 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Use of Diphtheria Toxoid-Tetanus Toxoid-Acellular Pertussis Vaccine as a Five-Dose SeriesSupplemental Recommendations of the Advisory Committee on Immunization Practices (ACIP)Please note: An erratum has been published for this article. To view the erratum, please click here. Advisory Committee on Immunization Practices Membership List, February 2000CHAIRMAN John F. Modlin, M.D. EXECUTIVE SECRETARY Dixie E. Snider, Jr., M.D., M.P.H. MEMBERS
Dennis A. Brooks, M.D., M.P.H. Richard D. Clover, M.D. David W. Fleming, M.D. Fernando A. Guerra, M.D. Charles M. Helms, M.D., Ph.D. David R. Johnson, M.D., M.P.H.
Chinh T. Le, M.D. Paul A. Offit, M.D. Margaret B. Rennels, M.D. Lucy S. Tompkins, M.D., Ph.D. Bonnie M. Word, M.D. EX-OFFICIO MEMBERS
William Egan, Ph.D. Geoffrey S. Evans, M.D. Michael A. Gerber, M.D. T. Randolph Graydon Martin G. Myers, M.D. Kristin Lee Nichol, M.D., M.P.H. Douglas A. Thoroughman, Ph.D. David H. Trump, M.D., M.P.H. LIAISON REPRESENTATIVES
American Academy of Family Physicians American Academy of Pediatrics American Association of Health Plans American College of Obstetricians and Gynecologists American College of Physicians American Hospital Association American Medical Association Association of Teachers of Preventive Medicine Biotechnology Industry Organization Canadian National Advisory Committee on Immunization Hospital Infection Control Practices Advisory Committee Infectious Diseases Society of America National Immunization Council and Child Health Program, Mexico National Medical Association National Vaccine Advisory Committee Pharmaceutical Research and Manufacturers of America
The following CDC staff members prepared this report: Melinda Wharton, M.D., M.P.H. in collaboration with the Advisory Committee on Immunization Practices Acellular Pertussis Vaccine Working Group Margaret B. Rennels, M.D. John F. Modlin, M.D. Karen M. Farizo, M.D. Use of Diphtheria Toxoid-Tetanus Toxoid-Acellular Pertussis Vaccine as a Five-Dose SeriesSupplemental Recommendations of the Advisory Committee on Immunization Practices (ACIP)SummaryFour vaccines containing diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) are currently licensed in the United States for use among infants and young children. As of October 2000, two products, ACEL-IMUNE® (a product of Lederle Laboratories) and Tripedia® (Aventis Pasteur, Inc.) were licensed for the five-dose DTaP vaccination series. Two other vaccines, Infanrix® (SmithKline Beecham Biologicals) and Certiva™ (North American Vaccine, Inc.) are licensed for the first four doses of the vaccination series, beginning with the primary series at ages 2, 4, and 6 months, and for completing the DTaP series among children who began the series with diphtheria and tetanus toxoids and whole-cell pertussis vaccine. This report supplements the statement from CDC's Advisory Committee on Immunization Practices regarding use of acellular pertussis vaccines and summarizes data regarding reactogenicity of acellular pertussis vaccines when administered as the fourth and fifth consecutive doses. Increases in the frequency and magnitude of local reactions at the injection site with increasing dose number have occurred for all currently licensed DTaP vaccines. Extensive swelling of the injected limb, sometimes involving the entire thigh or upper arm, after receipt of the fourth and fifth doses of DTaP vaccines has been demonstrated for multiple products from different manufacturers. Because data are insufficient regarding the safety, immunogenicity, and efficacy of using DTaP vaccines from different manufacturers in a mixed sequence, ACIP continues to recommend that, whenever feasible, the same brand of DTaP vaccine be used for all doses in the vaccination series. When the vaccine provider does not know or does not have available the type of DTaP vaccine previously administered, any of the licensed DTaP vaccines can be used to complete the vaccine series. INTRODUCTIONFour vaccines containing diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) are licensed for use among infants in the United States; two of these, ACEL-IMUNE® (a product of Lederle Laboratories) and Tripedia® (Aventis Pasteur, Inc.) are licensed for use as the complete five-dose series (Table). The other two licensed vaccines are approved for use for the first four doses of the five-dose series, beginning at ages 2, 4, and 6 months. Licensure of other DTaP vaccines as a five-dose series is anticipated. This report supplements previous recommendations regarding use of DTaP (1) and summarizes new Advisory Committee on Immunization Practices (ACIP) recommendations regarding DTaP vaccines as a five-consecutive--dose series. REACTOGENICITY OF DTaP VACCINES WHEN ADMINISTERED AS FOURTH AND FIFTH DOSES OF A SERIESData regarding use of a single DTaP vaccine for the complete five-dose series are limited, but available data demonstrate a substantial increase in the frequency and magnitude of local reactions after the fourth and fifth doses. Increases in the frequency of fever after the fourth dose have been reported also, although increased frequencies of other systemic reactions (e.g., fretfulness, drowsiness, or decreased appetite) have not been observed. Despite the increased reactogenicity of the fourth and fifth doses, acellular pertussis vaccines remain the preferred vaccines for preventing pertussis, diphtheria, and tetanus among children because of the improved safety profile when compared with whole-cell pertussis vaccines (2--5). Adverse Reactions After the Fourth Dose of DTaP When Administered as a Four-Dose SeriesIncreases in erythema, swelling, and pain at the injection site and increases in fever have been reported with the fourth dose as compared with the first dose for each of the currently licensed DTaP vaccines. These reactions typically have onset within 2 days of vaccination and resolve completely without sequelae (6). During 1991--1994, reactogenicity of ACEL-IMUNE administered as a four-dose series was assessed in an efficacy study in Germany (7). DTaP and diphtheria and tetanus toxoids and whole-cell pertussis vaccine (DTP) components of the study were randomized and double-blinded. Local and systemic reactions were reported on standard diary cards for 72 hours after each dose. Of 3,991 children who received the fourth dose of ACEL-IMUNE, 10% experienced erythema >0.9 in. (>2.4 cm), and 9% experienced induration >0.9 in. (>2.4 cm). After the first dose, only 2% of recipients were reported as experiencing erythema or induration of this magnitude. Fever >100.4 F (>38 C) was reported for 7% of recipients of the first dose, but after the fourth dose, 26% of recipients experienced fever >100.4 F (>38 C) (4,8). In an open-label trial (i.e., a study in which researchers and subjects know what vaccine and dose is being administered) in the United States, 109 infants who had previously received Tripedia at ages 2, 4, and 6 months received a fourth dose at age 1520 months (9). Reactions were assessed by parents at 6, 24, and 48 hours and daily thereafter for 14 days, and parents were asked to record daily on a standardized diary the presence or absence of injection-site tenderness, redness, or swelling. Of children receiving the fourth dose, 5.5% experienced fever >101 F (>38.3 C) within 72 hours of vaccination; 30.3%, injection site redness >1 in. (>2.54 cm); 29.4%, injection site swelling >1 in. (>2.54 cm); and 19.3%, injection site pain (9). In contrast, during the primary series study of 218 infants, no infants experienced fever >101 F (>38.3 C) after the first dose; 2%, erythema >1 in. (>2.54 cm); 2%, swelling >1 in. (>2.54 cm); and 10%, tenderness at the injection site (10). Of 22,505 children who had received three doses of Infanrix® (SmithKline Beecham Biologicals) at ages 3, 4, and 5 months during an open-label safety trial in Germany during April 1993--November 1994, 5,361 received a fourth dose at age 10--36 months (11). Standardized diaries reporting adverse events occurring within 3 days of vaccination were available for 1,809 children who had received the fourth dose. Age range of this subset of children was 14--28 months. Rates of redness, swelling, pain, and fever increased with successive doses. Redness >0.8 in. (>2 cm) increased from 0% after the first dose to 13.8% after the fourth dose; swelling >0.8 in. (>2 cm), from 0% to 11.4%; pain, from 2.0% to 26.3%; and fever >100.4 F (>38 C), from 6.3% to 26.4% (1113). Increases in the reactogenicity of the fourth dose of Certiva™ (North American Vaccine, Inc.) also have been reported. Fourth-dose data have been reported for 316 infants, a subset of >2,200 who received Certiva as a three-dose primary series during an open-label trial in the United States (14). Safety data were collected using standardized diary cards and telephone follow-up. Fever >100.4 F (>38 C) within 72 hours of vaccination increased in frequency from the first dose to the fourth dose, with fever reported among 1.5% of first-dose recipients and 10.5% of fourth-dose recipients. Frequency of redness >1.2 in. (>3 cm) increased from 0.2% after the first dose to 5.7% after the fourth dose; swelling >1.2 in. (>3 cm), from 0.6% to 4.5%; and tenderness or pain (any), from 5.9% to 19.0% (14). Adverse Reactions After the Fifth Dose of DTaP When Administered as a Five-Dose SeriesData regarding the reactogenicity of a fifth dose of DTaP administered after four doses of the DTaP vaccine are limited, but are available for three of the four currently licensed DTaP vaccines. These data demonstrate further increases in the local reactogenicity of the fifth dose compared with the fourth dose. No data are available regarding the frequency of adverse events after a fifth dose of Certiva. Data have been summarized from four clinical trials in the United States and Germany, during which 357 infants received a fifth dose of ACEL-IMUNE after having received four previous doses of the same vaccine. Case definitions of substantial erythema and induration varied by protocol, ranging from >0.8 in. (>2 cm) to >0.9 in. (>2.4 cm). However, substantial erythema within 72 hours after the fifth dose was reported for 20% of recipients; substantial induration for 14%; and tenderness for 38% (8). In a study in Germany during March--September 1998, of 580 children who received a fifth dose of Tripedia after four previous doses of the same vaccine, 31.0% experienced redness >2 in. (>5 cm) within 3 days of receipt of vaccine; 25.0% experienced swelling >2 in. (>5 cm); and 2.1% experienced severe pain (i.e., crying when the arm was moved) (15, Aventis Pasteur, Inc., unpublished data, January 2000). During a safety study in Germany, 413 children received a fifth dose of Infanrix after four previous doses of the same vaccine. During the 3 days after vaccination, redness >2 in. (>5 cm) was reported for 30.3% of recipients; swelling >2 in. (>5 cm), for 20.7%; and grade 3 pain (i.e., pain that prevented everyday activities and necessitated medical advice) for 1.6% of the 376 children for whom symptom sheets were completed (SmithKline Beecham Biologicals, unpublished data, February 2000). Limb Swelling After Booster Doses of DTaPSwelling involving the entire thigh or upper arm has been reported after booster doses of different acellular pertussis vaccines. Swelling of the entire thigh was reported among recipients of a booster dose of JNIH-6 (a two-component acellular pertussis vaccine produced by Biken [Japan] and comparable to the acellular pertussis component contained in Tripedia). During a study performed in Sweden during the 1980s, children who had previously received two or three doses of Biken acellular pertussis vaccine at age 6--8 months received a booster dose deep subcutaneously of the same vaccine at age 2 years. Certain children experienced substantial local reactions, including swelling of the entire thigh (16), although administration of vaccine subcutaneously could have influenced reaction rates in that study. Occurrence of extensive swelling involving the entire thigh of vaccinated children was reported among DTaP recipients in an open-label safety study in Germany during April 1993--November 1994, in which children who had previously received Infanrix at ages 3, 4, and 5 months received a fourth dose at age 10--36 months (11). Standardized diaries were available for 1,809 children, with data collected regarding the occurrence of specific solicited symptoms for 3 days after receipt of vaccine. Parents of the remaining 3,498 children were asked to report any symptoms occurring during the 28 days after vaccination; no specific symptoms were solicited. Among 5,361 vaccinees, an increase in thigh circumference was reported as an unsolicited reaction for 62 vaccinees (45 in the first group and 17 in the second group; frequency: 1.2%). One of six centers participating in the study accounted for a majority of these reports; at that center, this reaction was reported for 51 of 1,583 children (3.2%). Among 17 children whose thighs were measured, the mean increase in circumference was 0.9 in. (2.2 cm) (range: 0.2--2.0 in. [0.5--5 cm]). Swelling began within 48 hours of booster dose administration for 51 of 62 children; the mean duration of swelling was 3.9 days (range: 1--7 days). For a limited number of children, the swelling interfered with walking; but for the majority of children, no limitation of activity was experienced. None of the children were febrile. Pain when digital pressure was applied was reported for 51% of the children, and itching was observed among a limited number of children (11). In an analysis of the fourth- and fifth-dose follow-up studies from the Multicenter Acellular Pertussis Trial (MAPT) that examined 12 different DTaP vaccines, entire limb swelling was reported as an unsolicited reaction for 20 (2.0%) of 1,015 children who received four consecutive doses of the same DTaP (17). Entire thigh swelling was reported for 1 of 16 children receiving four consecutive doses of DTP and for 0 of 246 children receiving a booster dose of DTaP after three doses of DTP. Among children experiencing entire thigh swelling after the fourth dose, 70% were described as irritable, compared with 37% of fourth-dose recipients who did not experience entire thigh swelling. Erythema was reported for 60% of the vaccinees and pain for 60%; the corresponding frequencies among children without entire thigh swelling were 29% and 30%, respectively. Fever >100 F (>37.8 C) was reported for approximately 25% of both groups. Among the 20 children with entire thigh swelling, pain was judged to be mild for 7, moderate for 2, and severe for 3; pain was not reported for 8 of these 20 children. Of eight children whose swelling began on day 1, five experienced moderate or severe pain. A total of 12 children experienced swelling that began on day 2 or 3, none of whom experienced moderate or severe pain. Entire thigh swelling resolved completely and without sequelae among all 20 children (duration: 1--4 days among 11 children for whom duration was known). Among fifth-dose recipients, 0 of 121 children who had received the same DTaP vaccine experienced swelling of the entire upper arm; but such swelling occurred among 4 of 146 children (2.7%) who had received different DTaP vaccines during the five-dose series. Although the numbers of children receiving fourth and fifth doses during MAPT follow-up studies were limited, extensive limb swelling occurred after receipt of a fourth dose of 9 of the 12 DTaP vaccines included in the study (17). Recent studies of the fifth dose of Tripedia and Infanrix have also identified cases of extensive limb swelling. During an open-label trial performed in Germany during March--September 1998, swelling of the entire upper arm was reported as an unsolicited reaction for 14 of 490 children (2.9%) who had received a fifth dose of Tripedia (Aventis Pasteur, Inc., unpublished data, January 2000). For 13 of the 14 children, swelling began within 3 days of vaccination. Associated symptoms included redness for 10 of the 14 (71.4%) vaccinees, pain for 5 (35.7%), and fussiness for 2 (14.3%). Pain was graded as mild for all children for whom it was reported. No children had fever >100.4 F (>38 C), and only two children were evaluated during an office visit. Median duration of swelling was 4 days (range: 3--6 days) (Aventis Pasteur, Inc., unpublished data, January 2000). During an open-label trial in Germany of Infanrix as a fifth dose after four doses of Infanrix, parents or caretakers were informed of the possibility of limb swelling (SmithKline Beecham Biologicals, unpublished data, February and May 2000). Although limb swelling was not specifically solicited on diary cards, parents or caretakers were asked to contact the investigators if their children experienced such a reaction. Of 413 subjects enrolled, parents of 26 children contacted the investigators to report that their children had experienced swelling. A total of 3 of the 26 children (11%) had fever >99.5 F (>37.5 C) when measured axillarily or orally or >100.4 F (>38 C) when measured rectally. Other associated symptoms included pain for 23 (88.5%) vaccinees, which was diffuse for 6. Redness occurred for 26 (100%) vaccinees and was diffuse for 17. Warmth was experienced by 21 (80.8%) vaccinees and was diffuse for 13. Of the 26 children evaluated, one child experienced swelling extending from shoulder to elbow. That child experienced localized pain at the injection site and diffuse redness and warmth and was afebrile. For one child, swelling was assessed as grade 3 severity (i.e., prevented normal everyday activities and necessitated medical advice). For all vaccinees experiencing swelling, reaction began within 3 days of receipt of vaccine. Mean duration of swelling was 4 days (range: 1--10 days) (SmithKline Beecham Biologicals, unpublished data, February and May 2000). Pathogenesis of both substantial local reactions and limb swelling is unknown. In an analysis of data from the MAPT fourth- and fifth-dose follow-up studies, swelling >2 in. (>5 cm) after the fourth dose was associated with pertussis toxoid content of the vaccine administered; swelling after the fifth dose was associated with the aluminum content of the vaccine. Entire thigh swelling after the fourth dose was associated with diphtheria toxoid content of the vaccine. Prevaccination antibody levels to diphtheria, tetanus, or pertussis toxins were not predictive of this reaction. The inconsistent pattern of associations of vaccine content and swelling could indicate that the associations were a statistical artifact attributable to a limited sample size or to differential reporting of entire thigh swelling among the DTaP vaccine groups (17). SUPPLEMENTAL ACIP RECOMMENDATIONS FOR USING DTaP VACCINESData are limited regarding differences in reactogenicity among currently licensed acellular pertussis vaccines. Increases in frequency and magnitude of substantial local reactions at the injection site with increasing dose number have been reported for all currently licensed DTaP vaccines. Swelling of the thigh or entire upper arm after receipt of fourth and fifth doses of acellular pertussis vaccines has been documented for mul tiple products from different manufacturers. However, because reports of these reactions have generally not been solicited during safety studies, the frequency is unknown, and the absence of reports does not establish a lack of reaction after receipt of particular DTaP vaccines. Additionally, in the majority of studies of adverse events after receipt of the fourth and fifth doses of DTaP, participants represent a subset (a substantially limited subset in certain studies) of children who received the first three doses. Therefore, the observed frequencies of substantial swelling reactions might have been influenced by selection biases of unknown direction and magnitude. Data are insufficient to establish that mixed sequences of DTaP vaccines from different manufacturers are associated with higher or lower frequencies of these reactions than receipt of a single product for the entire DTaP series. Additional data regarding the reactogenicity of DTaP vaccines when administered as a five-dose series are needed. Whether children who experience entire limb swelling after a fourth dose of DTaP are at increased risk for this reaction after the fifth dose is unknown. Because reports to date indicate that the reactions are self-limited and in recognition of the benefits of the preschool dose of DTaP, a history of extensive swelling after the fourth dose should not be considered a contraindication for receipt of the fifth dose of the DTaP series. Parents or caregivers of children receiving the fourth and fifth doses of the DTaP series should be informed of the increases in reactogenicity that have been observed. Although available data demonstrate that these reactions are self-limited and resolve without sequelae, they might be clinically indistinguishable from other conditions (e.g., cellulitis) that require treatment. Therefore, providers must make decisions regarding evaluation and management of children with suspected reactions after DTaP vaccination on a case-by-case basis. Interchangeable Use of Acellular Pertussis VaccinesChildren who began the series with DTaP at age 2 months began eligibility to receive a fifth dose of DTaP during mid-2000. A child who began the series late and was vaccinated on an accelerated schedule might have become eligible for the fifth dose before then. Data are insufficient to document the safety, immunogenicity, and efficacy of using DTaP vaccines from different manufacturers in a mixed sequence. For this reason, the ACIP recommends that whenever feasible, the same brand of DTaP vaccine should be used for all doses of the vaccination series. However, the vaccine provider might not know or have available the type of DTaP vaccine previously administered to a child. Neither circumstance should present a barrier to administration of DTaP vaccine and any of the available licensed DTaP vaccines can be used to complete the vaccination series. References
Table 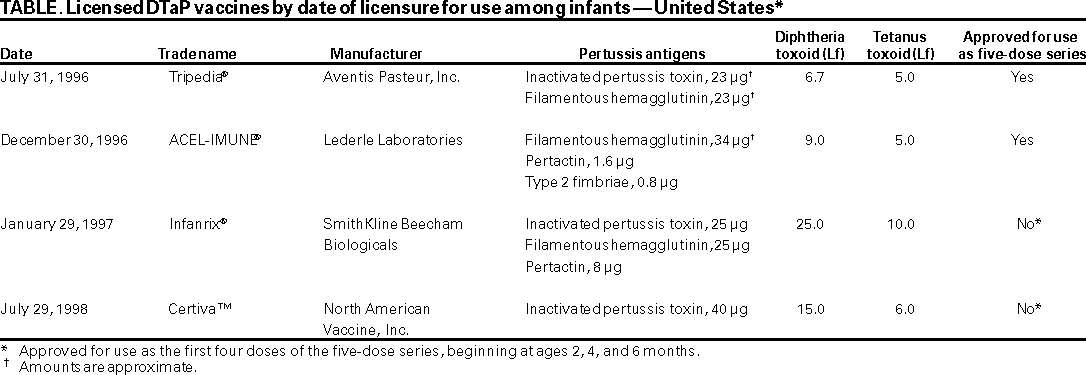 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/16/2000 |
|||||||||
This page last reviewed 5/2/01
|