 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Multistate Outbreak of Human Salmonella Infections Caused by Contaminated Dry Dog Food --- United States, 2006--2007During January 1, 2006--December 31, 2007, CDC collaborated with public health officials in Pennsylvania, other states, and the Food and Drug Administration (FDA) to investigate a prolonged multistate outbreak of Salmonella enterica serotype Schwarzengrund infections in humans. A total of 70 cases of S. Schwarzengrund infection with the outbreak strain (XbaI pulsed-field gel electrophoresis [PFGE] pattern JM6X01.0015) were identified in 19 states, mostly in the northeastern United States. This report describes the outbreak investigation, which identified the source of infection as dry dog food produced at a manufacturing plant in Pennsylvania. This investigation is the first to identify contaminated dry dog food as a source of human Salmonella infections. After handling pet foods, pet owners should wash their hands immediately, and infants should be kept away from pet feeding areas. On May 8, 2007, the Pennsylvania Bureau of Laboratories reported three cases of S. Schwarzengrund infection with indistinguishable PFGE patterns to CDC's PulseNet.* On June 9, 2007, after PulseNet identified cases in Ohio and other states, CDC's OutbreakNet† team was notified of a potential multistate outbreak of S. Schwarzengrund infections. During June 2007, the Pennsylvania Department of Health (PADOH) interviewed persons identified by PulseNet as infected with the outbreak strain of S. Schwarzengrund. These initial interviews suggested exposure to dogs or dry dog food as a possible source of infection. Thirteen infected persons from Pennsylvania were questioned about dog-related exposures: eight (62%) owned one or more dogs, and the other five reported regular contact with a dog. Seven of the eight persons who owned dogs were able to recall the types of dog food they had purchased recently. Several brands had been purchased, but persons in the households of six patients recalled purchasing dog food products made by manufacturer A. These interviews suggested exposure to dogs or dry dog foods as a possible source of infection. PADOH collected dog stool specimens and opened bags of dry dog food from the homes of the 13 Pennsylvania patients. The outbreak strain of S. Schwarzengrund was isolated from five of 13 dog stool specimens and two of 22 dry dog food specimens collected from the homes. The contaminated dry dog food bags were two different brands (brand A and brand B), both produced by manufacturer A at plant A in Pennsylvania. In July 2007, the Ohio Department of Health also interviewed persons infected with the outbreak strain of S. Schwarzengrund and collected two dog stool specimens from one patient's home. The outbreak strain of S. Schwarzengrund was isolated from one of the dog stool specimens. The dog recently had been fed brand A dry dog food, but the bag of dog food was no longer available for testing. Epidemiologic InvestigationA case was defined as a laboratory-confirmed infection with the outbreak strain of S. Schwarzengrund in a person residing in the United States who either had symptoms beginning on or after January 1, 2006, or (if the symptom onset date was unknown) had S. Schwarzengrund isolated from a specimen on or after January 1, 2006. During January 1, 2006--December 31, 2007, a total of 70 human cases of the outbreak strain of S. Schwarzengrund were reported to CDC via PulseNet from 19 states (Figures 1 and 2). The last reported illness onset date was October 1, 2007. No illness was reported in pets. The largest number of reported cases was in Pennsylvania (29 cases), followed by New York (nine) and Ohio (seven) (Figure 1). Among 61 ill persons whose age was available, the median age was 3 years (range: 1 month--85 years), and 24 (39%) were aged <1 year; of 45 persons whose sex was known, 22 (49%) were female. Of 38 ill persons for whom clinical information was available, 15 (39%) had bloody diarrhea; of 45 persons whose hospitalization status was known, 11 (24%) had been hospitalized. No deaths were reported. Case-Control StudyTo determine the source of infections caused by the outbreak strain of S. Schwarzengrund, the OutbreakNet team coordinated a multistate case-control study during July 17--September 28, 2007. Case-patient households were defined as those with at least one member infected with the outbreak strain of S. Schwarzengrund with an illness onset date or isolation date occurring during January 1, 2006--August 30, 2007. For each case-patient household, one to three geographically matched control households were recruited using a reverse--digit-dialing system. Persons in each case-patient and control household were asked whether they had been exposed to dry dog or dry cat food, which brands they usually purchased, and which brands they purchased in the 2 weeks before illness onset (for cases) or the 2 weeks before interview (for controls). Data were analyzed as a matched case-control study, and a multivariable logistic analysis was conducted to control for confounding from coexposures. One person was interviewed in each of 43 case-patient households and 144 control households in eight states: Delaware, Maine, Michigan, Minnesota, New York, North Dakota, Ohio, and Pennsylvania. Case-patient and control households were excluded from analysis where questions were not answered. Contact with a dog was reported by 34 (79%) persons in case-patient households compared with 86 (60%) persons in control households (matched odds ratio [mOR] = 2.7) (Table). Dry dog or cat food produced by manufacturer A usually was chosen for purchase by members of 19 (44%) case-patient households compared with 14 (10%) of control households (mOR = 7.8; 95% confidence interval [CI] = 2.6--27.8). Among the 19 persons in case-patient households who usually purchased manufacturer A pet food, 11 purchased brand A, three brand B, five brand C, and three brand D. All four brands were produced at plant A. Among the four brands, brand A typically was purchased by 11 (26%) persons in case-patient households compared with six (4%) persons in control households. In multivariable analysis, purchase of brand A was associated with illness (mOR = 23.7) (Table). In Pennsylvania alone, purchase of brand A also was associated with human illness in multivariable analysis (mOR = 15.4; CI = 2.1--infinity). Environmental InvestigationDuring 2007, plant A produced approximately 25 brands of dry pet food; specific distribution information for brands produced in plant A was not available. Plant A labeled these dry pet foods with a 1-year shelf life (i.e., sell-by date). On July 12, 2007, PADOH staff members visited plant A and collected 144 swabs of specimens from environmental surfaces; the outbreak strain of S. Schwarzengrund was isolated from one sample. FDA tested previously unopened bags of seven brands (brands E, F, G, H, I, J, and K) of dry dog food produced at plant A. Two brands of dry dog food (E and F) yielded the outbreak strain of S. Schwarzengrund. On August 21, 2007, manufacturer A announced a voluntary recall of 50-pound bags of brand E dry dog food and 5-pound bags of brand F dry dog food. On July 26, 2007, manufacturer A suspended operations at plant A for cleaning and disinfection. In mid-November 2007, plant A resumed normal operations. Reported by: A Ferraro, PhD, M Deasy, V Dato, MD, M Moll, MD, C Sandt, PhD, J Tait, B Perry, MS, L Lind, MPH, N Rea, PhD, R Rickert, MPH, C Marriott, MPH, C Teacher, MSN, P Fox, MS, K Bluhm, V Urdaneta, MD, S Ostroff, MD, Pennsylvania Dept of Health. E Villamil, MPH, P Smith, MD, Regional Epidemiology Program, New York State Dept of Health. Ohio Dept of Health. JL Austin, PulseNet; T Ayers, MS, S Alexander, DVM, RM Hoekstra, PhD, I Williams, PhD, Div of Foodborne, Bacterial, and Mycotic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; C Barton Behravesh, DVM, EIS Officer, CDC. Editorial Note:The laboratory and epidemiologic evidence in this investigation indicates that dry dog food produced by manufacturer A at plant A in Pennsylvania and sold under several brand names caused human illness during 2006--2007. Although previous reports in North America have associated Salmonella infection with certain pet treats, this report is the first to associate Salmonella with contaminated dry dog food. The case-control study found an association between infections in households and use of dry dog food or dry cat food produced by manufacturer A. In addition, the outbreak strain was isolated from 1) opened bags of dry dog food (brands A and B) that were produced in plant A by manufacturer A, 2) stool specimens from dogs in case-patient households that ate dry dog food produced in plant A, 3) an environmental sample from plant A, and 4) two bags (brands E and F) of previously unopened dry dog food produced in plant A. A voluntary recall of specific-sized bags of two brands of dry dog food issued by the manufacturer in August 2007 was based only on lot-specific testing of finished unopened bags found to be positive for Salmonella by official FDA testing. Other sizes of bags of the two brands of dry dog food, although produced at plant A, were not recalled. Other brands of dry dog or cat food produced at plant A, including brands associated epidemiologically and microbiologically with illness, also were not included in the recall. Plant A ceased operations during July--November 2007 to allow for cleaning and disinfection. However, because dry pet food has a 1-year shelf life and all contaminated products were not recalled, contaminated dry pet food might still be found in homes and could provide the potential for causing illness. Only an estimated 3% of Salmonella infections are laboratory-confirmed and reported to surveillance systems (2); therefore, this outbreak likely was larger than the 70 laboratory-confirmed cases identified. Most Salmonella infections are acquired by handling or consuming contaminated food products, particularly foods of animal origin. Infections also are acquired by direct and indirect contact with farm animals, reptiles, and occasionally pets. Investigations are ongoing to determine how persons might acquire Salmonella infections from dry pet food. Factors under review include the handling and storage of dry pet food, hand-washing practices, exposure of children to dry pet food, and location in the home where pets are fed. Although a specific source of contamination for the pet food from plant A was not identified, the plant equipment might have been contaminated, or contaminated ingredients might have been delivered to plant A. Dry pet foods typically are extruded, and production includes heat treatment, but the extruded food also is spray-coated with a taste enhancer, usually an animal fat. Outbreaks of human illness associated with animal-derived pet treats have been described previously in North America (3--6). These include outbreaks of Salmonella Infantis infection caused by contaminated pig ear pet treats (3,4), Salmonella Newport infection caused by contaminated pet treats containing dried beef (5), and Salmonella Thompson infections associated with contact with contaminated pet treats made from of beef or seafood (6). Follow-up investigations of these outbreaks demonstrated that pet treats were frequently contaminated with Salmonella organisms. After a 1999 outbreak in Canada, Salmonella organisms were isolated from 48 (51%) of 94 samples of pig ear pet treats purchased from local retail stores (5). During 1999--2000 in the United States, Salmonella strains were isolated from 65 (41%) of 158 samples of pig ear and other animal-derived pet treats purchased from retail stores (7). FDA regulates pet foods, treats, and supplements. If Salmonella is present, these products are considered adulterated under the Federal Food, Drug, and Cosmetic (FDC) Act.§ During January 1--July 27, 2007, at least 15 pet food products were recalled because of Salmonella contamination (8). On November 2, 2007, a single brand of pet vitamin was recalled voluntarily by the manufacturer because of possible Salmonella contamination (9). Salmonella contamination has not been identified in canned pet food, probably because the manufacturing process eliminates contamination. However, Salmonella contamination has been associated with raw pet food diets (10). Persons who suspect that contact with dry dog food has caused illness should consult their health-care providers. Most persons infected with Salmonella develop diarrhea, fever, and abdominal cramps 12--72 hours after infection, and Salmonella infection usually is diagnosed by culture of a stool sample. Illness typically lasts 4--7 days, and most persons recover without treatment. Infants, elderly persons, and persons with impaired immune systems are more likely than others to develop severe illness. To prevent Salmonella infections, persons should wash their hands for at least 20 seconds with warm water and soap immediately after handling dry pet foods, pet treats, and pet supplements, and before preparing food and eating. Infants should be kept away from pet feeding areas. Children aged <5 years should not be allowed to touch or eat pet food, treats, or supplements.¶ Acknowledgments This report is based, in part, on contributions by LS Kidoguchi, MPH, LM Gross, Bur of Communicable Disease; L Kornstein, PhD, B Tha, MS, Public Health Laboratory, New York City Dept of Health and Mental Hygiene. G Johnson, M Fage, Regional Epidemiology Program; D Nicholas, MPH, A Mears, MS, Food Protection; T Quinlan, Y Khachadourian, D Schoonmaker-Bopp, MS, L Armstrong, T Root, T Passaretti, K Musser, PhD, Wadsworth Center Laboratory, New York State Dept of Health. Food and Drug Admin. References
* PulseNet is the national molecular subtyping network for foodborne disease surveillance. † OutbreakNet is a national network of epidemiologists and other public health officials who investigate outbreaks of foodborne, waterborne, and other enteric illnesses in the United States. § Available at http://www.fda.gov/opacom/laws/fdcact/fdcact4.htm. ¶ Additional information available at http://www.cdc.gov/salmonella/schwarzengrund.html.
Figure 1 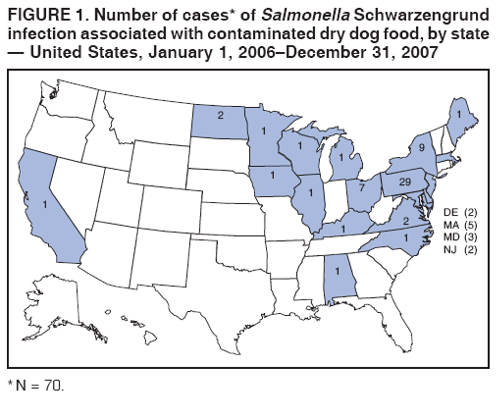 Return to top. Table 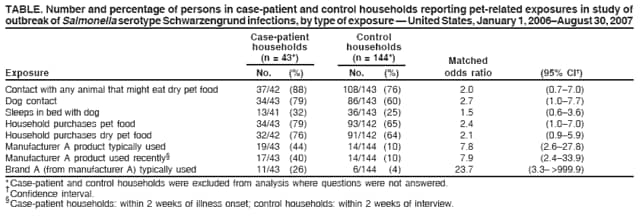 Return to top. Figure 2 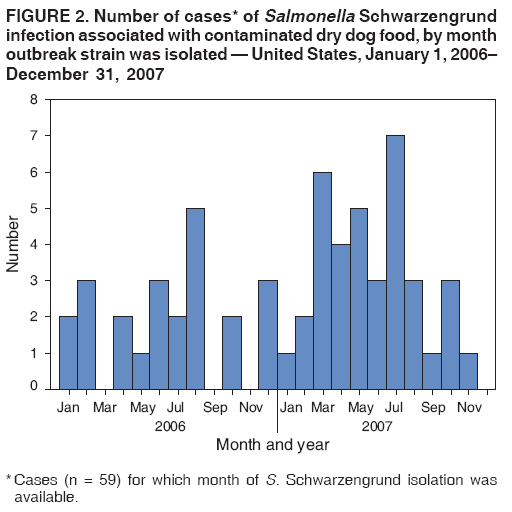 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/15/2008 |
|||||||||
|