Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Pseudo-Outbreak of Legionnaires Disease Among Patients Undergoing Bronchoscopy --- Arizona, 2008
Legionnaires disease (LD) is a potentially fatal form of pneumonia acquired by inhalation of aerosolized water containing Legionella bacteria. Legionella is a common cause of health-care--associated pneumonia, particularly in settings with hematopoietic stem-cell or solid-organ transplant recipients (1). On July 25, 2008, the Arizona Department of Health Services (ADHS) notified CDC of four patients who had Legionella cultured from specimens obtained during bronchoscopies performed at a medical center in Arizona. To characterize transmission and identify the source, ADHS and CDC began an investigation on August 1. This report summarizes the results of that investigation, which determined that the patients did not have LD and that nonsterile ice used to cool saline-filled syringes for bronchoalveolar lavage was the likely source of Legionella contamination of these clinical specimens. Ice was supplied by two ice machines, which became contaminated by heavy Legionella colonization within the center's potable water supply during a 6-month period (February--July 2008). Findings from the investigation underscore the importance of adherence to recommended infection control practices and surveillance for LD in health-care settings. Clinicians and endoscopy technicians should ensure that nonsterile items are not introduced during bronchoscopy procedures.
In May 2006, a hematopoietic stem-cell transplant patient at the medical center contracted LD, which was attributed to Legionella contamination of the center's potable water. After that incident was identified, the medical center began conducting routine clinical and environmental surveillance. Clinical surveillance included collection of respiratory specimens for Legionella culture during every bronchoscopy conducted at the center. Environmental surveillance included conducting routine cultures from samples of the center's potable water supply, in accordance with CDC guidelines (2). Specifically, semiannual testing was conducted in areas where oncology patients and hematopoietic stem-cell or solid-organ transplant recipients received care. Finally, to augment the routine chlorine disinfection of its water supply, a copper-silver ionization system was installed in August 2006.
The four apparent cases of LD occurred among patients who received bronchoscopy services at an endoscopy suite within the medical center. In the 12 months before the first patient's bronchoscopy on June 4, 2008, approximately 4,900 endoscopies had been performed in the suite, including 500 bronchoscopies. On July 21, 2008, as part of the clinical surveillance, the medical center's laboratory director reported to ADHS a cluster of four patients who had Legionella isolated from specimens obtained during bronchoscopies. ADHS notified CDC of the four apparent cases of LD on July 25, and the investigation began on August 1 (Figure). Investigators queried electronic laboratory records for Legionella-positive cultures from respiratory specimens collected at the medical center during January 2007--December 2008 to determine if additional unrecognized cases associated with bronchoscopies performed in the suite had occurred. No additional cases were identified. Investigators reviewed the medical records (i.e., demographic and clinical information, including microbiological testing, diagnostic imaging, and treatment) of the four patients to determine if they had clinical courses consistent with LD.
An environmental investigation also was conducted. Investigators reviewed test results from samples obtained during routine semiannual environmental surveillance from February and July to characterize the extent of Legionella contamination in the center's potable water supply. Additional environmental testing was conducted by sampling potable water to determine chlorine levels and identify sources of Legionella contamination where patient exposures might have occurred.
Clinical and Epidemiologic Investigation
The diagnosis of LD in the four patients was based on isolation of Legionella pneumophila from lavage specimens that had been collected as part of routine Legionella surveillance (Table 1). None of the patients had urine antigen or serologic testing for LD. All patients had undergone bronchoscopy using the same bronchoscope, and all received care at the medical center during May 31--July 31, 2008. None of the four patients had experienced fever or had a clinical course consistent with LD. Patients 1 and 3 had multiple organisms (methicillin-resistant Staphylococcus aureus, viridans group Streptococcus, or yeast) isolated from their lavage specimens, suggesting specimen contamination. Patient 2 had received empirical antimicrobial therapy for community-acquired pneumonia. Patients 1 and 3 had received levofloxacin specifically for LD; therapy was provided either because the patients had pulmonary abnormalities attributed to LD after Legionella was isolated or because a conservative therapeutic approach was elected because of the potential severity of LD, even if the disease was considered unlikely. The three hospitalized patients recovered from their underlying conditions. Patient 4 received outpatient services only and was not subsequently admitted to the center's health-care system nor did ADHS receive notification that he had received a diagnosis of LD at another health facility.
Environmental Investigation
Investigators believed bronchoscopy procedures were the most likely source of contamination and focused their investigation on bronchoscopy procedures and sterilization. In early July 2008, endoscopy technicians at the center began using cold saline flushes for bleeding control among patients undergoing bronchoalveolar lavage. One endoscopy technician reported using nonsterile ice to cool saline flushes in prefilled syringes. Ice for cooling was obtained from a primary ice machine in a nearby nursing station or from a back-up ice machine in a room used to prepare food and beverages. The ice was placed in a 16-ounce plastic tray. Although the tip of the prefilled saline syringe was placed directly into the ice bath, whether the tip was capped or uncapped could not be determined. Investigators identified no other source of nonsterile water used during bronchoscopies, or other pertinent breaches in infection control practices or bronchoscope sterilization or reprocessing.
To identify the specific contamination source, investigators collected biofilm swabs and 1-liter bulk water samples according to published procedures (3). During the investigation, environmental samples were taken from the bronchoscope, sink faucets in the endoscopy processing room, bays in the automatic endoscope reprocessing machine, reverse osmosis filter tubing and canisters, primary and back-up ice machines, and sink faucets and showerheads from two of the three hospitalized patients' rooms (Table 2). Legionella isolates from potable water samples that were routinely collected at the center in February and July of 2008, the clinical isolates from the four patients, and the environmental samples collected in August 2008 during the investigation were sent to CDC. At CDC, the Legionella laboratory cultured Legionella from the samples and analyzed the isolates, including serogrouping and sequence-based typing using seven gene fragments. Free chlorine concentrations in samples taken at distal locations in the medical center's potable water system also were measured to assess the amount of disinfectant present.
During the investigation, the review of sample results from routine environmental surveillance demonstrated that the potable water system had become heavily colonized with Legionella during a 6-month period, February--July 2008. Legionella had been isolated from one (1.3%) of 78 samples collected in February from sink faucets and showerheads in other patients' rooms. In contrast, Legionella (L. anisa, L. dumoffii, and L. pneumophila serogroups 1, 5, 6, and 8) was isolated from 42 of 117 (35.9%) potable water samples collected in July. No free chlorine was detected in the center's potable water supply during the investigation in August, indicating that disinfectant levels were inadequate to limit Legionella growth. L. pneumophila serogroup 8 was detected in both ice machines used by endoscopy staff (Table 2); serogroup 6 also was detected in the back-up machine. All four serogroup 8 isolates obtained from the four patients had sequence-based typing patterns that were identical to isolates from both ice machines. Before the investigation, L. pneumophila serogroup 6 also was detected in a specimen collected on July 25 by flushing sterile water through the bronchoscope that had been used for all four patients. Sequence-based typing patterns of isolates from the back-up ice machine matched the serogroup 6 bronchoscope isolate.
A series of control measures were established to prevent hospital-acquired LD, remediate contamination, and prevent subsequent Legionella colonization. High-risk patients, including hematopoietic stem-cell or solid-organ transplant recipients, were restricted from using potable water until point-of-use filters were installed on August 27. Chlorine injection into the cold water system was initiated on August 18, and the autochlorination system was reset to reach a routine, maximum disinfectant level of 1.5--2.0 ppm, which was within Environmental Protection Agency safety standards (4.0 ppm). To eliminate potential contamination from ice, the technicians began using a sterile, plastic bag to contain ice and serve as a barrier. By mid-August, endoscopy staff members were refrigerating saline bottles and had stopped using ice during bronchoscopies. In addition, the contaminated ice machines were disassembled for cleaning, including disinfection using a chlorine flush and replacement of filters. Extensive sampling performed on August 27 indicated that control and remediation efforts were effective; no L. pneumophila was detected among 115 potable water samples, and the potable water system continues to be routinely monitored semiannually by a commercially contracted Legionella specialist. No cases of LD have been detected at the medical center since the investigation.
Reported by: C Kioski, MPH, K Montefour, M Saubolle, PhD, T Johnson, MHA, J Faidley, M Williams, MSN, MBA, A Khalsa, M Rudinsky, MD, Banner Good Samaritan Medical Center, Banner Health, Phoenix; C Ogden, Office of Infectious Disease Svcs, Arizona Dept of Health Svcs. R Sunenshine, MD, Div of State and Local Readiness, Coordinating Office for Terrorism Preparedness and Emergency Response; L Hicks, DO, N Kozak, PhD, E Brown, M Buss, MS, B Fields, PhD, Div of Bacterial Diseases, National Center for Immunization and Respiratory Diseases; M Arduino, DrPH, Div of Healthcare Quality and Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases; B Silk, PhD, S Chen, PhD, EIS officers, CDC.
Editorial Note:
Water stagnation, low residual disinfection, and warm temperatures can promote Legionella colonization in large, complex potable water systems at hospitals and other facilities (4). Persons of advanced age, or who smoke, have chronic pulmonary disease, immunosuppression, malignancy, or certain other underlying conditions (e.g., end-stage renal disease or diabetes mellitus) are at increased risk for LD (2). This investigation determined that none of the four patients had LD and that the most likely cause of the pseudo-outbreak was the use of contaminated, nonsterile ice for cooling saline-filled syringes during bronchoalveolar lavage. Taken alone, certain radiographic evidence from the patients could be suggestive of LD; each had a pulmonary abnormality (e.g., infiltrate or nodule) or pneumonia diagnosis on admission that was an indication for the bronchoscopy. However, the clinical presentations and courses of illness for each patient were inconsistent with LD. None of the patients had high fever and severe illness, which are hallmarks of LD. Patient 1 did not have a definitive discharge diagnosis (i.e., acute and chronic respiratory failure), and her illness was not compatible with a diagnosis of LD. Patients 2 and 4 also had courses inconsistent with LD; patient 4 did not receive specific antimicrobial treatment for LD and subsequently was not admitted to the center's health-care system. Although patient 3 received a discharge diagnosis of legionellosis based on findings from the bronchoalveolar lavage specimen, he did not have a clinical course or radiographic findings compatible with LD. In addition, the isolation of multiple pathogens in patients 1 and 3 and scant Legionella growth in patients 3 and 4 suggest that the source of Legionella was contamination of the bronchoalveolar lavage specimens, and not infection.
Widespread Legionella colonization within the medical center's potable water system was documented between February and July of 2008, and the pseudo-outbreak was ultimately attributable to this colonization because the system supplied water to the ice machines. A copper-silver ionization system, which was installed in 2006 to prevent Legionella growth in the potable water system of the medical center, might have provided false assurances for Legionella control. The investigation did not determine whether pH or water temperatures were maintained within recommended ranges for optimum system functionality, but failures of copper-silver ionization systems have been reported elsewhere (5; personal communication, Carol Genese, New Jersey Department of Health and Senior Services, 2009). Ice machines were the only sources of L. pneumophila serogroup 6 and 8 identified that also were linked epidemiologically to bronchoscopy procedures. Sequence-based typing of Legionella isolates from the machines that supplied ice matched the patients' clinical isolates and the bronchoscope isolate. Although serogroup 8 also might have been present within the bronchoscope, drying likely prevented Legionella detection during the investigation. The bronchoscopy suite continued services during the period between the first patient's bronchoscopy on June 4 and the subsequent patients' bronchoscopies in July, but the absence of additional cases during that period remains unexplained. The gap in cases might have resulted from inconsistent supplying and use of ice from the two contaminated ice machines among the endoscopy technicians.
This is the second published report of Legionella contamination in clinical specimens associated with the use of nonsterile ice during bronchoscopies. In 2007, a similar pseudo-outbreak occurred among 13 patients whose bronchoalveolar lavage specimens were contaminated with L. pneumophila serogroup 8 by nonsterile ice for saline cooling during bronchoscopies (6). One actual case of a lower respiratory tract infection was subsequently attributed to Legionella infection, demonstrating that the use of nonsterile ice during bronchoscopies creates a risk for Legionella infection. Reports that Legionella amplification occurs between temperatures of 77°F (25°C) and 108°F (42°C) might have created the perception that ice could not support Legionella growth. Although low temperatures inhibit Legionella growth, the bacteria can remain viable in ice for extended periods (7).
If sterile ice is not available for use during bronchoscopy, precautions should be taken to ensure that nonsterile ice does not directly contact equipment or patient specimens (e.g., refrigeration of the saline bottle or use of a sterile bag containing ice as a barrier). Ice machines can be reservoirs for Legionella contamination and should be disinfected. Health-care facilities should regularly monitor and address conditions that can promote Legionella colonization of the potable water supply (e.g., inadequate levels of halogen-based disinfectants). Because of the inherent risk for infection associated with reuse of medical devices (8), health-care facilities also should adhere to guidelines on proper use, reprocessing, and high-level disinfection or sterilization of medical equipment; regularly inspect and test reusable devices; and conduct surveillance for clusters of unusual infections to ensure patient safety (9,10).
References
- Kool JL, Fiore AE, Kioski CM, et al. More than 10 years of unrecognized nosocomial transmission of Legionnaires' disease among transplant patients. Infect Control Hosp Epidemiol 1998;19:898--904.
- CDC. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR 2004;53(No. RR-3).
- CDC. Procedures for the recovery of Legionella from the environment. Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www.cdc.gov/legionella/files/legionellaprocedures-508.pdf.
- Kool JL, Bergmire-Sweat D, Butler JC, et al. Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial legionnaires' disease: a cohort study of 15 hospitals. Infect Control Hosp Epidemiol 1999;20:798--805.
- Graman PS, Fine L, Hardy D. Nosocomial legionellosis and the emergence of Legionella pneumophila (Lp) in a water system during treatment with copper-silver ionization. Abstract K-4110. In: 48th Annual ICAAC/IDSA 46th Annual Meeting abstracts book; October 25--28, 2008; Washington, DC. Washington, DC: American Society for Microbiology, 2008: 573. Available at http://www.icaacidsa2008.org/documents/icaacidsaabstracts2008.pdf.
- Schuetz AN, Hughes RL, Howard RM, et al. Pseudo-outbreak of Legionella pneumophila serogroup 8 infection associated with a contaminated ice machine in a bronchoscopy suite. Infect Control Hosp Epidemiol 2009;30:461--6.
- Paszko-Kolva C, Shahamat M, Colwell RR. Effect of temperature on survival of Legionella pneumophila in the aquatic environment. Microb Releases 1993;2:73--9.
- Weber DJ, Rutala WA. Lessons from outbreaks associated with bronchoscopy. Infect Control Hosp Epidemiol 2001;22:403--8.
- Rutala WA, Weber DJ, HICPAC. Guideline for disinfection and sterilization in healthcare facilities, 2008. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/disinfection_nov_2008.pdf.
- CDC. Guidelines for environmental infection control in health care facilities. MMWR 2003;52(No. RR-10).
FIGURE. Timeline of events preceding and during an investigation of a pseudo-outbreak of Legionnaires disease (LD) among patients undergoing bronchoscopy at a medical center --- Arizona, 2008
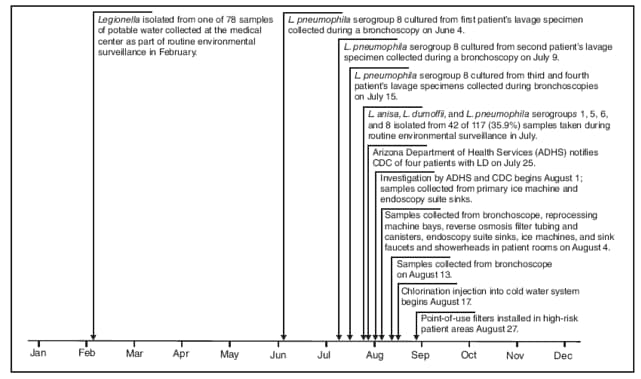
Alternative Text: The figure above shows the timeline of events preceding and during an investigation of a pseudo-outbreak of Legionnaires disease among four patients undergoing bronchoscopy at an Arizona medical center during 2008.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/13/2009


