 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pneumonia Hospitalizations Among Young Children Before and After Introduction of Pneumococcal Conjugate Vaccine --- United States, 1997--2006Streptococcus pneumoniae is the leading bacterial cause of community-acquired pneumonia hospitalizations and an important cause of bacteremia and meningitis, especially among young children and older adults (1,2). A 7-valent pneumococcal conjugate vaccine (PCV7) was licensed and the Advisory Committee on Immunization Practices formulated recommendations for its use in infants and children in February 2000 (2). Vaccination coverage rapidly increased during the second half of 2000, in part through funding by CDC's Vaccines for Children program. Subsequently, active population- and laboratory-based surveillance demonstrated substantial reductions in invasive pneumococcal disease (IPD) among children and adults (3). In addition, decreases in hospitalizations and ambulatory-care visits for all-cause pneumonia also were reported (4,5). To gauge whether the effects of PCV7 on reducing pneumonia continue, CDC is monitoring pneumonia hospitalizations by using data from the Nationwide Inpatient Sample. This report provides an update for 2005 and 2006, the most recent years for which information is available. In 2005 and 2006, the incidence rates for all-cause pneumonia hospitalizations among children aged <2 years were 9.1 per 1,000 and 8.1 per 1,000, respectively. In 2006, the rate for all-cause pneumonia among children aged <2 years was approximately 35% lower than during 1997--1999. Most of this decrease occurred soon after the vaccine was licensed in 2000, and the rates have remained relatively stable since then. The rate for all-cause pneumonia among children aged 2--4 years did not change after PCV7 licensure and has remained stable. Continued monitoring of pneumonia-related hospitalizations among children is needed to track the effects of pneumococcal immunization programs. The Nationwide Inpatient Sample contains data on inpatient stays from states that participate in the Healthcare Cost and Utilization Project, sponsored by the Agency for Healthcare Research and Quality. The project is a stratified probability sample of U.S. acute-care hospitals and the largest all-payer inpatient-care database available in the United States. In 2006, this database recorded information from approximately 8 million hospitalizations (approximately 20% of all U.S. hospitalizations) from 1,045 hospitals in 38 states. Data are weighted to generate national estimates while accounting for complex sampling design (6). For this analysis, all-cause pneumonia hospitalization was defined as a record in which International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 480--486 (pneumonia) or 487.0 (influenza with pneumonia) were assigned as the primary diagnosis. Trends in hospitalizations for nonpneumonia acute respiratory illness (ARI) also were evaluated to assess the possibility that, after PCV7 introduction, practitioners were less likely to assign a pneumonia code for respiratory conditions in a vaccinated child and more likely to make other respiratory diagnoses. A nonpneumonia ARI hospitalization was defined as a record with any of the following ICD-9-CM codes assigned as the primary diagnosis: 381--383 (otitis media and mastoiditis), 460--466 (acute respiratory infections, including acute bronchitis, bronchiolitis, acute nasopharyngitis, sinusitis, pharyngitis, tonsillitis, laryngitis, tracheitis, and other acute upper respiratory infections), 487 (influenza, excluding 487.0), 490 (bronchitis), 491 (chronic bronchitis), or 493 (asthma). Some of these diagnoses, such as asthma, bronchiolitis, or acute bronchitis generally are not considered to be caused by S. pneumoniae. Hospitalization rates among children aged <2 years and 2--4 years were calculated by dividing the total number of yearly hospitalizations by age-specific population denominators from U.S. census data. Baseline rates before introduction of PCV7 were defined as the average annualized rates during 1997--1999; incidence rate ratios (RRs) were calculated by dividing estimated rates for 2006 by the baseline rates. Point estimates and 95% confidence intervals (CIs) were calculated using outcome-specific Poisson regression models that accounted for the Nationwide Inpatient Sample sampling design. Rate differences between baseline and 2006 rates were multiplied by age-specific census data to estimate changes in the absolute number of hospitalizations during 2006. To examine changes in the distribution of causes of hospitalization after introduction of PCV7, the proportion of all nonbirth-related hospitalizations that were coded as pneumonia and nonpneumonia ARI among children aged <2 years during 1997--1999 and 2006 were calculated. In 2005, a total of 74,559 children aged <2 years were hospitalized in the United States for all-cause pneumonia, and 67,430 were hospitalized in 2006, accounting for approximately 8% of yearly nonbirth-related hospitalizations in this age group. The rates of all-cause pneumonia hospitalization per 1,000 children aged <2 years were 9.1 in 2005 and 8.1 in 2006. Although the rate of all-cause pneumonia in 2005 was higher than in 2004 (8.0), this increase was not statistically significant. The 2005 and 2006 rates were 27% and 35% lower than the baseline rate of 12.5 per 1,000 (Table). For 2006, the rate reduction represented an estimated 36,300 fewer pneumonia hospitalizations among children aged <2 years during 2006, compared with the average annual number of hospitalizations during 1997--1999. Among children aged 2--4 years, the rate of all-cause pneumonia hospitalization did not change significantly during the study years (Table, Figure). Among children aged <2 years, the rate of nonpneumonia ARI hospitalizations was 24.6 per 1,000 in 2005 and 21.9 per 1,000 in 2006. The rate in 2006 represented a significant decline from the rate of 28.1 during the baseline period (RR = 0.8). For 2006, this rate reduction represented an estimated 51,500 fewer nonpneumonia ARI hospitalizations among children aged <2 years during 2006 compared with the average annual number of hospitalizations during 1997--1999. Among children aged 2--4 years, the rate of nonpneumonia ARI hospitalizations was 6.5 per 1,000 in 2005 and 5.6 per 1,000 in 2006. The 2006 rate was not significantly different compared with the baseline period (RR = 1.0). Annual rates for all nonbirth-related hospitalizations among children aged <2 years were 120 per 1,000 children in 2005 and 100 per 1,000 children in 2006, compared with 117 per 1,000 children during the baseline period. The proportion of total annual nonbirth-related hospitalizations coded as pneumonia was 8% in 2006, compared with 11% during the baseline period (p<0.001). The proportion of such hospitalizations coded as nonpneumonia ARI was 22% in 2006, compared with 24% during the baseline period (p=0.005). Reported by: CG Grijalva, MD, MR Griffin, MD, Vanderbilt Univ, Nashville, Tennessee. JP Nuorti, MD, Respiratory Diseases Br, National Center for Immunization and Respiratory Diseases; ND Walter, MD, EIS Officer, CDC. Editorial Note:The results of this analysis cannot, by themselves, establish a causal relationship between the advent of PCV7 and trends in childhood pneumonia hospitalizations. However, the updated analysis of national hospital discharge data suggests that reductions in all-cause pneumonia hospitalizations among U.S. children aged <2 years after routine PCV7 use have been sustained and that the benefits of PCV7 might extend beyond the documented changes in IPD (3) to hospitalizations for pneumonia. Moreover, rates of nonpneumonia ARI also declined after introduction of PCV7, indicating that the decreases in pneumonia hospitalizations likely were not the result of a shift in coding of respiratory hospitalizations to nonpneumonia ARI codes. In addition, the analysis suggests that the declines were unlikely to result from a reduction in total hospitalization rates. The transient increase in all-cause pneumonia rates from 2004 to 2005 might reflect increased circulation of respiratory viruses or other seasonal variation. Although many nonpneumonia ARI diagnoses traditionally have not been considered manifestations of S. pneumoniae infection, recent data indicate that the pneumococcus might contribute to a wider range of childhood respiratory illness than previously thought. A randomized clinical trial performed in child care centers in Israel suggested that immunization with a 9-valent pneumococcal conjugate vaccine reduced reported episodes of upper respiratory infections, lower respiratory infections, and otitis media by 15%, 16%, and 17%, respectively (7). Furthermore, in a trial of 9-valent pneumococcal conjugate vaccine among South African children, vaccinated children had 45% fewer influenza A--associated pneumonia episodes than unvaccinated children, suggesting that S. pneumoniae might be a copathogen in illnesses diagnosed as influenza (8). Although rates of IPD have decreased substantially among children aged 2--4 years after PCV7 introduction (3), a reduction in all-cause pneumonia hospitalizations was not observed in this age group. The reasons for this are unknown but might be associated with lower overall rates of pneumococcal infection in this age group. In addition, other etiologic agents are becoming more common causes of pneumonia in children aged >2 years (1). The findings in this report are subject to at least three limitations. First, identification of hospitalizations for pneumonia and nonpneumonia ARI was based on ICD-9-CM codes and might be subject to misclassification, despite internal quality control and validation for consistency within the Nationwide Inpatient Sample. Second, establishing the etiology of pneumonia is difficult. Nationwide Inpatient Sample data are deidentified before public release and chart reviews cannot be performed to confirm recorded diagnoses. Because most pneumococcal pneumonias are classified as pneumonias without further characterization, this report provides an estimate of the effect of PCV7 on all-cause pneumonia without regard to pneumococcal serotypes. Furthermore, serotyping is not part of routine diagnostic work-ups, and this information would not be recorded in medical charts. However, the decrease in nonpneumonia ARI hospitalizations among children aged <2 years suggests that the decreases in pneumonia hospitalizations were unlikely to result from a shift in coding of pneumonia to nonpneumonia ARI codes. Finally, factors other than shifts in coding could affect hospitalization rates. Reduced clinician concerns for severe pneumococcal disease among immunized children, for example, might lead to outpatient treatment rather than hospitalization. However, other data indicate that ambulatory-care visits for pneumonia among children aged <2 years also have decreased since introduction of PCV7 (5). In addition, the proportion of all hospitalizations that were attributable to pneumonia or nonpneumonia ARI decreased significantly, suggesting that the declines were unlikely to result from a secular reduction in overall hospitalization rate. Despite the substantial morbidity associated with childhood pneumonia, no pneumonia-specific prospective population-based surveillance system exists for monitoring trends in the incidence of pneumonia hospitalizations or pneumonia-related ambulatory-care visits in the United States. Monitoring childhood pneumonia is important for the evaluation of effects of current and future pneumococcal immunization programs. Increases in pneumococcal disease caused by serotypes not included in PCV7 could result in some increase in pneumonia, even though observed increases in non-PCV7 serotype IPD have been modest thus far (9). In addition, extended-valency pneumococcal conjugate vaccines are expected to be licensed by late 2009 to early 2010 and might further reduce pneumonia rates. Finally, vaccination of children against influenza, as recommended by the Advisory Committee on Immunization Practices, is increasing and also might reduce pneumonia hospitalization rates (10). References
Table 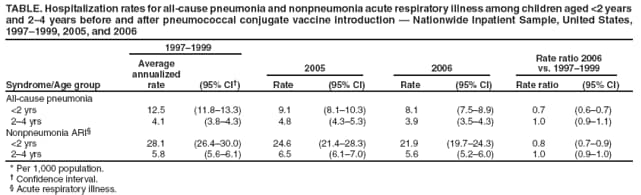 Return to top. Figure 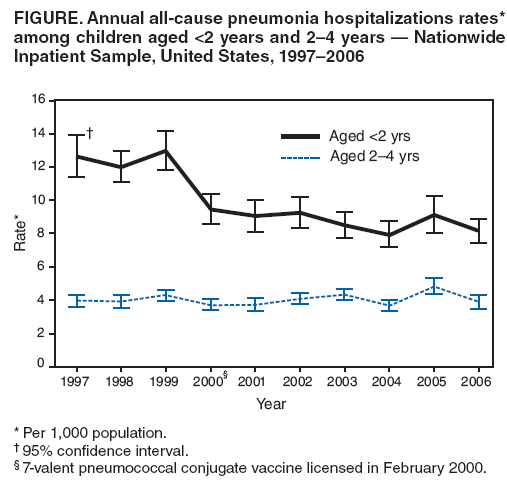 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 1/14/2009 |
|||||||||
|