 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Lower Extremity Disease Among Persons Aged >40 Years With and Without Diabetes --- United States, 1999--2002Lower extremity disease (LED), including peripheral arterial disease (PAD) and peripheral insensate neuropathy (PN), is a chronic condition that disproportionately affects older persons and persons with diabetes. LED can result in disabling foot complications (e.g., ulcers, infection, gangrene, or amputation) (1,2). PAD has been associated with increased risk for cardiovascular morbidity (3) and mortality (4,5). For this report, CDC analyzed data collected during 1999--2002 from the National Health and Nutrition Examination Survey (NHANES) to update previously published estimates of the prevalence of LED among persons aged >40 years with and without diabetes (6). The results of this analysis indicated that approximately 18% of persons aged >40 years had LED and that LED was twice as prevalent among persons with diabetes as among those without diabetes. Approximately two thirds of persons with LED and half of those with both diabetes and LED were asymptomatic. Multiple complications of LED can be prevented if LED is detected early (1,2). Increasing knowledge among clinicians and the public of the prevalence of LED and associated risk factors might lead to early detection, intervention, and treatment to prevent disabling consequences. NHANES is an ongoing, cross-sectional survey of representative samples of the civilian, noninstitutionalized U.S. population (aged >2 months for the 1988--1994 surveys and all ages for the 1999--2002 surveys). For the 1999--2000 and 2001--2002 NHANES surveys, participants were administered detailed in-person home interviews followed by standardized health examinations in a mobile exam center (MEC). In the MEC, persons aged >40 years received noninvasive tests for PAD (i.e., ankle-brachial blood pressure measurements) and PN (i.e., monofilament testing of foot sensation) and examinations for foot abnormalities and lesions by trained health technicians. LED was defined as 1) PAD (ankle-brachial blood pressure index [ABI] of <0.9 in either leg), 2) PN (one or more insensate areas in either foot), 3) self-reported history of a foot ulcer or sore on a leg or foot that took >4 weeks to heal, or 4) observed foot lesions or foot/toe amputation. PAD cases were classified as symptomatic if participants answered "yes" when asked whether they ever had calf pain in either leg while walking. PN cases were classified as symptomatic if participants reported having numbness/loss of feeling or painful sensations/tingling in their feet during the preceding 3 months. Diabetes was defined as self-report of a physician's previous diagnosis. Women with diabetes diagnosed only during pregnancy were classified as without diabetes. Details of these measurements and exclusion criteria have been described previously (6). Complete PAD, PN, and LED data were collected for 5,071, 5,313, and 4,929 persons with diabetes data, respectively. All analyses used examination weights to account for the unequal probability of selection, oversampling, and survey nonresponse. Age-adjusted estimates were made (using the direct method) to the 2000 U.S. census population using three age groups: 40--59, 60--74, and >75 years. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, and Mexican-American. Estimates were not shown separately for persons of other racial/ethnic populations, although these persons were included in totals and strata by other characteristics. Among U.S. adults aged >40 years, approximately 5.0% had PAD (Figure); approximately two thirds of these persons were asymptomatic, and approximately one fourth (1.4%; 95% confidence interval [CI] = 1.0--1.8) had severe PAD (i.e., ABI of <0.7 in either leg). Approximately 12.9% had PN; approximately three fourths of these persons were asymptomatic, and one fourth (3.3%; CI = 2.6-4.0) had severe PN (i.e., three or more insensate areas). Approximately 4% of persons reported a foot ulcer or were observed to have a current foot lesion or toe/foot amputation. Overall, approximately 18.6% of the U.S. adult population aged >40 years had at least one LED condition (i.e., PAD, PN, history of ulcer, current foot lesion, or amputation), among whom two thirds were asymptomatic. The percentage of adults with PN or with any LED who were symptomatic was greater among persons with diagnosed diabetes than among persons without diagnosed diabetes. Among persons with PN, 42% of those with diabetes were symptomatic, compared with 21% of those without diabetes. Among persons with any LED, 53% of those with diabetes were symptomatic, compared with 31% of those without diabetes. However, among persons with PAD, approximately one third were symptomatic regardless of diabetes status. Among adults aged >40 years, prevalence of LED was higher among persons aged >75 years (40.8%) and 60--74 years (26.2%) than among persons aged 40--59 years (12.3%). Prevalence of LED also was higher among men than among women (23.1% versus 16.6%) (Table) and higher among non-Hispanic blacks than among non-Hispanic whites or Mexican-Americans (27.0% versus 19.1% and 21.1%, respectively). Among all age, sex, and racial/ethnic subpopulations, the age-adjusted prevalence of any LED was 1.5--1.8 times greater among adults with diagnosed diabetes than among those without diabetes (Table). Reported by: R Paulose-Ram, PhD, Q Gu, MD, M Eberhardt, PhD, National Center for Health Statistics; E Gregg, PhD, L Geiss, MA, M Engelgau, MD, Div of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC. Editorial Note:The findings in this report indicate that approximately one fifth of the U.S. adult population aged >40 years has LED, and the majority of cases are asymptomatic; prevalence of LED is approximately twice as high among persons with diagnosed diabetes as among those without diabetes. These results highlight the importance of improved detection and prevention of asymptomatic and symptomatic LED among both persons with and without diabetes. In 2003, the Prevention of Atherothrombotic Disease Network identified five steps to improve PAD treatment and outcomes: 1) increase awareness of PAD and its consequences, 2) identify persons with symptomatic PAD, 3) screen for patients at high risk, 4) improve treatment for symptomatic PAD cases, and 5) increase early detection of asymptomatic cases (2). In 2003, the American Diabetes Association (ADA) recommended PAD screening for all persons with diabetes aged >50 years, including those without symptoms (7). Early detection and control of diabetes and coexisting risk factors for peripheral neuropathy (e.g., smoking or hypertension) can prevent, delay, or slow progression of diabetic neuropathy (8). In 1993, the Diabetes Control Complications Trial (DCCT) demonstrated that tight glycemic control can reduce the risk for developing clinical neuropathy (9). ADA has adopted the DCCT standards for tight glycemic control in persons with type 1 diabetes (8). Several foot-related conditions, including PN and PAD, are associated with increased risk for amputation (1). Because early detection and aggressive care of foot ulcers and lesions can reduce risk for amputation, ADA also recommends an extensive annual foot examination for all persons with diabetes (8). The findings in this report are subject to at least four limitations. First, NHANES samples the noninstitutionalized population and does not include persons in nursing homes and other institutions. Second, within the NHANES sample, 17% were missing ABI measurements and 12% were missing PN measurements (e.g., because of participant refusal or equipment failure); these persons might have had LED. However, nonresponse analyses were performed and adjustment procedures were conducted. Estimates computed with the adjusted weights (based on age, sex, race/ethnicity, and diabetes status) produced only minor differences in point and variance estimates (0.1%--0.6%); therefore, all estimates in this report were based on the original 4-year examination weights. Third, 9% of the PAD sample had ABI measurements performed on only one foot and might have been misclassified as without PAD even if the other foot had disease. Finally, although foot lesions and lower extremity amputations were identified by trained health technicians, the causes of these conditions were not determined. Advanced age and diabetes are strong risk factors for PAD and PN. As the U.S. population ages and the prevalence of diabetes increases, the public health burden associated with PAD and PN will increase. NHANES provides the first nationally representative data on the prevalence of these diseases and should inform policy makers, clinicians, and researchers regarding the magnitude of LED to guide programs addressing prevention and treatment. CDC also provides resources and technical assistance to state and territorial diabetes control and prevention programs to increase awareness and understanding of diabetes, improve and monitor the quality of diabetes care, and promote early detection of diabetes complications. In addition, CDC collaborates with the National Institutes of Health in administering the National Diabetes Education Program, which seeks to increase public and professional awareness regarding diabetes and proper foot care. Information for persons with diabetes regarding how to prevent problems and take better care of their feet is available at http://www.cdc.gov/diabetes/consumer/problems.htm. References
Table 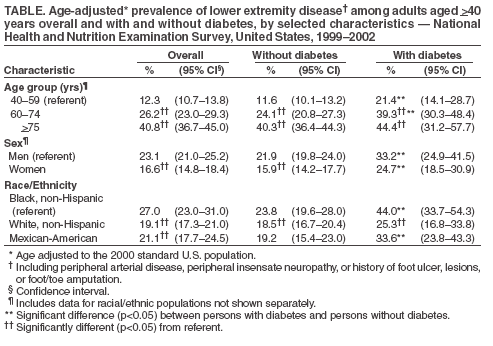 Return to top. Figure 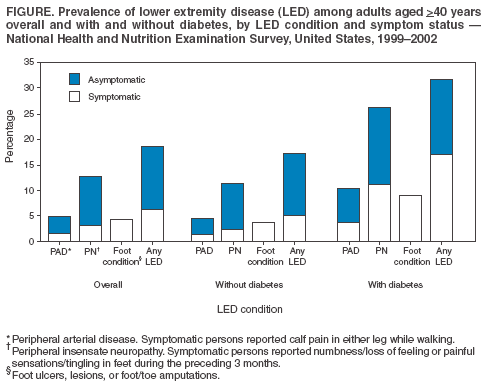 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 11/17/2005 |
|||||||||
|