 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Measles --- United States, January 1--April 25, 2008Measles, a highly contagious acute viral disease, can result in serious complications and death. As a result of a successful U.S. vaccination program, measles elimination (i.e., interruption of endemic measles transmission) was declared in the United States in 2000 (1). The number of reported measles cases has declined from 763,094 in 1958 to fewer than 150 cases reported per year since 1997 (1). During 2000--2007,* a total of 29--116 measles cases (mean: 62, median: 56) were reported annually. However, during January 1--April 25, 2008, a total of 64 confirmed measles cases were preliminarily reported to CDC, the most reported by this date for any year since 2001. Of the 64 cases, 54 were associated with importation of measles from other countries into the United States, and 63 of the 64 patients were unvaccinated or had unknown or undocumented vaccination status. This report describes the 64 cases and provides guidance for preventing measles transmission and controlling outbreaks through vaccination, infection control, and rapid public health response. Because these cases resulted from importations and occurred almost exclusively in unvaccinated persons, the findings underscore the ongoing risk for measles among unvaccinated persons and the importance of maintaining high levels of vaccination. Measles cases in the United States are reported by state health departments preliminarily to CDC, and confirmed cases are reported officially via the National Notifiable Disease Surveillance System, using standard case definitions† and case classifications. Cases are considered importation associated if they are 1) acquired outside the United States (i.e., international importation) or 2) acquired inside the United States and either epidemiologically linked via a chain of transmission to an importation or accompanied by virologic evidence of importation (i.e., a chain of transmission from which a measles virus is identified that is not endemic in the United States). Other cases in the United States are classified as having an unknown source. During January 1--April 25, 2008, a total of 64 preliminary confirmed measles cases were reported from the following areas: New York City (22 cases), Arizona (15), California (12), Michigan and Wisconsin (four each), Hawaii (three), and Illinois, New York state, Pennsylvania, and Virginia (one each) (Figure). Patients ranged in age from 5 months to 71 years; 14 patients were aged <12 months, 18 were aged 1--4 years, 11 were aged 5--19 years, 18 were aged 20--49 years, and three were aged >50 years, including one U.S. resident born before 1957.§ Fourteen (22%) patients were hospitalized; no deaths were reported. Transmission occurred in both health-care and community settings. One of the 44 patients for whom transmission setting was known was an unvaccinated health-care worker who was infected in a hospital. Seventeen (39%) were infected while visiting a health-care facility, including a child aged 12 months who was exposed in a physician's office when receiving a routine dose of measles, mumps, and rubella (MMR) vaccine. Fifty-four (84%) of the 64 measles cases were importation associated: 10 (16%) of the 64 were importations (five in visitors to the United States and five in U.S. residents traveling abroad) from Switzerland (three), Israel (three), Belgium (two), and India and Italy (one each); 29 (45%) cases were epidemiologically linked to importations; and 15 (23%) cases had virologic evidence of importation. The remaining 10 (16%) cases were from unknown sources; however, all occurred in communities with importation-associated cases. Specimens from 14 patients were genotyped at CDC, and four different genotypes were identified: three from Arizona (genotype D5), three from California (D5), five from New York City (one in a case epidemiologically linked to an imported case from Belgium and four in cases in communities where importations from Israel had occurred; all D4), two from Wisconsin (H1), and one from Michigan (D5). Fifty-six of the 64 measles cases reported in 2008 have occurred in five outbreaks (defined as three or more cases linked in time or place). In New York City, an outbreak of 22 cases has been reported, including four importations and 18 other cases (10 importation associated). In Arizona, 15 cases have been reported; the index patient was an unvaccinated adult visitor from Switzerland. In San Diego, California, 11 cases have been reported, and an additional case spread to Hawaii; the index patient in the San Diego outbreak was an unvaccinated child who had traveled to Switzerland. In Michigan, four cases have been reported; the index patient was an unvaccinated youth aged 13 years with an unknown source of infection. In Wisconsin, four cases have been reported; the index patient was a person aged 37 years with unknown vaccination status who likely was exposed to a Chinese visitor with measles-compatible illness. Sixty-three of the 64 patients were unvaccinated or had unknown or undocumented¶ vaccination status, and one patient had documentation of receiving 2 doses of MMR vaccine. None of the five patients who were visitors to the United States had been vaccinated. Among the 59 patients who were U.S. residents, 13 were aged <12 months and too young to be vaccinated routinely, seven were children aged 12--15 months and had not yet received vaccination, 21 were children aged 16 months--19 years, including 14 (67%) who claimed exemptions because of religious or personal beliefs (Table). Among the 18 patients aged >20 years, 14 had unknown or undocumented vaccination status, two had claimed exemptions and acquired measles in Europe, one had evidence of immunity because of birth before 1957, and one had documentation of receiving 2 doses of MMR vaccine. Of the five U.S. residents with measles who were vaccine eligible and had traveled abroad, all were unvaccinated. One was a child aged 15 months who was not vaccinated before travel, and two were adults who were unvaccinated because of personal belief exemptions. For two adults, the reason for not being vaccinated was unknown. Reported by: SB Redd, PK Kutty, MD, AA Parker, MSN, MPH, CW LeBaron, MD, AE Barskey, MPH, JF Seward, MBBS, JS Rota, PA Rota, PhD, L Lowe, PhD, WJ Bellini, PhD, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:Although ongoing measles transmission was declared eliminated in the United States in 2000 (1) and in the World Health Organization (WHO) Region of the Americas in 2002 (2), approximately 20 million cases of measles occur each year worldwide. The 2008 upsurge in measles cases serves as a reminder that measles is still imported into the United States and can result in outbreaks unless population immunity remains high through vaccination. Among the 64 confirmed measles cases, prior vaccination could be documented for only one person. Before introduction of measles vaccination in 1963, approximately 3 to 4 million persons had measles annually in the United States; approximately 400--500 died, 48,000 were hospitalized, and 1,000 developed chronic disability from measles encephalitis (1). Even after elimination of endemic transmission in 2000, imported measles has continued to create a substantial U.S. public health burden; of the 501 measles cases reported during 2000--2007, one in four patients was hospitalized, and one in 250 died (1). Thus far in 2008, five U.S. residents and five visitors have been documented as acquiring measles abroad. Of these 10 persons, nine acquired measles in the WHO European Region. These importations likely are related to an increase in 2008 in measles activity in Europe. In Switzerland, approximately 2,250 measles cases have been reported since November 2006. The Swiss measles outbreak started in Lucerne, where the measles vaccination coverage level in children is 78%, and spread across the country, predominantly affecting children aged 5--15 years who were unvaccinated because of parental opposition to vaccination.** In Israel (which is included in the WHO European Region), a measles outbreak with approximately 1,000 cases is ongoing (Ministry of Health, Israel, unpublished data, 2008), and measles transmission is occurring in other European countries, predominantly among populations opposed to vaccination. This situation prompted travel advisories to be issued in the United States and Europe.†† Health-care providers should advise patients who travel abroad of the importance of measles vaccination and should consider the diagnosis of measles in persons with clinically compatible illness who have traveled abroad recently or have had contact with travelers. The limited size of recent measles outbreaks in the United States has resulted from highly effective measles and MMR vaccines, preexisting high vaccination coverage levels in preschool and school-aged children, and a rapid and effective public health response. All children should receive 2 doses of MMR vaccine, with the first dose recommended at age 12--15 months and the second dose at age 4--6 years. Unless they have other documented evidence of measles immunity,§§ all adults should receive at least 1 dose. Two doses are recommended for international travelers aged >12 months, health-care personnel, and students at secondary and postsecondary educational facilities. Infants aged 6--11 months should receive 1 dose before travel abroad (3). During a measles outbreak, the vaccination response should be guided by the epidemiology of the outbreak and the outbreak setting and might include offering 1 dose of measles or MMR vaccine to infants aged 6--11 months, offering the second dose to preschool-aged children provided that 28 days have elapsed since the first dose, and recommending 1 dose to health-care workers born before 1957 unless they show other evidence of immunity. Patients with measles frequently seek medical care, and emergency departments are common sites of measles transmission (4). To prevent transmission of measles in health-care settings, patients should be asked to wear a surgical mask (if tolerated) for source containment, airborne infection-control precautions (5) should be followed stringently, and patients should be placed in a negative air-pressure room as soon as possible. If a negative air-pressure room is not available, the patient should be placed in a room with the door closed. Measles cases should be investigated, patients isolated promptly, and specimens obtained for laboratory confirmation and viral genoptying. Case contacts without documented evidence of measles immunity should be vaccinated, offered immune globulin, or asked to quarantine themselves at home from the fifth day after their first exposure to the twenty-first day after their last exposure. Contacts with measles-compatible symptoms should be managed in a manner that will prevent further spread (3,5). Health-care personnel place themselves and their patients at risk if they are not protected against measles. In accordance with current recommendations, health-care personnel should have documented evidence of measles immunity¶¶ readily available at their work location (3). If this documentation is not available when measles is introduced, major costs and disruptions to health-care operations can result from the need to exclude potentially infected staff members and rapidly ensure immunity for others (6). Many of the measles cases in children in 2008 have occurred among children whose parents claimed exemption from vaccination because of religious or personal beliefs and in infants too young to be vaccinated. Forty-eight states currently allow religious exemptions to school vaccination requirements, and 21 states allow exemptions based on personal beliefs.*** During 2002 and 2003, nonmedical exemption rates were higher in states that easily granted exemptions than states with medium or difficult exemption processes (7); in such states, the process of claiming a nonmedical exemption might require less effort than fulfilling vaccination requirements (8). Although national vaccination levels are high,††† unvaccinated children tend to be clustered geographically or socially, increasing their risk for outbreaks (6,9). An upward trend in the mean proportion of school children who were not vaccinated because of personal belief exemptions was observed from 1991 to 2004 (7). Increases in the proportion of persons declining vaccination for themselves or their children might lead to large-scale outbreaks in the United States, such as those that have occurred in other countries (e.g., United Kingdom and Netherlands) (10). Ongoing measles virus transmission has been eliminated in the United States, but the risk for imported disease and outbreaks remains. High vaccination coverage in the United States has limited the spread of imported measles in 2008. Nevertheless, the measles outbreaks in 2008 illustrate the risk created by importation of disease into clusters of persons with low vaccination rates, both for the unvaccinated and those who come into contact with them. References
* Based on MMWR surveillance summaries (2000--2006) and CDC unpublished provisional data as of December 31, 2007. † Measles clinical case definition: an illness characterized by a generalized maculopapular rash, a temperature of >101°F (>38.3°C) and cough, coryza, or conjunctivitis. A case is considered confirmed if it is laboratory confirmed (using serologic or virologic methods) or if it meets the clinical case definition and is epidemiologically linked to a confirmed case. § The other two cases in persons aged >50 years occurred in a U.S. resident aged 50 years and a visitor from Switzerland aged 71 years. ¶ Two adults in the Arizona outbreak reported receipt of 1 and 2 vaccine doses, respectively, but lacked documentation. ** World Health Organization. Measles and rubella surveillance bulletin. Geneva, Switzerland: World Health Organization; 2008. Available at http://www.euro.who.int/vaccine/publications/20080401_1. †† U.S. travel advisories available at http://wwwn.cdc.gov/travel/contentmeasles.aspx. European travel advisories available at http://ecdc.europa.eu/health_topics/measles/080423_travel_advice.html. §§ Laboratory evidence of immunity, documentation of physician-diagnosed measles, or birth before 1957. ¶¶ Documented receipt of 2 doses of live measles virus vaccine, laboratory evidence of immunity, documentation of physician-diagnosed measles, or birth before 1957. *** Institute for Vaccine Safety. Vaccine exemptions. Baltimore, MD: Johns Hopkins Bloomberg School Public Health; 2007. Available at http://www.vaccinesafety.edu/cc-exem.htm. ††† CDC. Statistics and surveillance: immunization coverage in the U.S. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm. Table 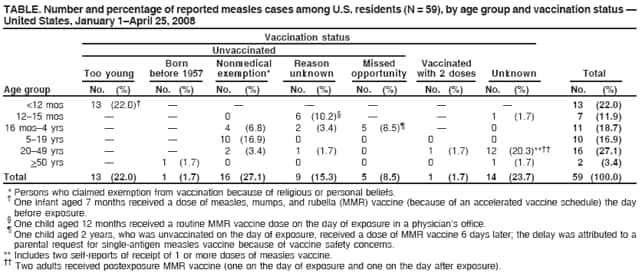 Return to top. Figure 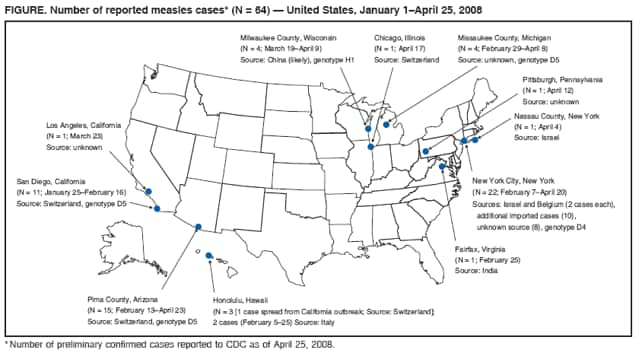 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/1/2008 |
|||||||||
|