 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of Polio in Adults --- Namibia, 2006After 10 years with no detected wild poliovirus (WPV) transmission in Namibia, an outbreak of poliomyelitis cases occurred in 2006. The outbreak was traced to importation from neighboring Angola of WPV type 1 (WPV1) that originated in India. As of October 2, 2006, a total of 19 cases of polio, with paralysis onset between early May and June 26, had been confirmed by isolation of WPV1 from stool specimens, primarily from young adult males; six of the patients died. This report describes outbreak investigation and response activities and provides an update on routine and supplemental immunization activities (SIAs)* and acute flaccid paralysis (AFP) surveillance in Namibia. Outbreak Investigation and ResponseOn May 8, 2006, a man aged 39 years from the Hardap region, approximately 400 km southeast of the capital city of Windhoek, was admitted to a Windhoek hospital after onset of AFP 2 days earlier. On June 5, the Regional Reference Poliovirus Laboratory at the National Institute of Communicable Diseases in South Africa reported isolation of WPV1 in the patient's stool specimens. AFP surveillance was intensified, and as of October 2, 2006, a total of 306 AFP cases had been reported for the year (Figure 1). Of the 306 AFP cases, 19 cases were confirmed as polio through WPV1 isolation, with the most recent onset of paralysis occurring on June 26. Of the other 287 AFP cases, 201 were classified as nonpolio AFP, and the National Polio Expert Committee classified seven cases as polio compatible. Another 66 AFP cases, with inadequate† stool specimens, all virus-negative, are pending classification, including some that subsequently might be classified as polio compatible; 13 additional cases are pending laboratory results and subsequent classification. In addition to the single case reported from the Hardap region, WPV-confirmed cases were reported from two densely populated areas: 1) informal settlements (i.e., areas with temporary substandard housing, poor sanitation, and crowding) in the Katutura vicinity of Windhoek in the Khomas region (14 WPV cases), and 2) three adjacent regions bordering Angola: Ohangwena and Omusati, with one case each, and Oshana, with two cases (Figure 2). Compared with patients with nonpolio AFP, the WPV patients more often reported having contact with persons from Angola during the 3 months preceding paralysis onset (Fisher's exact test, p = 0.007). All WPV-confirmed cases occurred in persons aged >14 years (range: 14--51 years), with 14 (74%) of 19 confirmed cases in persons aged 15--29 years. Seventeen (89%) of the 19 patients were male. Six patients with confirmed WPV died (case-fatality ratio [CFR]: 32%); four of the six who died had respiratory symptoms requiring ventilator support, and at least one other patient developed respiratory difficulty shortly before death. In response to the outbreak of WPV cases, the Namibia Ministry of Health and Social Services (MoHSS) activated the National Health Emergency Management Committee to coordinate activities. Three nationwide SIAs were held during June 21--23, July 18--20, and August 22--24, 2006, using both house-to-house and fixed-post vaccine delivery strategies. Because most patients were adults, the first two SIAs targeted the entire population of Namibia (i.e., adults and children of all ages); the third round targeted only children aged <5 years. Monovalent oral poliovirus vaccine type 1 (mOPV1) was administered during the first two SIAs; trivalent OPV (tOPV) was administered during the third SIA, along with measles vaccine and distribution of vitamin A supplements. Based on the number of vaccine doses administered and current population estimates, close to 100% of the target populations were reached during all three SIAs. Postcampaign monitoring conducted in nine of the 13 regions determined vaccination coverage of >95%. The second and third SIAs were conducted after onset of the last reported confirmed case of polio, which occurred on June 26, 5 days after the first nationwide SIA (Figure 1). Previous Outbreaks in NamibiaNamibia reported no polio cases from 1990 until May 1993, when an outbreak of 53 WPV1 cases (27 virologically confirmed and 26 clinically compatible) occurred. Seventy-nine percent of patients in the 1993 outbreak were aged <5 years. A smaller WPV1 outbreak with 27 cases occurred in the northern regions of Namibia during 1994--1995 (1). Both outbreaks were linked by genetic sequencing to WPV imported from Angola. The most recent reported WPV case before 2006 occurred in September 1995. Immunization ActivitiesThe Namibian Expanded Program on Immunization (EPI) was established in June 1990, the year Namibia gained independence from South Africa. Public health services, including immunization, had been severely disrupted by conflict during 1966--1989. Immunization services improved after 1990, with survey estimates for infant coverage with 3 doses of oral poliovirus vaccine (OPV3), increasing from 37% in 1989 to 76% in 2000 (Table). However, during 1989--2000, coverage estimates varied among regions (2,7,8); for example, OPV3 coverage varied by region from 48% to 78% in 1992 (7). Since 2000, annual national estimates of coverage with OPV3 have ranged from 64% to 83% (Table), with continued variation among regions. OPV3 coverage exceeded 80% in 20 (61%) of 33 districts in 2004 and 10 (30%) of 33 districts in 2005. In addition to routine immunization, annual SIAs have been held since 1996, targeting children aged <5 years. AFP SurveillanceAlthough most AFP cases are nonpolio (i.e., resulting from causes other than poliomyelitis), meeting goals for AFP surveillance helps to ensure that the surveillance system is sensitive enough to detect poliomyelitis cases should they occur. AFP surveillance is evaluated by two key indicators: sensitivity of reporting (target: nonpolio AFP rate of >1.0 case per 100,000 children aged <15 years§) and completeness of specimen collection (target: two adequate stool specimens collected from >80% of all AFP cases). During 2001--2005, national nonpolio AFP rates in Namibia exceeded >1.0 case per 100,000 persons aged <15 years (2.6 in 2004 and 2.0 in 2005). With the increase in AFP reporting during the outbreak, AFP rates in 2006 have exceeded 2.0 cases in all regions. Nationally, adequate stool specimens were obtained from >80% of persons with AFP during 2003--2005. However, adequate stool collection during January 1--October 2, 2006, was 67%, and exceeded 80% in only four of Namibia's 13 regions. Genetic sequencing determined that the WPV1 in the Namibia outbreak belongs to the same cluster as the virus detected in 2005 in both Angola and the Democratic Republic of Congo, which had been imported into Angola from India. Sequencing indicated that this outbreak virus had been circulating for up to 2 years in the southwest subregion of Africa before detection in 2005. Consistent with recent undetected circulation and ongoing surveillance gaps, Angola reported 10 WPV1 cases in 2005 but none in 2006 until reporting a case with onset June 27, 2006. The genetic sequence relationships among the Namibian isolates indicate that spread of the virus occurred from a single-source importation. Reported by: World Health Organization (WHO) Namibia Office, Windhoek; Inter-Country Program Office, WHO, Harare; Regional Office of WHO for Africa, Harare, Zimbabwe; Polio Eradication Group, WHO, Geneva, Switzerland. National Institute of Communicable Diseases Laboratory, Johannesburg, South Africa. Global Immunization Div and Div of Viral Diseases, National Center for Immunization and Respiratory Diseases (proposed), CDC. Editorial Note:This 2006 outbreak underscores the ongoing threat of WPV importations into polio-free areas, the ability of WPV to spread to susceptible populations of any age, and the need for polio-free countries to maintain high levels of preparedness for the timely detection of and response to importations. In this outbreak, virus importation from Angola was indicated by sequencing data, the frequency of cross-border contacts between population groups from Namibia and Angola, and the higher frequency of contact with Angolan residents by patients with confirmed WPV infection compared with patients with nonpolio AFP. The Namibia outbreak illustrates that populations of any age with low immunity against poliovirus are at risk. This outbreak primarily affected young adults born before 1990, an age group consisting of persons who either had not been vaccinated for polio or had been vaccinated incompletely. For the most part, this group also would not have been covered by EPI SIAs conducted four times a year during 1990--1995 and targeted to children aged <5 years. Increasing vaccination coverage among children aged <5 years in the early 1990s would have reduced transmission of WPV, decreasing opportunities for older, unvaccinated persons to acquire natural immunity, a factor possibly contributing to the high attack rate in older age groups. Previous polio outbreaks among adults included a large outbreak (138 paralytic cases, 69 confirmed WPV1 cases) in Albania in 1996, with an attack rate of 10 per 100,000 persons among adults aged 19--25 years who had been vaccinated with OPV that might have been stored without refrigeration for prolonged periods (3). Outbreaks affecting adults also have occurred among religious groups with low vaccination acceptance (4). The CFR was 32% in this outbreak involving young adults. High CFRs in young adults during polio outbreaks have been reported previously. In an outbreak in Cape Verde in 2000, the CFR was 57% among persons aged >15 years (5). In the 1996 outbreak in Albania, the CFR was highest (18%) among persons aged 19--25 years (3). In the 2006 outbreak in Namibia, at least five of the six patients who died had respiratory symptoms, and four required ventilator support, suggesting that bulbar paralysis might have contributed to the high CFR. During 2004 and 2005, AFP surveillance systems in Namibia and Angola, at the national level, surpassed the key indicators for sensitivity of reporting and completeness of specimen collection. However, WPV circulation in the southwest African subregion escaped detection for approximately 2 years, suggesting considerable AFP surveillance quality gaps at the subnational level. During the 2006 outbreak, only four of Namibia's 13 regions have met the 80% stool adequacy standard. Surveillance training targeting district and regional MoHSS staff members was held in July, August, and September 2006. Maintaining sensitive surveillance and stool adequacy levels in Namibia and surrounding countries is critical to rapid detection of WPV virus circulation. WHO's Advisory Committee on Polio Eradication recommends that any polio-free country that detects imported WPV conduct at least three large-scale, house-to-house SIAs using type-specific mOPV, initiating the first within 28 days of confirmation, and continuing with at least two additional SIAs after the last virus is detected (6). Namibia followed these recommendations, conducting the first SIA round, which targeted the entire population, within 3 weeks of laboratory confirmation of the first WPV-confirmed case and 46 days after the first onset of paralysis. The last known WPV-confirmed case occurred less than a week after the first SIA, with no WPV-confirmed cases reported since then, although the 66 cases with inadequate stool specimen collection pending review and classification by the National Polio Expert Committee are of concern. Additional SIA rounds in Namibia will be necessary if more WPV cases are detected. The risk for continuing WPV spread from Angola south to Namibia or north to the Democratic Republic of Congo remains high until circulation in Angola is interrupted. SIAs in Angola are planned for November 16--18, 2006. The increase in the number of WPV cases reported from endemic countries (particularly Nigeria and India) in 2006 underscores the continuing threat of importations from polio-endemic countries and the necessity for full implementation of outbreak response recommendations (6) by all polio-free countries until poliovirus transmission is interrupted globally. Acknowledgment The findings in this report were based, in part, on data provided by the Namibia Ministry of Health and Social Services. References
* Nationwide mass campaigns during a short period in which 2 doses of oral poliovirus vaccine are administered to all persons in the target age group, regardless of vaccination history, with an interval of 4--6 weeks between doses. † AFP cases with inadequate stool specimens are those that lack the following: two stool specimens collected at least 24 hours apart within 14 days of paralysis onset and shipped to the laboratory in good condition. Adequate stool specimens meet these criteria. § In 2006, this indicator was changed to two cases per 100,000 children aged <15 years.
Figure 1  Return to top. Figure 2 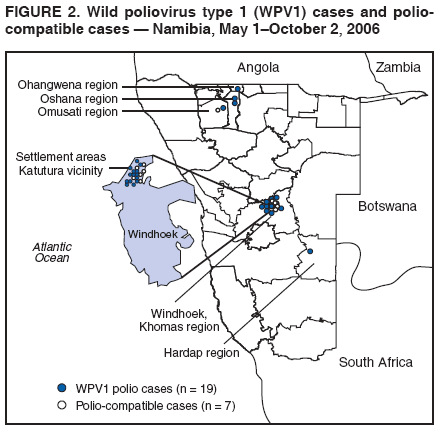 Return to top. Table 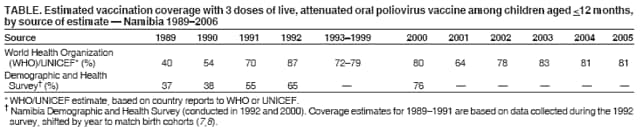 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 11/8/2006 |
|||||||||
|