Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Update: Infections With a Swine-Origin Influenza A (H1N1) Virus --- United States and Other Countries, April 28, 2009
Since April 21, 2009, CDC has reported cases of respiratory infection with a swine-origin influenza A (H1N1) virus (S-OIV) transmitted through human-to-human contact (1,2). This report updates cases identified in U.S. states and highlights certain control measures taken by CDC. As of April 28, the total number of confirmed cases of S-OIV infection in the United States had increased to 64, with cases in California (10 cases), Kansas (two), New York (45), Ohio (one), and Texas (six). CDC and state and local health departments are investigating all reported U.S. cases to ascertain the clinical features and epidemiologic characteristics. On April 27, CDC distributed an updated case definition for infection with S-OIV (Box).
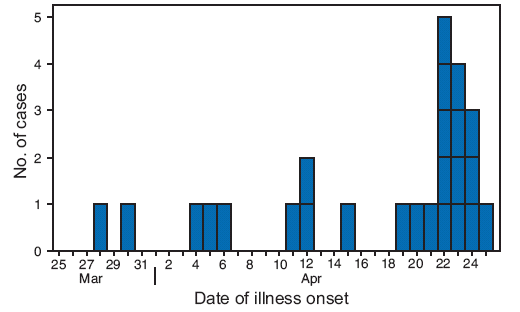
Of the 47 patients reported to CDC with known ages, the median age was 16 years (range: 3--81 years), and 38 (81%) were aged <18 years; 51% of cases were in males. Of the 25 cases with known dates of illness onset, onset ranged from March 28 to April 25 (Figure). To date, no deaths have been reported among U.S. cases, but five patients are known to have been hospitalized. Of 14 patients with known travel histories, three had traveled to Mexico; 40 of 47 patients (85%) have not been linked to travel or to another confirmed case. Information is being compiled regarding vaccination status of infected patients, but is not yet available. According to the World Health Organization (WHO), as of April 27, a total of 26 confirmed cases of S-OIV infection had been reported by Mexican authorities. Canada has reported six cases and Spain has reported one case.*
Emergency Use Authorizations
If an emerging public health threat is identified for which no licensed or approved product exists, the Project BioShield Act of 2004 authorizes the Food and Drug Administration (FDA) commissioner to issue an Emergency Use Authorization (EUA) so that promising countermeasures can be disseminated quickly for the protection and safety of the U.S. population (3).
In response to the current public health emergency involving swine-origin influenza, FDA issued four EUAs on April 27 to allow emergency use of
- oseltamivir (Tamiflu) and zanamivir (Relenza) for the treatment and prophylaxis of influenza (two EUAs),
- disposable N95 respirators for use by the general public, and
- the rRT-PCR Swine Flu Panel for diagnosis.
Oseltamivir is FDA-approved for treatment and prevention of influenza in adults and children aged ≥1 year. Zanamivir is FDA-approved for treatment of influenza in adults and children aged ≥7 years who have been symptomatic for <2 days, and for prevention of influenza in adults and children aged ≥5 years. The EUA allows the use of oseltamivir for treatment of influenza in children aged <1 year and prevention of influenza in children aged 3 months--1 year. Additionally, traditional prescribing and dispensing requirements might not be met. Under the scope and conditions of current EUAs, mass dispensing of both antiviral medications will be allowed per state and/or local public health authority.
FDA has authorized use of certain N95 respirators to help reduce wearer exposure to pathogenic biological airborne particulates during a public health emergency involving S-OIV. On April 27, CDC published guidelines for the use of N95 respirators. For example, respirators should be considered for use by persons for whom close contact with an infectious person is unavoidable. This can include selected individuals who must care for a sick person (e.g., family member with a respiratory infection) at home. Additional information is available at http://www.cdc.gov/swineflu/masks.htm.
Currently, no FDA-cleared tests specifically for the S-OIV strain exist in the United States or elsewhere. For this purpose and to meet the significant increase in demand for influenza testing throughout the country, CDC has developed the rRT-PCR Swine Flu Panel to expand and maintain the operational capabilities of public health or other qualified laboratories by providing a detection tool for the presumptive presence of S-OIV.
Control Measures at Ports of Entry and Travel Warning for Mexico
CDC, in collaboration with industry and federal partners, is continuing to conduct routine illness detection at ports of entry with heightened awareness for travelers who might be infected with S-OIV. During April 19--27, 15 cases of illness in travelers entering the United States from Mexico that were clinically consistent with S-OIV infection were detected. Of these 15 cases, two were laboratory confirmed as swine-origin influenza A (H1N1). Nine travelers remain in isolation pending completion of evaluation, and four travelers were released to complete travel after influenza virus infection was ruled out.
WHO has declared a Public Health Emergency of International Concern. As part of its responsibilities under the International Health Regulations, CDC is prepared to implement additional screening measures for international flights, if deemed necessary, to prevent exportation of S-OIV. In addition, CDC in collaboration with the U.S. Department of Homeland Security, is distributing travelers health alert notices to all persons traveling to countries with confirmed cases of S-OIV infection.
CDC has recommended that U.S. travelers avoid nonessential travel to Mexico (http://wwwn.cdc.gov/travel/contentswineflumexico.aspx). However, CDC might revise its travel guidance as the outbreak in Mexico evolves and is characterized more completely. Travelers who cannot delay travel to Mexico should visit http://www.cdc.gov/travel and follow the posted recommendations to reduce their risk for infection.
Nonpharmaceutical Community Mitigation
CDC has issued interim guidance for nonpharmaceutical community mitigation efforts in response to human infections with S-OIV (http://www.cdc.gov/swineflu/mitigation.htm). Current recommendations for isolation of patients with cases of S-OIV, household contacts, school dismissal, and other social distancing interventions also are available at http://www.cdc.gov/swineflu/mitigation.htm and will be updated as the situation evolves.
Reported by: Strategic Science and Program Unit, Coordinating Center for Infectious Diseases; Div of Global Migration and Quarantine, National Center for Preparedness, Detection, and Control of Infectious Diseases; Influenza Div, National Center for Immunization and Respiratory Diseases, CDC Influenza Emergency Response Team, CDC.
References
- CDC. Swine influenza A (H1N1) infection in two children---southern California, March--April 2009. MMWR 2009;58:400--2.
- CDC. Update: swine influenza A (H1N1) infections---California and Texas, April 2009. MMWR;58(In press).
- Nightingale SL, Prasher JM, Simonson S. Emergency Use Authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerg Infect Dis 2007;13:1046--51.