 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Use of Anthrax Vaccine in the United StatesRecommendations of the Advisory Committee on Immunization PracticesAdvisory Committee on Immunization Practices Membership List, October 2000 CHAIRMAN EXECUTIVE SECRETARY MEMBERS
Richard D. Clover, M.D. Fernando A. Guerra, M.D. Charles M. Helms, M.D., Ph.D. David R. Johnson, M.D., M.P.H. Chinh T. Le, M.D. Paul A. Offit, M.D. Margaret B. Rennels, M.D. Lucy S. Tompkins, M.D., Ph.D. Bonnie M. Word, M.D. EX OFFICIO MEMBERS James E. Cheek, M.D., M.P.H. Geoffrey S. Evans, M.D. T. Randolph Graydon Martin G. Myers, M.D. Carole Heilman, M.D. Karen Midthun, M.D. Martin G. Myers, M.D. Kristin Lee Nichol, M.D., M.P.H. LIAISON REPRESENTATIVES
American Academy of Pediatrics Jon Abramson, M.D. American Association of Health Plans American College of Obstetricians and Gynecologists American College of Physicians American Hospital Association American Medical Association Association of Teachers of Canadian National Advisory Committee on Immunization Healthcare Infection Control Practices Advisory Committee Infectious Diseases Society of America National Immunization Council and Child Health Program, Mexico National Medical Association National Vaccine Advisory Committee Pharmaceutical Research and Manufacturers of America The following CDC staff members prepared this report: Lisa D. Rotz, M.D. Summary These recommendations concern the use of aluminum hydroxide adsorbed cell-free anthrax vaccine (Anthrax Vaccine Adsorbed [AVA], BioPort Corporation, Lansing, MI) in the United States for protection against disease caused by Bacillus anthracis. In addition, information is included regarding the use of chemoprophylaxis against B. anthracis. INTRODUCTIONAnthrax is a zoonotic disease caused by the spore-forming bacterium Bacillus anthracis (1,2). The disease most commonly occurs in wild and domestic mammals (e.g., cattle, sheep, goats, camels, antelope, and other herbivores)(2). Anthrax occurs in humans when they are exposed to infected animals or tissue from infected animals or when they are directly exposed to B. anthracis (3--5). Depending on the route of infection, anthrax disease can occur in three forms: cutaneous, gastrointestinal, and inhalation (2). B. anthracis spores can remain viable and infective in the soil for many years. During this time, they are a potential source of infection for grazing livestock, but generally do not represent a direct infection risk for humans. Grazing ruminants become infected when they ingest these spores. Consequently, humans can become infected with B. anthracis by skin contact, ingestion, or inhalation of B. anthracis spores originating from animal products of infected animals. Direct skin contact with contaminated animal products can result in cutaneous anthrax. Ingestion of infected and undercooked or raw meat can result in oropharyngeal or gastrointestinal forms of the disease. Inhalation of aerosolized spores associated with industrial processing of contaminated wool, hair, or hides can result in inhalation anthrax. Person-to-person transmission of inhalation anthrax has not been confirmed. Estimation of the true incidence of human anthrax worldwide is difficult because reporting of anthrax cases is unreliable (6). However, anthrax occurs globally and is most common in agricultural regions with inadequate control programs for anthrax in livestock. In these regions, anthrax affects domestic animals, which can directly or indirectly infect humans, and the form of anthrax that occurs in >95% of cases is cutaneous. These regions include South and Central America, Southern and Eastern Europe, Asia, Africa, the Caribbean, and the Middle East (6). The largest recent epidemic of human anthrax occurred in Zimbabwe during 1978--1980; 9445 cases occurred, including 141 (1.5%) deaths (4). In the United States, the annual incidence of human anthrax has declined from approximately 130 cases annually in the early 1900s to no cases during 1993--2000. The last confirmed case of human anthrax reported in the United States was a cutaneous case reported in 1992. Most cases reported in the United States have been cutaneous; during the 20th century, only 18 cases of inhalation anthrax were reported, the most recent in 1976 (7). Of the 18 cases of inhalation anthrax reported in the United States since 1950, two occurred in laboratory workers. No gastrointestinal cases have been reported in the United States. Anthrax continues to be reported among domestic and wild animals in the United States. The incidence of anthrax in U.S. animals is unknown; however, reports of animal infection have occurred among the Great Plains states from Texas to North Dakota (8--10). In addition to causing naturally occurring anthrax, B. anthracis has been manufactured as a biological warfare agent, and concern exists that it could be used as a biological terrorist agent. B. anthracis is considered one of the most likely biological warfare agents because of the ability of B. anthracis spores to be transmitted by the respiratory route, the high mortality of inhalation anthrax, and the greater stability of B. anthracis spores compared with other potential biological warfare agents (11--14). Anthrax has been a focus of offensive and defensive biological warfare research programs for approximately 60 years. The World Health Organization estimated that 50 kg of B. anthracis released upwind of a population center of 500,000 could result in 95,000 deaths and 125,000 hospitalizations (15). The infectious dose of B. anthracis in humans by any route is not precisely known. Based on data from studies of primates, the estimated infectious dose by the respiratory route required to cause inhalation anthrax in humans is 8,000--50,000 spores (7,16,17). The influence of the bacterium strain or host factors on this infectious dose is not completely understood. Primary and secondary aerosolization of B. anthracis spores are important considerations in bioterrorist acts involving deliberate release of B. anthracis. Primary aerosolization results from the initial release of the agent. Secondary aerosolization results from agitation of the particles that have settled from the primary release (e.g., as a result of disturbance of contaminated dust by wind, human, or animal activities.) In the generation of infectious aerosols, the aerosol is composed of two components that have differing properties: particles larger than 5 microns and particles 1--5 microns in diameter. Particles >5 microns in diameter quickly fall from the atmosphere and bond to any surface. These particles require large amounts of energy to be resuspended. Even with use of highly efficient dissemination devices (i.e., devices able to disseminate a high concentration of agent into the environment), the level of environmental contamination with the larger, bound particles is estimated to still be too low to represent a substantial threat of secondary aerosolization (18--20). Particles 1--5 microns in diameter behave as a gas and move through the environment without settling. Environmental residue is not a concern from this portion of the aerosol (21). Disease The symptoms and incubation period of human anthrax vary depending on the route of transmission of the disease. In general, symptoms usually begin within 7 days of exposure (1). Cutaneous Most (>95%) naturally occurring B. anthracis infections are cutaneous and occur when the bacterium enters a cut or abrasion on the skin (e.g., when handling contaminated meat, wool, hides, leather, or hair products from infected animals). The reported incubation period for cutaneous anthrax ranges from 0.5 to 12 days (1,6,22). Skin infection begins as a small papule, progresses to a vesicle in 1--2 days, and erodes leaving a necrotic ulcer with a characteristic black center. Secondary vesicles are sometimes observed. The lesion is usually painless. Other symptoms might include swelling of adjacent lymph glands, fever, malaise, and headache. The case-fatality rate of cutaneous anthrax is 20% without antibiotic treatment and <1% with antibiotic treatment (1,23,24). Gastrointestinal The intestinal form of anthrax usually occurs after eating contaminated meat and is characterized by an acute inflammation of the intestinal tract. The incubation period for intestinal anthrax is suspected to be 1--7 days. Involvement of the pharynx is characterized by lesions at the base of the tongue or tonsils, with sore throat, dysphagia, fever, and regional lymphadenopathy. Involvement of the lower intestine is characterized by acute inflammation of the bowel. Initial signs of nausea, loss of appetite, vomiting, and fever are followed by abdominal pain, vomiting of blood, and bloody diarrhea (25). The case-fatality rate of gastrointestinal anthrax is unknown but is estimated to be 25%--60% (1,26,27). Inhalation Inhalation anthrax results from inspiration of 8,000--50,000 spores of B. anthracis. Although the incubation period for inhalation anthrax for humans is unclear, reported incubation periods range from 1 to 43 days (28). In a 1979 outbreak of inhalation anthrax in the former Soviet Union, cases were reported up to 43 days after initial exposure. The exact date of exposure in this outbreak was estimated and never confirmed, and the modal incubation period was reported as 9--10 days. This modal incubation period is slightly longer than estimated incubation periods reported in limited outbreaks of inhalation anthrax in humans (29). However, the incubation period for inhalation anthrax might be inversely related to the dose of B. anthracis (30,31). In addition, the reported administration of postexposure chemoprophylaxis during this outbreak might have prolonged the incubation period in some cases. Data from studies of laboratory animals suggest that B. anthracis spores continue to vegetate in the host for several weeks postinfection, and antibiotics can prolong the incubation period for developing disease (28--30,32). These studies of nonhuman primates, which are considered to be the animal model that most closely approximates human disease, indicate that inhaled spores do not immediately germinate within the alveolar recesses but reside there potentially for weeks until taken up by alveolar macrophages. Spores then germinate and begin replication within the macrophages. Antibiotics are effective against germinating or vegetative B. anthracis but are not effective against the nonvegetative or spore form of the organism. Consequently, disease development can be prevented as long as a therapeutic level of antibiotics is maintained to kill germinating B. anthracis organisms. After discontinuation of antibiotics, if the remaining nongerminated spores are sufficiently numerous to evade or overwhelm the immune system when they germinate, disease will then develop. This phenomenon of delayed onset of disease is not recognized to occur with cutaneous or gastrointestinal exposures. Initial symptoms can include sore throat, mild fever, and muscle aches. After several days, the symptoms can progress to severe difficulty breathing and shock. Meningitis frequently develops. Case-fatality estimates for inhalation anthrax are based on incomplete information regarding the number of persons exposed and infected. However, a case-fatality rate of 86% was reported following the 1979 outbreak in the former Soviet Union, and a case-fatality rate of 89% (16 of 18 cases) was reported for inhalation anthrax in the United States (8,28,29). Records of industrially acquired inhalation anthrax in the United Kingdom, before the availability of antibiotics or vaccines, document that 97% of cases were fatal. PATHOGENESISB. anthracis evades the immune system by producing an antiphagocytic capsule. In addition, B. anthracis produces three proteins --- protective antigen (PA), lethal factor (LF), and edema factor (EF) --- that act in binary combinations to form two exotoxins known as lethal toxin and edema toxin (33--35). PA and LF form lethal toxin; PA and EF form edema toxin. LF is a protease that inhibits mitogen-activated protein kinase-kinase (36). EF is an adenylate cyclase that generates cyclic adenosine monophosphate in the cytoplasm of eukaryotic cells (37,38). PA is required for binding and translocating LF and EF into host cells. PA is an 82 kD protein that binds to receptors on mammalian cells and is critical to the ability of B. anthracis to cause disease. After binding to the cell membrane, PA is cleaved to a 63 kD fragment that subsequently binds with LF or EF (39). LF or EF bound to the 63KD fragment undergoes receptor-mediated internalization, and the LF or EF is translocated into the cytosol upon acidification of the endosome. After wound inoculation, ingestion, or inhalation, spores infect macrophages, germinate, and proliferate. In cutaneous and gastrointestinal infection, proliferation can occur at the site of infection and the lymph nodes draining the infection site. Lethal toxin and edema toxin are produced and respectively cause local necrosis and extensive edema, which is a major characteristic of the disease. As the bacteria multiply in the lymph nodes, toxemia progresses, and bacteremia may ensue. With the increase in toxin production, the potential for widespread tissue destruction and organ failure increases (40). CONTROL AND PREVENTIONReducing the Risk for Exposure Worldwide, anthrax among livestock is controlled through vaccination programs, rapid case detection and case reporting, and burning or burial of animals suspected or confirmed of having the disease. Human infection is controlled through reducing infection in livestock, veterinary supervision of slaughter practices to avoid contact with potentially infected livestock, and restriction of importation of hides and wool from countries in which anthrax occurs. In countries where anthrax is common and vaccination coverage among livestock is low, humans should avoid contact with livestock and animal products that were not inspected before and after slaughter. In addition, consumption of meat from animals that have experienced sudden death and meat of uncertain origin should be avoided (1,4). Vaccination Protective Immunity Before the mechanisms of humoral and cellular immunity were understood, researchers demonstrated that inoculation of animals with attenuated strains of B. anthracis led to protection (41,42). Subsequently, an improved vaccine for livestock, based on a live unencapsulated avirulent variant of B. anthracis, was developed (43,44). Since then, this vaccine has served as the principal veterinary vaccine in the Western Hemisphere. The use of livestock vaccines was associated with occasional animal casualties, and live vaccines were considered unsuitable for humans. In 1904, the possibility of using acellular vaccines against B. anthracis was first suggested by investigators who discovered that injections of sterilized edema fluid from anthrax lesions provided protection in laboratory animals (45,46). This led to exploration of the use of filtrates of artificially cultivated B. anthracis as vaccines (47--51) and thereby to the human anthrax vaccines currently licensed and used in the United States and Europe today. The first product --- an alum-precipitated cell-free filtrate from an aerobic culture --- was developed in 1954 (52,53). Alum is the common name for aluminum potassium sulfate. This vaccine provided protection in monkeys, caused minimal reactivity and short-term adverse events in humans, and was used in the only efficacy study of human vaccination against anthrax in the United States. In the United States, during 1957--1960, the vaccine was improved through a) the selection of a B. anthracis strain that produced a higher fraction of PA under microaerophilic conditions, b) the production of a protein-free media, and c) the use of aluminum hydroxide rather than alum as the adjuvant (50,51). This became the vaccine approved for use in the United States --- anthrax vaccine adsorbed (AVA [patent number 3,208,909, September 28, 1965]). Passive immunity against B. anthracis can be transferred using polyclonal antibodies in laboratory animals (54); however, specific correlates for immunity against B. anthracis have not been identified (55--57). Evidence suggests that a humoral and cellular response against PA is critical to protection against disease following exposure (49,57--59). Anthrax Vaccine Adsorbed AVA, the only licensed human anthrax vaccine in the United States, is produced by BioPort Corporation in Lansing, Michigan, and is prepared from a cell-free filtrate of B. anthracis culture that contains no dead or live bacteria (60). The strain used to prepare the vaccine is a toxigenic, nonencapsulated strain known as V770-NP1-R (50). The filtrate contains a mix of cellular products including PA (57) and is adsorbed to aluminum hydroxide (Amphogel, Wyeth Laboratories) as adjuvant (49). The amount of PA and other proteins per 0.5mL dose is unknown, and all three toxin components (LF, EF, and PA) are present in the product (57). The vaccine contains no more that 0.83 mg aluminum per 0.5mL dose, 0.0025% benzethonium chloride as a preservative, and 0.0037% formaldehyde as a stabilizer. The potency and safety of the final product is confirmed according to U.S. Food and Drug Administration (FDA) regulations (61). Primary vaccination consists of three subcutaneous injections at 0, 2, and 4 weeks, and three booster vaccinations at 6, 12, and 18 months. To maintain immunity, the manufacturer recommends an annual booster injection. The basis for the schedule of vaccinations at 0, 2, and 4 weeks, and 6, 12, and 18 months followed by annual boosters is not well defined (52,62,63; Table 1). Because of the complexity of a six-dose primary vaccination schedule and frequency of local injection-site reactions (see Vaccine Safety), studies are under way to assess the immunogenicity of schedules with a reduced number of doses and with intramuscular (IM) administration rather than subcutaneous administration. Immunogenicity data were collected from military personnel who had a prolonged interval between the first and second doses of anthrax vaccine in the U.S. military anthrax vaccination program. Antibody to PA was measured by enzyme-linked immunosorbent assay (ELISA) at 7 weeks after the first dose. Geometric mean titers increased from 450 µg/mL among those who received the second vaccine dose 2 weeks after the first (the recommended schedule, n = 22), to 1,225 for those vaccinated at a 3-week interval (n = 19), and 1,860 for those vaccinated at a 4-week interval (n = 12). Differences in titer between the routine and prolonged intervals were statistically significant (p <0.01). Subsequently, a small randomized study was conducted among military personnel to compare the licensed regimen (subcutaneous injections at 0, 2, and 4 weeks, n = 28) and alternate regimens (subcutaneous [n = 23] or intramuscular [n=22] injections at 0 and 4 weeks). Immunogenicity outcomes measured at 8 weeks after the first dose included geometric mean IgG concentrations and the proportion of subjects seroconverting (defined by an anti-PA IgG concentration of >25 µg/mL). In addition, the occurrence of local and systemic adverse events was determined. IgG concentrations were similar between the routine and alternate schedule groups (routine: 478 µg/mL; subcutaneous at 0 and 4 weeks: 625 µg/mL; intramuscular at 0 and 4 weeks: 482 µg/mL). All study participants seroconverted except for one of 21 in the intramuscular (injections at 0 and 4 weeks) group. Systemic adverse events were uncommon and similar for the intramuscular and subcutaneous groups. All local reactions (i.e., tenderness, erythema, warmth, induration, and subcutaneous nodules) were significantly more common following subcutaneous vaccination. Comparison of the three vaccination series indicated no significant differences between the proportion of subjects experiencing local reactions for the two subcutaneous regimens but significantly fewer subcutaneous nodules (p<0.001) and significantly less erythema (p = 0.001) in the group vaccinated intramuscularly (P. Pittman, personal communication, USAMRIID, Ft. Detrick, MD). Larger studies are planned to further evaluate vaccination schedule and route of administration. At this time, ACIP cannot recommend changes in vaccine administration because of the preliminary nature of this information. However, the data in this report do support some flexibility in the route and timing of anthrax vaccination under special circumstances. As with other licensed vaccines, no data indicate that increasing the interval between doses adversely affects immunogenicity or safety. Therefore, interruption of the vaccination schedule does not require restarting the entire series of anthrax vaccine or the addition of extra doses. Vaccine Efficacy The efficacy of AVA is based on several studies in animals, one controlled vaccine trial in humans (64), and immunogenicity data for both humans and lower mammalian species (47,49,57,65). Vaccination of adults with the licensed vaccine induced an immune response measured by indirect hemagglutination in 83% of vaccinees 2 weeks after the first dose and in 91% of vaccinees who received two or more doses (57,65). Approxi mately 95% of vaccinees seroconvert with a fourfold rise in anti-PA IgG titers after three doses (57,65). However, the precise correlation between antibody titer (or concentration) and protection against infection is not defined (57). The protective efficacy of the alum-precipitated vaccine (the original form of the PA filtrate vaccine) and AVA (adsorbed to aluminum hydroxide) have been demonstrated in several animal models using different routes of administration (49--52,57,62,63,66--69). Data from animal studies (except primate studies) involve several animal models, preparations, and vaccine schedules and are difficult to interpret and compare. The macaque model (Rhesus monkeys, Macaca mulatta) of inhalation anthrax is believed to best reflect human disease (31), and the AVA vaccine has been shown to be protective against pulmonary challenge in macaques using a limited number of B. anthracis strains (52,62,70--73) (Table 2). In addition to the studies of macaques, a study was published in 1962 of an adjuvant controlled, single-blinded, clinical trial among mill workers using the alum-precipitated vaccine --- the precursor to the currently licensed AVA. In this controlled study, 379 employees received the vaccine, 414 received the placebo, and 340 received neither the vaccine nor the placebo. This study documented a vaccine efficacy of 92.5% for protection against anthrax (cutaneous and inhalation combined), based on person time of occupational exposure (64). During the study, an outbreak of inhalation anthrax occurred among the study participants. Overall, five cases of inhalation anthrax occurred among persons who were either placebo recipients or did not participate in the controlled part of the study. No cases occurred in anthrax vaccine recipients. No data are available regarding the efficacy of anthrax vaccine for persons aged <18 years and >65 years. Duration of Efficacy The duration of efficacy of AVA is unknown in humans. Data from animal studies suggest that the duration of efficacy after two inoculations might be 1--2 years (57,62,72). Vaccine Safety Data regarding adverse events associated with use of AVA are derived from information from three sources. These sources are a) prelicensure investigational new drug data evaluating vaccine safety, b) passive surveillance data regarding adverse events associated with postlicensure use of AVA, and c) several published studies (64,74,75). Prelicensure Adverse Event Surveillance Local Reactions. In AVA prelicensure evaluations, 6,985 persons received 16,435 doses: 9,893 initial series doses and 6,542 annual boosters (74). Severe local reactions (defined as edema or induration >120 mm) occurred after 1% of vaccinations. Moderate local reactions (defined as edema and induration of 30 mm--120 mm) occurred after 3% of vaccinations. Mild local reactions (defined as erythema, edema, and induration <30 mm) occurred after 20% of vaccinations. In a study of the alum precipitated precursor to AVA, moderate local reactions were documented in 4% of vaccine recipients and mild reactions in 30% of recipients (64). Systemic Reactions. In AVA prelicensure evaluations, systemic reactions (i.e., fever, chills, body aches, or nausea) occurred in <0.06% (in four of approximately 7,000) of vaccine recipients (74). In the study of the alum precipitated precursor to AVA, systemic reactions occurred in 0.2% of vaccine recipients (64). Postlicensure Adverse Event Surveillance Data regarding potential adverse events following anthrax vaccination are available from the Vaccine Adverse Event Reporting System (VAERS) (75). From January 1, 1990, through August 31, 2000, at least 1,859,000 doses of anthrax vaccine were distributed in the United States. During this period, VAERS received 1,544 reports of adverse events; of these, 76 (5%) were serious. A serious event is one that results in death, hospitalization, or permanent disability or is life-threatening. Approximately 75% of the reports were for persons aged <40 years; 25% were female, and 89% received anthrax vaccine alone. The most frequently reported adverse events were injection-site hypersensitivity (334), injection-site edema (283), injection-site pain (247), headache (239), arthralgia (232), asthenia (215), and pruritis (212). Two reports of anaphylaxis have been received by VAERS. One report of a death following receipt of anthrax vaccine has been submitted to VAERS; the autopsy final diagnosis was coronary arteritis. A second fatal report, submitted after August 31, 2000, indicated aplastic anemia as the cause of death. A causal association with anthrax vaccine has not been documented for either of the death reports. Serious adverse events infrequently reported (<10) to VAERS have included cellulitis, pneumonia, Guillain-Barré syndrome, seizures, cardiomyopathy, systemic lupus erythematosus, multiple sclerosis, collagen vascular disease, sepsis, angioedema, and transverse myelitis (CDC/FDA, unpublished data, 2000). Analysis of VAERS data documented no pattern of serious adverse events clearly associated with the vaccine, except injection-site reactions. Because of the limitations of spontaneous reporting systems, determining causality for specific types of adverse events, with the exception of injection-site reactions, is often not possible using VAERS data alone. Published Studies About Adverse Events Adverse events following anthrax vaccination have been assessed in several studies conducted by the Department of Defense in the context of the routine anthrax vaccination program. At U.S. Forces, Korea, data were collected at the time of anthrax vaccination from 4,348 service personnel regarding adverse events experienced from a previous dose of anthrax vaccine. Most reported events were localized, minor, and self-limited. After the first or second dose, 1.9% reported limitations in work performance or had been placed on limited duty. Only 0.3% reported >1 day lost from work; 0.5% consulted a clinic for evaluation; and one person (0.02%) required hospitalization for an injection-site reaction. Adverse events were reported more commonly among women than among men. A second study at Tripler Army Medical Center, Hawaii, assessed adverse events among 603 military health-care workers. Rates of events that resulted in seeking medical advice or taking time off work were 7.9% after the first dose; 5.1% after the second dose; 3.0% after the third dose; and 3.1% after the fourth dose. Events most commonly reported included muscle or joint aches, headache, and fatigue (10). However, these studies are subject to several methodological limitations, including sample size, the limited ability to detect adverse events, loss to follow-up, exemption of vaccine recipients with previous adverse events, observational bias, and the absence of unvaccinated control groups (10). No studies have definitively documented occurrence of chronic diseases (e.g., cancer or infertility) following anthrax vaccination. In an assessment of the safety of anthrax vaccine, the Institute of Medicine (IOM) noted that published studies reported no significant adverse effects of the vaccine, but the literature is limited to a few short-term studies (76). One published follow-up study of laboratory workers at Fort Detrick, Maryland, concluded that, during the 25-year period following receipt of anthrax vaccine, the workers did not develop any unusual illnesses or unexplained symptoms associated with vaccination (77,78). IOM concluded that, in the peer-reviewed literature, evidence is either inadequate or insufficient to determine whether an association exists between anthrax vaccination and long-term adverse health outcomes. IOM noted that few vaccines for any disease have been actively monitored for adverse effects over long periods and encouraged evaluate of active long-term monitoring studies of large populations to further evaluate the relative safety of anthrax vaccine. Such studies are under way by the Department of Defense. CDC has conducted two epidemiologic investigations of the health concerns of Persian Gulf War (PGW) veterans that examined a possible association with vaccinations, including anthrax vaccination. The first study, conducted among Air Force personnel, evaluated several potential risk factors for chronic multisymptom illnesses, including anthrax vaccination. Occurrence of a chronic multisymptom condition was significantly associated with deployment to the PGW but was not associated with specific PGW exposures and also affected nondeployed veterans (79). The ability of this study to detect a significant difference was limited. The second study focused on comparing illness among PGW veterans and controls. The study documented that the self-reported prevalence of medical and psychiatric conditions was higher among deployed PGW veterans than nondeployed veterans. In this study, although a question was asked about the number of vaccinations received, no specific questions were asked about the anthrax vaccine. However, the study concluded that the relation between self-reported exposures and conditions suggests that no single exposure is related to the medical and psychiatric conditions among PGW military personnel (80). In summary, current research has not documented any single cause of PGW illnesses, and existing scientific evidence does not support an association between anthrax vaccine and PGW illnesses. No data are available regarding the safety of anthrax vaccine for persons aged <18 years and >65 years. Management of Adverse Events Adverse events can occur in persons who must complete the anthrax vaccination series because of high risk of exposure or because of employment requirements. Several protocols have been developed to manage specific local and systemic adverse events (available at www.anthrax.osd.mil). However, these protocols have not been evaluated in randomized trials. Reporting of Adverse Events Adverse events occurring after administration of anthrax vaccine --- especially events that are serious, clinically significant, or unusual --- should be reported to VAERS, regardless of the provider's opinion of the causality of the association. VAERS forms can be obtained by calling (800) 822-7967. Information about VAERS and how to report vaccine adverse events is available from http://www.vaers.org>, <http://www.fda.gov/cber/vaers/vaers.htm> or <http://www.cdc.gov/nip/>. PRECAUTIONS AND CONTRAINDICATIONSVaccination During Pregnancy No studies have been published regarding use of anthrax vaccine among pregnant women. Pregnant women should be vaccinated against anthrax only if the potential benefits of vaccination outweigh the potential risks to the fetus. Vaccination During Lactation No data suggest increased risk for side effects or temporally related adverse events associated with receipt of anthrax vaccine by breast-feeding women or breast-fed children. Administration of nonlive vaccines (e.g., anthrax vaccine) during breast-feeding is not medically contraindicated. Allergies Although anaphylaxis following anthrax vaccination is extremely rare and no anaphylaxis deaths associated with AVA have been reported, this adverse event can be life threatening. AVA is contraindicated for persons who have experienced an anaphylactic reaction following a previous dose of AVA or any of the vaccine components. Previous History of Anthrax Infection Anthrax vaccine is contraindicated in persons who have recovered from anthrax because of previous observations of more severe adverse events among recipients with a vaccine history of anthrax than among nonrecipients. The vaccine is also contraindicated in persons with a history of an anaphylactic reaction to the vaccine. Illness In the context of the routine preexposure program, vaccination of persons with moderate or severe acute illness should be postponed until recovery. This prevents superimposing the adverse effects of the vaccine on the underlying illness or mistakenly attributing a manifestation of the underlying illness to the vaccine. Vaccine can be administered to persons who have mild illnesses with or without low-grade fever. RECOMMENDATIONS FOR USE OF AVAPreexposure Vaccination Occupational and Laboratory Exposures Routine vaccination with AVA is indicated for persons engaged a) in work involving production quantities or concentrations of B. anthracis cultures and b) in activities with a high potential for aerosol production (81). Laboratorians using standard Biosafety Level 2 practices in the routine processing of clinical samples are not at increased risk for exposure to B. anthracis spores. The risk for persons who come in contact in the workplace with imported animal hides, furs, bone meal, wool, animal hair, or bristles has been reduced by changes in industry standards and import restrictions (82). Routine preexposure vaccination is recommended only for persons in this group for whom these standards and restrictions are insufficient to prevent exposure to anthrax spores. Routine vaccination of veterinarians in the United States is not recommended because of the low incidence of animal cases. However, vaccination might be indicated for veterinarians and other high-risk persons handling potentially infected animals in areas with a high incidence of anthrax cases. Bioterrorism Preparedness Although groups initially considered for preexposure vaccination for bioterrorism preparedness included emergency first responders, federal responders, medical practitioners, and private citizens, vaccination of these groups is not recommended. Recommendations regarding preexposure vaccination should be based on a calculable risk assessment. At present, the target population for a bioterrorist release of B. anthracis cannot be predetermined, and the risk of exposure cannot be calculated. In addition, studies suggest an extremely low risk for exposure related to secondary aerosolization of previously settled B. anthracis spores (28,83). Because of these factors, preexposure vaccination for the above groups is not recommended. For the military and other select populations or for groups for which a calculable risk can be assessed, preexposure vaccination may be indicated. Options other than preexposure vaccination are available to protect personnel working in an area of a known previous release of B. anthracis. If concern exists that persons entering an area of a previous release might be at risk for exposure from a re-release of a primary aerosol of the organism or exposure from a high concentration of settled spores in a specific area, initiation of prophylaxis should be considered with antibiotics alone or in combination with vaccine as is outlined in the section on postexposure prophylaxis. Postexposure Prophylaxis --- Chemoprophylaxis and Vaccination Penicillin and doxycycline are approved by FDA for the treatment of anthrax and are considered the drugs of choice for the treatment of naturally occurring anthrax (14,83,84). In addition, ciprofloxacin and ofloxacin have also demonstrated in vitro activity against B. anthracis (14,85). On the basis of studies that demonstrated the effectiveness of ciprofloxacin in reducing the incidence and progression of inhalation anthrax in animal models, FDA recently approved the use of ciprofloxacin following aerosol exposure to B. anthracis spores to prevent development or progression of inhalation anthrax in humans. Although naturally occurring B. anthracis resistance to penicillin is rare, such resistance has been reported (86). As of November 2000, no naturally occurring resistance to tetracyclines or ciprofloxacin had been reported. Antibiotics are effective against the germinated form of B. anthracis but are not effective against the spore form of the organism. Following inhalation exposure, spores can survive in tissues for months without germination in nonhuman primates (30,87). This phenomenon of delayed vegetation of spores resulting in prolonged incubation periods has not been observed for routes of infection other than inhalation. In one study, macaques were exposed to four times the LD50 dose* of anthrax spores, and the pro portion of spores that survived in the lung tissue was estimated to be 15%--20% at 42 days, 2% at 50 days, and <1% at 75 days (8). Although the LD50 dose for humans is believed to be similar to that for nonhuman primates, the length of persistence of B. anthracis spores in human lung tissue is not known. The prolonged incubation period reported in the Soviet Union outbreak of inhalation anthrax suggests that lethal amounts of spores might have persisted up to 43 days after initial exposure. Although postexposure chemoprophylaxis with tetracycline was reportedly initiated during this outbreak, the duration of therapy was not reported. Currently, ciprofloxacin is the only antibiotic approved by FDA for use in reducing the incidence or progression of disease after exposure to aerosolized B. anthracis. Although postexposure chemoprophylaxis using antibiotics alone has been effective in animal models, the definitive length of treatment is unclear. Several studies have demonstrated that short courses (5--10 days) of postexposure antibiotic therapy are not effective at preventing disease when large numbers of spores are inhaled (7,30). Longer courses of antibiotics may be effective (87). The study findings indicate that seven of 10, nine of 10 and eight of nine macaques exposed to 240,000--560,000 anthrax spores (8 times the LD50) survived when treated for 30 days with penicillin, doxycycline, or ciprofloxacin, respectively. All animals survived while undergoing antibiotic prophylaxis. Three animals treated with penicillin died on days 9, 12, and 20 after antibiotics were discontinued (days 39, 42, and 50 after exposure). A single animal in the doxycycline group died of inhalation anthrax 28 days after discontinuing treatment (day 58), and one animal in the ciprofloxacin group died 6 days after discontinuation of therapy (day 36). In addition, studies have demonstrated that antibiotics in combination with postexposure vaccination are effective at preventing disease in nonhuman primates after exposure to B. anthracis spores (30,87). Vaccination alone after exposure was not protective. Because the current vaccine is labeled for use in specifically defined preexposure situations only, no FDA-approved labeling addresses the optimal number of vaccinations for postexposure prophylaxis use of the vaccine. An estimated 83% of human vaccinees develop a vaccine-induced immune response after two doses of the vaccine and >95% develop a fourfold rise in antibody titer after three doses (57,65). Although the precise correlation between antibody titer and protection against disease is not clear, these studies of postexposure vaccine regimens used in combination with antibiotics in nonhuman primates have consistently documented that two to three doses of vaccine were sufficient to prevent development of disease once antibiotics were discontinued. Only one study has directly compared antibiotics plus vaccine with a longer course of antibiotics following aerosol exposure (87). This study documented no significant difference in survival for animals treated with doxycycline alone for 30 days or animals treated with 30 days of doxycycline plus two doses of anthrax vaccine postexposure (nine of 10 versus nine of nine, p = 0.4). However, the study suggests a possible benefit of postexposure combination of antibiotics with vaccination. Following Inhalation Exposure Postexposure prophylaxis against B. anthracis is recommended following an aerosol exposure to B. anthracis spores. Such exposure might occur following an inadvertent exposure in the laboratory setting or a biological terrorist incident. Aerosol exposure is unlikely in settings outside a laboratory working with large volumes of B. anthracis, textile mills working with heavily contaminated animal products, or following a biological terrorism or warfare attack. Following naturally occurring anthrax among livestock, cutaneous and rare gastrointestinal exposures among humans are possible, but inhalation anthrax has not been reported. Because of the potential persistence of spores following a possible aerosol exposure, antibiotic therapy should be continued for at least 30 days if used alone, and although supporting data are less definitive, longer antibiotic therapy (up to 42--60 days) might be indicated. If vaccine is available, antibiotics can be discontinued after three doses of vaccine have been administered according to the standard schedule (0, 2, and 4 weeks) (Table 3). Because of concern about the possible antibiotic resistance of B. anthracis used in a bioterrorist attack, doxycycline or ciprofloxacin can be chosen initially for antibiotic chemoprophylaxis until organism susceptibilities are known. Antibiotic chemoprophylaxis can be switched to penicillin VK or amoxicillin once antibiotic susceptibilities are known and the organism is found to be penicillin susceptible with minimum inhibitory concentrations (MICs) attainable with oral therapy. Although the shortened vaccine regimen has been effective when used in a postexposure regimen that includes antibiotics, the duration of protection from vaccination is not known. Therefore, if subsequent exposures occur, additional vaccinations might be required. Following Cutaneous or Gastrointestinal Exposure No controlled studies have been conducted in animals or humans to evaluate the use of antibiotics alone or in combination with vaccination following cutaneous or gastrointestinal exposure to B. anthracis. Cutaneous and rare gastrointestinal exposures of humans are possible following outbreaks of anthrax in livestock. In these situations, on the basis of pathophysiology, reported incubation periods, current expert clinical judgment, and lack of data, postexposure prophylaxis might consist of antibiotic therapy for 7--14 days. Antibiotics could include any of those previously mentioned in this report and in Table 3. RESEARCH AGENDAThe following research priorities should be considered regarding anthrax vaccine: immunogenicity, evaluation of changes in use of the current vaccine, human safety studies, postexposure prophylaxis, antibiotic susceptibility and treatment studies, and safety of anthrax vaccine in clinical toxicology studies among pregnant animals. Immunogenicity Regarding the immunogenicity of AVA, priority research topics include a) identifying a quantitative immune correlate(s) of protection in relevant animal species (especially rabbits and nonhuman primates) and b)defining the quantitative relation between the vaccine-elicited immune response in these animal species and humans. Specifically, such information could help to provide scientific justification for changing the schedule and route of administration of the existing vaccine. Evaluating Changes in the Current Vaccine Schedule and Route Studies evaluating the effects of variations in use of the current anthrax vaccine should include a definitive clinical evaluation comparing the intramuscular and subcutaneous routes of administration and an assessment of the effects of reducing the number of inoculations required for protection. Both immunogenicity and safety of these changes should be evaluated. Information about the efficacy and safety of AVA use in children and elderly persons is needed. Information about safety of the vaccine during pregnancy is also needed. In addition, research to develop the next generation of anthrax vaccines should continue. Human Safety Studies To assess the safe use of anthrax vaccine in humans, the Advisory Committee on Immunization Practices (ACIP) recommends several areas of research. Adverse event surveillance through VAERS should be enhanced, which could include development of electronic reporting capability and implementation of strategies to facilitate reporting. In addition, the influence of lot-to-lot variations in the vaccine on rates of adverse events should be evaluated. Other safety issues related to use of anthrax vaccine that should be addressed include development and evaluation of pretreatment strategies to decrease short-term adverse events; assessment of risk factors for adverse events, including sex and preexisting antibody levels; and analysis of differences in rates of occurrence of adverse events by route of anthrax transmission and method of vaccine administration (intramuscular, subcutaneous, or jet injector). Because the role of repeated inoculations in local and systemic reactions remains unclear, further research is needed regarding this subject. In addition, the feasibility of studies to evaluate longer term and systemic adverse events should be determined. Postexposure Prophylaxis Although a substantial benefit of postexposure antibiotics in preventing development of inhalation anthrax has been demonstrated in macaques, further research is needed to determine the optimal number of days of administration of those antibiotics and any additional benefit of receiving the anthrax vaccine in combination with antibiotics. This is a high priority for the current federal initiative regarding bioterrorism preparedness. Determining alternative antibiotics for children and pregnant women should be an important part of this research. Antibiotic Susceptibility and Treatment Studies Studies are needed that assess in vitro susceptibility of B. anthracis strains to azithromycin, erythromycin, and other antibiotics that are practical for children and elderly persons. In addition, treatment trials in animals for antibiotic alternatives to penicillin and doxycycline are recommended. Safety of Anthrax Vaccine in Clinical Toxicology Studies Among Pregnant Animals To assess the safety of anthrax vaccine use during human pregnancy, ACIP recommends that regulatory toxicology studies be conducted in pregnant animals. The study findings could provide baseline data for further studies of the safety of AVA use in pregnant women. References
*LD50=a lethal dose of 50%; defined as the dose of a product that will result in the death of 50% of a population exposed to that product. Table 1 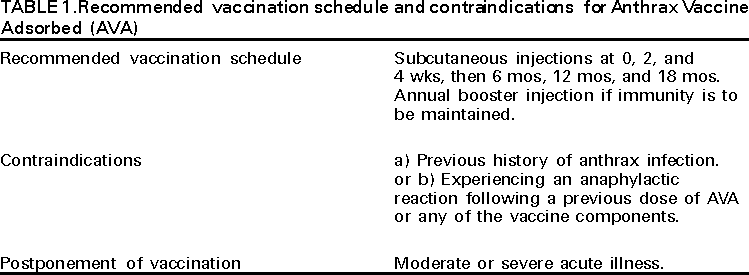 Return to top. Table 2 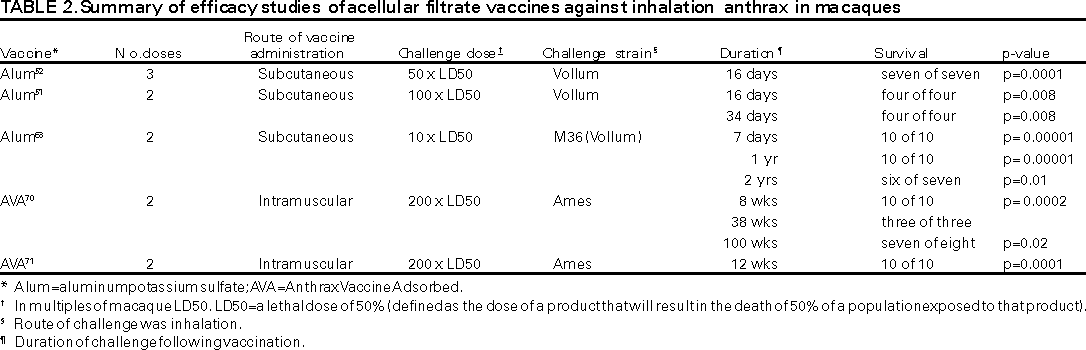 Return to top. Table 3  Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/13/2000 |
|||||||||
This page last reviewed 5/2/01
|