 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommendations from an Ad Hoc Meeting of the WHO Measles and Rubella Laboratory Network (LabNet) on Use of Alternative Diagnostic Samples for Measles and Rubella SurveillanceLaboratory confirmation of measles and rubella is an important component of disease surveillance in all settings. Because the use of clinical diagnosis for surveillance is unreliable, case-based laboratory confirmation of disease is critically important in settings with measles or rubella elimination goals. The World Health Organization (WHO) Measles and Rubella Laboratory Network (LabNet) was established in 2000 to provide a standardized testing and reporting structure and a comprehensive, external quality-assurance program (1). LabNet currently consists of 679 laboratories serving 166 countries. However, measles and rubella surveillance remains incomplete in certain areas because of difficulties with the collection and transport of serum specimens. Recently, LabNet evaluated two alternative sampling approaches to serum samples, the use of dried blood spots (DBS) and oral fluid (OF) samples. Both of these approaches have potential to be useful tools for measles and rubella control programs. In June 2007, WHO convened an ad hoc meeting in Geneva, Switzerland, to review available data and provide recommendations on use of DBS and OF samples for measles and rubella diagnostics. Attendees included LabNet staff members and scientists who had been conducting studies to evaluate use of these alternative diagnostic samples. The attendees concluded that 1) although serum-based diagnostics remain the "gold standard," the use of these two alternative sampling techniques would not adversely affect routine measles and rubella surveillance and might enhance surveillance; 2) regions in the elimination phase* that already have established serum-based testing for rash illness surveillance would not likely benefit from converting to DBS or OF sampling methods, except in special circumstances; and 3) DBS or OF sampling are viable options for measles and rubella surveillance in all regions, especially where patients might resist venipuncture for blood collection, or where special challenges exist with transport or refrigeration of diagnostic samples. Background on Use of Alternative Diagnostic SamplesConventional laboratory confirmation of suspected cases of measles and rubella is based on the detection of virus-specific immunoglobulin M (IgM) in a single serum sample collected soon after the onset of symptoms (2). In addition, detection of viral RNA by reverse transcription--polymerase chain reaction (RT-PCR), usually in a throat swab or urine sample, and subsequent genotyping of strains is valuable for diagnosis and molecular epidemiology (2). Accurate laboratory results for detection of IgM and viral RNA are dependent on proper collection, processing, shipment, and storage of clinical samples and use of accurate tests performed by a proficient laboratory. However, collection of blood samples by venipuncture, particularly from children, can be a challenge, and the sustained refrigeration required for diagnostic samples during transport is not always achievable. In these situations, alternatives to serum collection can be useful. DBS has been used for various epidemiologic studies for the detection of measles- and rubella-specific IgG and IgM antibodies and viral RNA (3--5). Antibody and viral RNA are sufficiently stable on DBS at <98.6°F (<37.0°C) to allow this sample collection method to be used for case confirmation or molecular epidemiology in areas where sample refrigeration is not feasible. OF has been used in similar studies and for the national measles, mumps, and rubella (MMR) surveillance program in the United Kingdom (UK) for approximately 10 years (6,7). OF is easy to collect, and collection is more acceptable to the population (6), thereby enabling health-care workers to obtain more complete sampling for suspected cases. Evaluations Comparing Alternative Diagnostic Samples with Serum-Based DiagnosticsSince 2001, LabNet reference laboratories in Australia, Cote d'Ivoire, Netherlands, Turkey, Uganda, the UK, and the United States have been working to 1) determine IgM and RNA stability in DBS and OF samples and 2) optimize the methods for IgM antibody assay and protocols for RNA detection in DBS and OF samples (8--10). This work has provided data on sensitivity and specificity of OF and DBS samples compared with serum and also has identified logistic challenges in implementing alternative sampling techniques. Three different types of data were available for review during the ad hoc meeting. First, beginning in 2001, LabNet laboratories conducted studies that collected OF, DBS, and corresponding serum samples from persons with suspected measles or rubella during outbreaks and tested the samples for the presence of measles- or rubella-specific IgM antibodies. Second, LabNet reviewed data from the MMR surveillance program in the UK, where 1,000--3,000 OF samples have been collected annually during the past decade. Third, LabNet reviewed data from seven countries in the WHO African Region that used DBS sampling methods for routine measles and rubella surveillance during 2005--2007. DBS was either the only sample collected (Sierra Leone) or was collected in conjunction with routine serum collection (Burkina Faso, the Democratic Republic of Congo, Ethiopia, Ghana, Senegal, and Zambia). Standard protocols for sample collection and laboratory testing recommended by LabNet were used (2). Data from all three sources indicated that the sensitivity and specificity of DBS and OF for detecting measles and rubella virus--specific IgM parallels that of serum; however, a moderate decline in sensitivity for detecting rubella virus--specific IgM in OF during the first 4--5 days after disease onset was observed (Figures 1 and 2; Table). Detection of RNA in serum and DBS was shown to be possible with nested or real-time RT-PCR (but not conventional RT-PCR) if samples are collected within 5--7 days after rash onset. This procedure has proven invaluable for collecting viral sequence information where urine or throat swabs were not available. In the MMR surveillance program in the UK, using OF, the rate of measles RNA detection by nested RT-PCR ranged from 80% to 90% when collected during the first week after rash onset, and reached 50% at 3--4 weeks after rash onset. Conventional RT-PCR was sensitive for up to 2 weeks after rash onset, but was still considered useful. For rubella, testing for both IgM and RNA in OF samples substantially increased the sensitivity of surveillance for confirming cases during the first 4--5 days after rash onset, when many rubella cases are not yet IgM positive. Results of evaluations comparing OF and DBS with serum sampling indicated that OF and DBS sampling have a potential role in improving measles and rubella surveillance. Compared with serum collection, these sampling procedures provide:
However, use of OF and DBS sampling also has some disadvantages compared with serum collection, in particular:
RecommendationsHaving considered the evidence described in this report, participants in the ad hoc meeting made the following recommendations. No single alternative sampling technique has been shown to be optimal for surveillance under every circumstance, and serum should still be considered the "gold standard" for IgM detection. However, DBS and OF sampling techniques are viable options for measles and rubella surveillance (5--10), especially where challenges with specimen transport or refrigeration exist or where patients might resist venipuncture. Alternative sampling techniques would not adversely affect routine measles and rubella surveillance (provided adequate training and resources are provided) and might enhance surveillance through:
Regions in the elimination phase that already have established a serum-based rash illness surveillance system would not likely benefit from changing to DBS or OF sampling methods except in special circumstances, such as in settings where:
Implications for Measles and Rubella Surveillance in the United StatesElimination of indigenous measles and rubella virus was declared in the United States in 2000 and 2004, respectively.† High-quality measles and rubella surveillance including timely collection of diagnostic samples for laboratory confirmation, along with sustained high coverage with a combined MMR vaccine, have been critical in achieving that public health success. At present, routine measles and rubella surveillance in the United States will continue to rely upon already established diagnostic methods, including serum-based assays for detection of virus-specific antibodies and on nasopharyngeal swab or urine samples for virus detection. References
* As of 2008, four out of six World Health Organization regions have measles elimination goals: the Region of the Americas (by 2000; measles declared eliminated since late 2002), the European Region (by 2010), the Eastern Mediterranean Region (by 2010), and the Western Pacific Region (by 2012). In addition, two regions have rubella elimination goals: the Region of the Americas and the European Region (both by 2010). † Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5718a5.htm and http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5411a5.htm. Figure 1 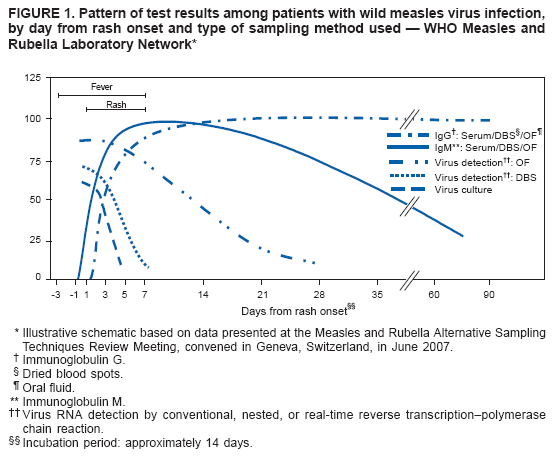 Return to top. Figure 2 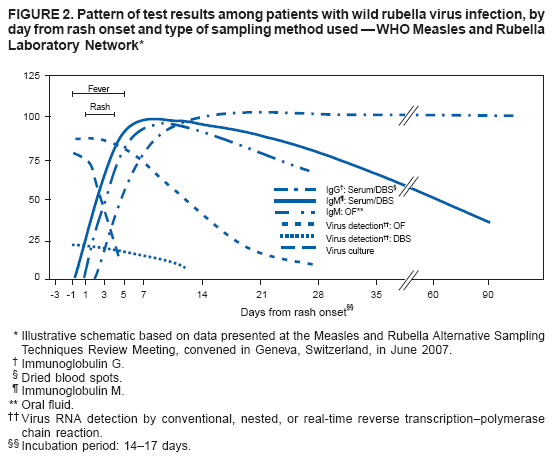 Return to top. Table 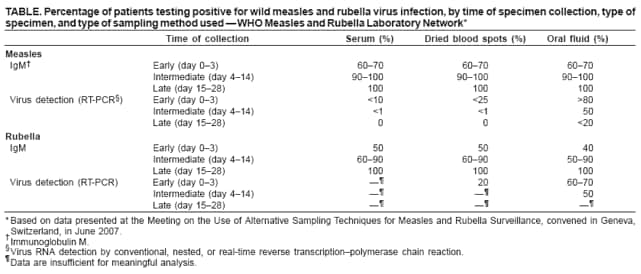 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/19/2008 |
|||||||||
|