Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Receipt of Influenza Vaccine During Pregnancy Among Women With Live Births --- Georgia and Rhode Island, 2004--2007
Pregnant women are at increased risk for complications from influenza (1--3). Since 2004, the Advisory Committee on Immunization Practices (ACIP) and American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice have recommended that all pregnant women be vaccinated with the trivalent inactivated vaccine during any trimester of pregnancy (4,5). To assess the percentage of women who were vaccinated during pregnancy among women with recent live births, CDC analyzed data from the Pregnancy Risk Assessment and Monitoring System (PRAMS) from Georgia and Rhode Island, the two states that collected this information on the PRAMS survey. This report summarizes the results, which showed that in Georgia, the prevalence of influenza vaccination during the woman's most recent pregnancy increased from 10.4% in 2004 to 15.5% in 2006. In Rhode Island, vaccination prevalence increased from 21.9% in 2004 to 33.4% in 2007. During 2006 in Georgia, the most common reasons for not receiving vaccination were, "I don't normally get the flu vaccination" (69.4%), and "my physician did not mention anything about a flu vaccine during my pregnancy" (44.5%). Increased efforts are needed to assess vaccine coverage during pregnancy and to educate providers and pregnant women about ACIP and ACOG recommendations on providing intramuscular, inactivated influenza vaccine during any trimester of pregnancy.
PRAMS is a population-based surveillance system that collects data on a wide range of maternal behaviors and experiences before, during, and after pregnancy. PRAMS surveys currently are administered by 37 states, New York City, and one tribal-state partnership in South Dakota. Each month, participating states or entities use birth certificate data to select a stratified random sample of 100--300 women with recent live births. A questionnaire is mailed to the women 2--6 months after delivery. The participating entities use a standard questionnaire, to which they can add questions. From 2004 to 2007, Georgia and Rhode Island included questions about influenza vaccination on their surveys. Responses from Georgia for 3 years (2004--2006; N = 5,231) and Rhode Island for 4 years (2004--2007; N = 5,499) were analyzed. Variables included receipt of influenza vaccination in women during pregnancy, demographics, and health-care service use indicators. Response rates for the years of data examined for Georgia were 70.0% for 2004, 70.2% for 2005, and 70.8% for 2006; rates for Rhode Island were 75.5% for 2004, 75.1% for 2005, 72.5% for 2006, and 72.1% for 2007.
Women whose influenza vaccination status was missing (229 for Georgia and 163 for Rhode Island) were excluded. PRAMS data were weighted to take into account complex survey design, nonresponse, and noncoverage for each state. Data were analyzed to estimate influenza vaccination prevalence and 95% confidence intervals. Chi-square tests were used to determine statistical significance and statistical software were used to account for the complex sampling strategy.
Surveys conducted by both states inquired about influenza vaccination coverage by asking the question, "Did you get a flu vaccination during your most recent pregnancy?" The Georgia survey included a follow-up question for women who reported not being vaccinated to assess their reasons. The question included was, "What were your reasons for not getting a flu vaccination during your most recent pregnancy?" A list of reasons with a choice of yes/no response included items on receipt of provider advice, perceptions of vaccine safety, and timing of pregnancy. The Rhode Island survey included a question on provider advice, "At any time during your pregnancy, did a doctor, nurse, or other health-care worker offer you a flu vaccination or tell you to get one?"
In both states, most of the increase in influenza vaccination coverage was observed from 2004 to 2005 (Figure); in Georgia, coverage increased 62.5%, from 10.4% to 16.9%, and in Rhode Island, coverage increased 37.4%, from 21.9% to 30.1%. Vaccination prevalence remained mostly stable during 2005--2006, but with a further 10.0% increase observed in Rhode Island from 2006 to 2007. Prevalence of influenza vaccination during pregnancy in the two states varied by state and demographically (Table 1).
In Rhode Island, the prevalence of women who reported receiving advice about influenza vaccine or an offer of vaccination increased from 33.0% during 2004 to 47.7% during 2007 (p<0.001). In 2007, among respondents who reported receiving vaccination advice from a health-care provider, the prevalence of those who also were vaccinated was 65.7%. In 2007, Rhode Island data showed that among women who did not report receiving advice from their health-care provider about influenza vaccine, only 4.6% reported receiving influenza vaccination.
In Georgia, previous vaccination history, provider advice, perceptions of safety, and timing of pregnancy were among the reasons unvaccinated women cited for not getting the influenza vaccine (Table 2). The top reasons cited were "I don't normally get the flu vaccination" (69.4%) and "my physician did not mention anything about a flu vaccine during my pregnancy" (44.5%); 28.1% were worried about the safety of the influenza vaccine for their infant and 27.1% were worried about the safety for themselves.
Reported by: IB Ahluwalia, PhD, L Harrison, MPH, D Jamieson, MD, Div of Reproductive Health, National Center on Chronic Disease Prevention and Health Promotion; SA Rasmussen, MD, Div of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, CDC.
Editorial Note:
Despite evidence that maternal vaccination with influenza vaccine protects infants from influenza-like illness during the first 6 months of life (6), recent national data show that pregnant women have the lowest rates of coverage among all adult populations recommended to receive influenza vaccination (7). During 2004--2007, influenza vaccination prevalence increased significantly in both states among women with recent live births. The increases in coverage partially could be related to changes in ACIP and ACOG recommendations in 2004, when the recommendation for pregnant women changed from vaccine administration to women who would be in their second or third trimester during influenza season to administration of vaccine any time during pregnancy (4,5). Also, increased media reporting about vaccination of high-risk populations during the 2004--2005 influenza vaccine shortage might have increased awareness among pregnant women and their providers, perhaps resulting in an increase in influenza vaccination prevalence (8).
Interventions focusing on providers and pregnant women might address barriers to influenza vaccination experienced by both (3,9). In July 2006, Rhode Island passed a law* requiring the Rhode Island health department to purchase vaccine and distribute it to physicians who enroll in the Immunize for Life adult immunization program.† By enacting specific legislation, Rhode Island increased vaccine availability for health-care providers and perhaps alerted providers and pregnant women about the importance of immunizing pregnant women with influenza vaccine. The Rhode Island experience with vaccine distribution might be a useful example for other states on the effectiveness of working with health-care providers to supply influenza vaccine for pregnant women.
Approximately 25% of women in Georgia cited being in their first trimester as a reason for not getting the influenza vaccine, identifying a need to educate pregnant women about the importance of getting the influenza vaccine, even during the earliest phases of pregnancy. These women and their physicians might not have been aware of current ACIP and ACOG recommendations that women who are pregnant during influenza season should be vaccinated, irrespective of trimester. Providers should be educated about these recommendations, about influenza risks for pregnant women, and about interventions to prevent severe illness in this population (3--5,9).
The findings in this report are subject to at least three limitations. First, PRAMS data on influenza vaccination were only available from two states, and these findings might not be generalizable to all women with live births in the United States. Second, PRAMS data are self-reported by women 2--4 months postpartum and therefore they might be subject to recall bias. Finally, information on provider recommendations was assessed by maternal report; data from health-care providers regarding their practice related to influenza vaccine might have shown different results.
Because the seasonal influenza vaccine is unlikely to provide protection against pandemic influenza A (H1N1) infection (10), ACIP recommends that pregnant women receive both vaccine formulations during the 2009--10 influenza season. The trivalent inactivated seasonal influenza vaccine is now available and the influenza A (H1N1) 2009 monovalent vaccine is expected to become available in mid-October (10).
Acknowledgments
This report is based, in part, on contributions by H Kim, PhD, and R Cain, Rhode Island Dept of Health Center for Health Data and Analysis; C Hoban, PhD, and D Goodman, PhD, Georgia Dept of Community Health, Office of Epidemiology, Evaluation, and Health Information; A Harrison, Science Applications International Corporation Contractor; and S Gupta, MPH, Div of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
References
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 2006;12:1638--43.
- Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis 2008;14:95--100.
- Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev 2006;28:47--53.
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR 2008;57(No. RR-7).
- American College of Obstetricians and Gynecologists' Committee on Obstetric Practice. ACOG committee opinion number 305, November 2004. Influenza vaccination and treatment during pregnancy. Obstet Gynecol 2004;104:1125--6.
- Zaman K, Roy E, Arifeen SE, Rahman M, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555--64.
- Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989--2005. Vaccine 2008;26:1786--93.
- Brewer NT, Hallman WK. Subjective and objective risk as predictors of influenza vaccination during the vaccine shortage of 2004--2005. Clin Infect Dis 2006;43:1379--86.
- CDC. Influenza vaccination in pregnancy: practices among obstetrician-gynecologists---United States, 2003--04 influenza season. MMWR 2005;54:1050--2.
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-10).
* Routine childhood and adult immunization vaccines. Title 23, Chapter 23-1, Sect. 23-1-44 (2006). Available at http://www.rilin.state.ri.us/statutes/title23/23-1/23-1-44.htm.
† Additional information available at http://www.health.ri.gov/immunization/immunizeforlife.php.
FIGURE. Influenza vaccination coverage during most recent pregnancy among women with recent live births* --- Pregnancy Risk Assessment and Monitoring System, Georgia and Rhode Island, 2004--2007
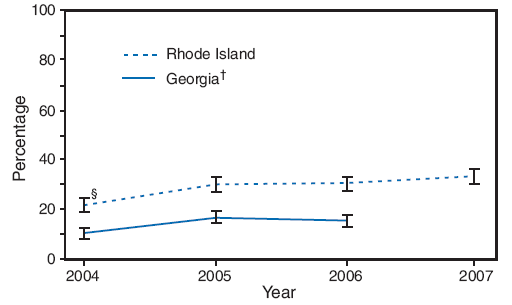
* Based on response to the question, "Did you get a flu vaccination during your most recent pregnancy?"
† 2007 data for Georgia were not available. Percentages are weighted.
§ 95% confidence interval.
Alternative Text: The figure above shows influenza vaccination coverage during the most recent pregnancy among women with recent live births in Georgia and Rhode Island from 2004-2007 from the Pregnancy Risk Assessment and Monitoring System. In both states, most of the increase in influenza vaccination coverage was observed from 2004 to 2005; in Georgia, coverage increased from 10.4% to 16.9%, and in Rhode Island, coverage increased from 21.9% to 30.1%. Vaccination prevalence remained mostly stable during 2005-2006, but with a further increase observed in Rhode Island from 2006 to 2007.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/9/2009


