regarding local testing options should be provided to all potential vaccinees, including sites where testing is performed at no cost. The recently licensed rapid HIV test might facilitate availability of HIV testing to potential vaccinees (46). Simultaneous Administration of Smallpox Vaccine with Other VaccinesSimultaneously administering the most widely used live and inactivated vaccines has produced seroconversion rates and rates of adverse reactions similar to those observed when the vaccines are administered separately (47--50). Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines. An inactivated vaccine can be administered either simultaneously or at any time before or after a different inactivated vaccine or live vaccine. The immune response to one live-virus vaccine might be impaired if administered within 30 days of another live-virus vaccine, if not administered simultaneously (51,52). To minimize the potential risk for interference, parenterally administered live vaccines not administered on the same day should be administered >4 weeks apart, whenever possible. If parenterally administered live vaccines are separated by <4 weeks, the vaccine administered second should not be counted as a valid dose and should be repeated. The repeat dose should be administered >4 weeks after the last, invalid dose (37). Smallpox vaccine can be administered at the same time as certain other vaccines, with levels of safety and efficacy comparable to those observed when the vaccines are administered separately (53). Vaccines that have been documented to be effective when administered simultaneously with smallpox vaccine include oral polio vaccine, bacille of Calmette-Guérin (BCG) vaccine, yellow fever vaccine, measles vaccine, and diphtheria and tetanus toxoids and whole-cell pertussis vaccine (53). However, no data exist regarding simultaneous administration of smallpox vaccine with other vaccines now routinely administered to children and adults in the United States. Varicella vaccine virus lesions might be confused with vaccinia lesions if the vaccines were administered simultaneously. In uncontrolled trials of persons aged >13 years, approximately 1,600 vaccinees who received 1 dose and 955 who received 2 doses of varicella vaccine were monitored for 42 days for adverse events (Merck and Co., Inc. Varivax [Package insert]. West Point, PA: Merck and Co., 1995). After the first and second doses, a nonlocalized rash consisting of a median number of five lesions developed in 5.5% and 0.9% of vaccinees, respectively, and occurred at a peak of 7--21 days and 0--23 days postvaccination, respectively (54). Smallpox vaccine can be administered simultaneously with any inactivated vaccine (e.g., influenza vaccine) to encourage appropriate receipt of all indicated vaccines (e.g., among such populations as health-care workers). With the exception of varicella vaccine, smallpox vaccine can be administered simultaneously with other live-virus vaccines. To avoid confusion in ascertaining which vaccine might have caused postvaccination skin lesions or other adverse events, and facilitate managing such events, varicella vaccine and smallpox vaccine should only be administered >4 weeks apart. Timing of Tuberculosis Screening and Smallpox VaccinationSuppression of tuberculin skin test (purified protein derivative [PPD]) reactivity has been demonstrated after administration of smallpox vaccine (55), as has been observed after administration of other parenteral live-virus vaccines (37). Health-care workers scheduled to receive an annual PPD skin test should not receive the skin test for 1 month after smallpox vaccination to prevent possible false-negative reactions. Reporting and Managing Adverse EventsPersons with progressive vaccinia, eczema vaccinatum, and severe generalized vaccinia or inadvertent inoculation might benefit from therapy with VIG or cidofovir, although the latter has not been approved by FDA for this indication. Suspected cases of these illnesses or other serious adverse events after smallpox vaccination should be reported immediately to state health departments. VIG and cidofovir are available from CDC for treatment of adverse events among smallpox vaccine recipients and their contacts under IND protocols. Recommendations regarding treatment of adverse events have been published recently (56). Additionally, serious adverse events after smallpox vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS). Reports can be submitted through a secure Internet-based system at https://secure.vaers.org/VaersDataEntryintro.htm. Printable VAERS forms are located online at http://www.vaers.org/pdf/vaers_form.pdf, or postage-paid forms can be obtained by calling 800-822-7967 (toll-free). Submission of VAERS reports by Internet is encouraged to expedite processing and data entry. Completed forms can be faxed to 877-721-0366 (toll-free) or mailed to P.O. Box 1100, Rockville, MD 20894-1100. Additional information related to VAERS reporting can be obtained by calling 800-822-7967 or by e-mail at info@vaers.org. Future DirectionsACIP will review these recommendations periodically or more urgently, if necessary. These reviews will include new information or developments related to smallpox disease, smallpox vaccines (including licensure of additional smallpox vaccines), risk of smallpox attack, smallpox vaccine adverse events, and the experience gained in the implementation of these recommendations. Revised recommendations will be developed as needed. Acknowledgments The preparers of this report are grateful for the assistance of the following persons: Walter Orenstein, M.D., CDC/National Immunization Program; Dixie Snider, M.D., CDC/Office of the Director; J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center; Sheila Fallon-Friedlander, M.D., San Diego School of Medicine and the University of California; Julie R. Kenner, M.D., Ph.D., U.S. Department of Veterans Affairs, The National Jewish Medical and Research Center, and the University of Colorado Health Services Center; Jon M. Hanifin, M.D., Oregon Health and Science University; Sheryl Lyss, M.D., and Hoyt G. Wilson, Ph.D., CDC/National Center for Chronic Disease Prevention and Health Promotion; Ida Onorato, M.D., and Allyn Nakashima, M.D., CDC/National Center for HIV, STD, and TB Prevention; Linda Chiarello, M.S., and Gloria Kovach, CDC/National Center for Infectious Diseases; and Demetria Gardner, CDC/National Immunization Program. References
* Further information regarding the supplement approval and labeling for Dryvax is located at http://www.fda.gov/cber/products/smalwye102502.htm. † Poster copies of this algorithm are available from state health departments and at http://www.bt.cdc.gov/agent/smallpox/diagnosis. Copies of the poster can be ordered at https://www2.cdc.gov/nchstp_od/PIWeb/niporderform.asp. § Additional information regarding surveillance activities after laboratory confirmation of a smallpox outbreak is located in CDC's Smallpox Response Plan and Guidelines (http://www.bt.cdc.gov/agent/smallpox/response-plan/index.asp). ¶ This might involve creating regional teams of subspecialists (e.g., local medical consultants with smallpox experience, dermatologists, ophthalmologists, pathologists, surgeons, anesthesiologists in facilities where intensivists [i.e., physicians who are board-certified in a medical specialty and who receive special training in critical care] are not trained in anesthesia) to deliver consultative services. ** FDA guidance is available at http://www.fda.gov/cber/gdlns/smpoxdefquar.htm. Advisory Committee on Immunization Practices
Chairman: John F. Modlin, M.D., Professor of Pediatrics and Medicine, Dartmouth Medical School, Lebanon, New Hampshire.
ACIP Bioterrorism Working Group Chair: John F. Modlin, M.D., Dartmouth Medical School, Lebanon, New Hampshire.
Heathcare Infection Control Practices Advisory Committee
Chair: Robert A. Weinstein, M.D., Cook County Hospital, Chicago, Illinois.
HICPAC Bioterrorism Working Group Chair: Jane D. Siegel, M.D., University of Texas Southwestern Medical Center, Dallas, Texas.
* Non-HICPAC members. Box  Return to top. Table 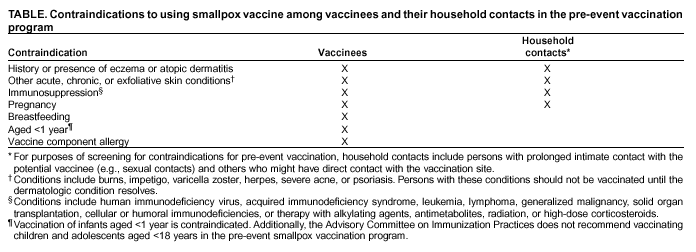 Return to top. Figure 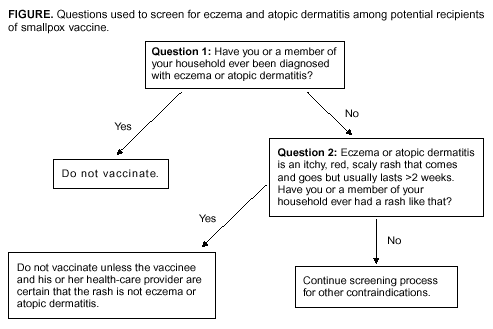 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/14/2003 |
|||||||||
This page last reviewed 3/14/2003
|