 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Simian Malaria in a U.S. Traveler --- New York, 2008Four species of intraerythrocytic protozoa of the genus Plasmodium (P. falciparum, P. vivax, P. ovale, and P. malariae) are known to cause malaria in humans. However, recent reports from Asia suggest the possibility that a fifth malaria species, Plasmodium knowlesi, is emerging as an important zoonotic human pathogen. Although more than 20 species of Plasmodium can infect nonhuman primates, until recently, naturally acquired human infections of simian malaria were viewed as rare events lacking public health significance. When viewed by light microscopy (the gold standard for laboratory diagnosis of malaria), many of the simian species are almost indistinguishable from the four Plasmodium species that cause infection in humans (Table). Molecular techniques, such as polymerase chain reaction (PCR) amplification and microsatellite analysis, are needed for definitive species determination. This report describes the first recognized case of imported simian malaria in several decades in the United States, diagnosed in 2008 in a patient from New York who had traveled to the Philippines. Atypical features of the parasite seen on light microscopy triggered further molecular testing, which confirmed the diagnosis of P. knowlesi. To date, all simian malaria species have been susceptible to chloroquine treatment. Molecular analysis of certain malaria parasites isolated from ill travelers returning to the United States from Asia or South America can more accurately assess the burden of simian malaria parasite infections in humans. The first recognized case of naturally acquired simian malaria was a 1965 case of P. knowlesi infection in an employee of the U.S. Army who had returned home from an assignment in Southeast Asia (1); subsequent reports were few and unconfirmed. In 2002, investigators in Malaysia noted an increasing number of P. malariae cases with atypical features, including increased clinical severity and higher parasitemia (2). By using a nested PCR assay, more than 50% of these malaria cases were determined to be P. knowlesi; none were P. malariae, as originally determined by microscopy (2). In a retrospective evaluation by the same investigators during 2001--2006, 28% of 960 specimens from patients in Sarawak, Malaysian Borneo, were found to be P. knowlesi, after being morphologically diagnosed most often as P. malariae (3). The group also reported four unusual fatalities attributed to severe malaria caused by P. malariae that was later confirmed as P. knowlesi by PCR. Additional cases of naturally occurring P. knowlesi infection in humans have been reported from Singapore (4), the Thai-Burma border (5), the Philippines (6), Yunnan Province in China (7), and Finland, where a returning traveler from Malaysia was misdiagnosed initially as having infection with P. falciparum (8). Case ReportIn the recent U.S. case, a woman aged 50 years with no previous history of malaria who was born in the Philippines but had lived in the United States for 25 years, returned to her home country to visit friends and relatives on October 17, 2008. While there, she stayed on the island of Palawan in a cabin located at the edge of a forested area known to be a habitat for long-tailed macaques. She had not taken malaria chemoprophylaxis and had not used any mosquito-avoidance measures, both of which are recommended preventive measures for travelers to this area. The woman returned to the United States on October 30, 2008, and noted the onset of a headache. Fever and chills ensued, and symptoms persisted for several days, after which she sought medical attention. In the emergency department, she was noted to be hypotensive and to have thrombocytopenia. Examination of thick and thin malaria smears (Figure 1) was ordered, and an initial, erroneous diagnosis of babesiosis was made by a laboratory technician. Upon review by the laboratory supervisor the following morning, the diagnosis was reassessed as malaria with 2.9% of red cells parasitized. However, the atypical appearance of the Plasmodium sp. seen in the smears prevented a species-specific diagnosis. The woman was treated successfully with atovaquone-proguanil and primaquine for Plasmodium of undetermined species. An ethylenediaminetetraacetic acid (EDTA) blood tube and two stained smears were sent to New York state's Wadsworth Center Parasitology Reference Laboratory for confirmation of malaria and molecular determination of species by PCR. The Wadsworth Center confirmed the presence of atypical rings and schizonts of a Plasmodium species (Figure 1), but conventional PCR targeting the small subunit (SSU) of rRNA did not yield a product consistent with any of the four species of Plasmodium known to infect humans. The specimen also was negative for the variants of P. ovale, which are commonly seen in Southeast Asia. However, primers specific for the SSU rDNA of the genus Plasmodium yielded a 1,055-bp PCR product that was sequenced and noted to be a 99% match over its full length to the SSU rRNA gene from P. knowlesi (H strain) (9). These data confirmed that the infection was caused by P. knowlesi. Reported by: JG Ennis, AE Teal, A Habura, PhD, S Madison-Antenucci, PhD, JS Keithly, PhD, Div of Infectious Diseases, New York State Dept of Health. PM Arguin, MD, JW Barnwell, PhD, WE Collins, PhD, S Mali, MPH, L Slutsker, MD, A Dasilva, DSc, Div of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; J Hwang, MD, EIS Officer, CDC. Editorial Note:Several conditions need to coincide for simian species of Plasmodium to infect humans: 1) human erythrocytes must be susceptible to invasion by simian parasites, 2) humans must be near or in forests where nonhuman simians are infected, and 3) anopheline mosquitoes that feed on both humans and nonhuman simians must be present (10). Many areas in Asia and South America have overlapping populations of nonhuman primates that serve as reservoirs for simian malaria and competent Anopheles mosquito vectors that are necessary to transmit the infection to humans (Table, Figure 2) (1). For P. knowlesi in Asia, the normal hosts are long-tailed and pig-tailed macaques and mitered-leaf monkeys, which are found with Anopheles mosquito vectors of the Leucosphyrus group, enabling transmission of infection (1). Other simian malaria species known to infect humans include P. simium and P. brasilianum in South America and P. cynomolgi and P. inui in Asia (1,10). Most simian malaria infections in humans can cause mild or moderate disease but often are self-limited, not requiring antimalarial therapy (1). However, P. knowlesi, with its 24-hour asexual replication cycle, can result in large parasite burden and severe, life-threatening disease (3). Severe malaria imported from Asia should alert the physician to the possibility of infection with P. knowlesi. Health-care providers also should consider hospitalization if the patient with malaria reports travel to forested areas of Asia, where P. knowlesi transmission occurs. Simian Plasmodium species are susceptible to all available antimalarials in the United States. Although definitive diagnosis as a simian species of Plasmodium cannot be made in time to guide selection of antimalarials at the initiation of therapy, treatment for undetermined Plasmodium species will effectively treat all simian species. Use of current treatment and chemoprophylaxis guidelines are appropriate for treating and preventing simian malaria infections in humans. Health-care providers of patients with malaria and laboratories that diagnose malaria imported from Asia or non-falciparum malaria from South America should refer appropriate specimens to a Clinical Laboratory Improvement Amendments (CLIA)- verified state health reference laboratory or CDC's Division of Parasitic Diseases Reference Laboratory for species confirmation by molecular testing. In the United States, approximately 1,500 malaria cases are reported each year, almost all imported from areas where malaria is endemic; approximately 200 of these cases are imported from Asia or South America. In the United States, the potential for not recognizing a Plasmodium infection of simian origin is high because diagnosis usually relies on microscopic examination of Giemsa-stained smears rather than diagnosis by molecular techniques. Only a few laboratories (including state and federal public health reference and commercial laboratories) routinely use molecular assays, and even fewer have the capacity to confirm simian species. The substantial number of recent human cases of simian malaria reported in Malaysia and the wider region (including the travel-associated case described in this report) underscores the need to define the scope and magnitude of the problem (2--8). Persons wishing to send specimens for species confirmation by CDC should collect pretreatment blood in EDTA or acid citrate dextrose blood collection tubes. Instructions and specimen submission forms are available online at http://www.cdc.gov/malaria/smscs.htm. Contact information for local or state health department laboratories is available at http://www.aphl.org/aboutaphl/aboutphls/pages/memberlabs.aspx. As with all suspected cases of malaria, health-care providers with questions regarding diagnosis or treatment should call the CDC Malaria Hotline at 770-488-7788 (Monday--Friday, 8:30 a.m. to 4:30 p.m., EST). Health-care providers seeking emergency consultation after hours should call 770-488-7100 and request to speak with a CDC Malaria Branch clinician. References
Figure 1 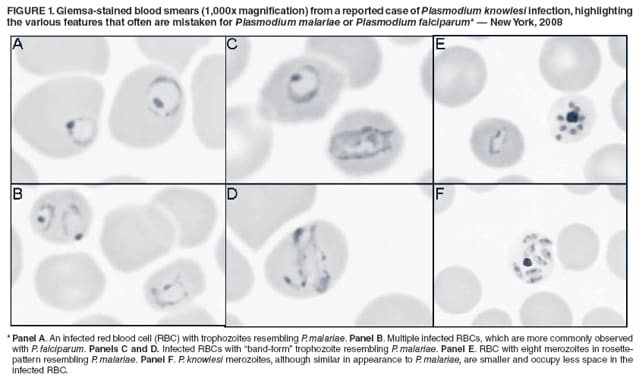 Return to top. Figure 2 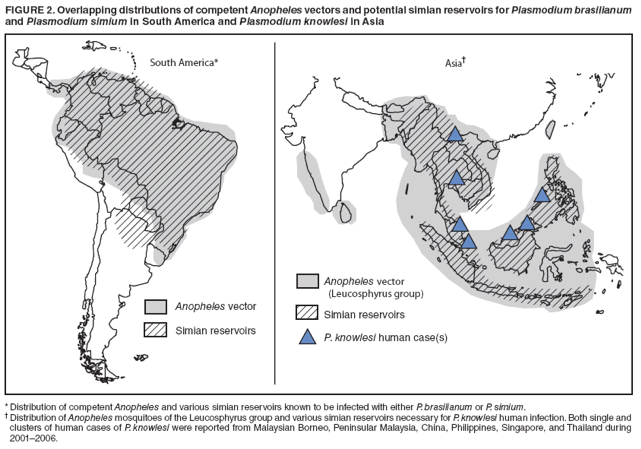 Return to top. Table  Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/12/2009 |
|||||||||
|