 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Atypical Reactions Associated With Heroin Use --- Five States, January--April 2005
Please note:
An
erratum has been published for this article. To view the erratum, please
click here. New JerseyDuring January 28--February 2, 2005, nine cases of atypical reactions after heroin use were reported to the New Jersey Poison Information and Education System (NJPIES). The reports originated from hospitals in four New Jersey counties. The reported route of exposure was intranasal in six patients, intravenous in two, and unknown in one. Tachycardia (89%), hyperglycemia (78%), palpitations (78%), and hypokalemia (78%) were the most common signs, symptoms, and laboratory findings; six patients (67%) had all four. In addition, multiple patients had nausea, hypotension, chest pain, venous hyperoxia, lactic acidosis, agitation, and anxiety. Early in the investigation, cyanide was suspected as the adulterant responsible for the atypical reactions because several patients had venous hyperoxia and lactic acidosis. However, the uncharacteristic responses of the patients to antidotal therapy (i.e., sodium thiosulfate and sodium nitrite) for cyanide, presence of signs not typically associated with cyanide poisoning (e.g., hypokalemia), and negative cyanide levels made cyanide an unlikely etiology. Law enforcement personnel with the New Jersey State Police responded to the outbreak and tested samples of the heroin involved; the presence of clenbuterol, a b2 adrenergic receptor agonist, was reported. Information regarding the atypical reactions to heroin use was disseminated by NJPIES and local public health agencies to the general public, public health agencies in neighboring states, national toxicology organizations, and federal agencies. One patient reported atypical symptoms on multiple occasions after using heroin but only sought medical attention after seeing a flyer informing heroin users of suspected drug adulteration. Case 1. The first reported patient was a man aged 21 years who went to the emergency department (ED) of a New Jersey hospital January 28, 2005, complaining of chest pain, palpitations, and shortness of breath, which had begun soon after intranasal exposure to what he believed was heroin. While in the ED, his highest recorded heart rate was 137 beats per minute (bpm), and his lowest recorded systolic blood pressure was 69 mmHg. On physical examination, the patient had tachycardia, tachypnea, pale skin, and mydriasis (dilated pupils). Laboratory studies revealed the following serum values: potassium, 2.2 mmol/L (reference range: 3.5--5.3 mmol/L); glucose, 243 mg/dL (reference range: 65--115 mg/dL); CO2, 13 mmol/L (reference range: 22--32 mmol/L); an elevated anion gap; and an elevated lactate level (1). An electrocardiogram (ECG) revealed ischemic changes. The patient required intravenous fluid replacement, potassium supplementation, and an intravenous calcium channel blocker for persistent tachycardia. His laboratory, ECG, and vital sign abnormalities resolved during his 4 days in the intensive care unit. The patient left against medical advice on the fifth day of hospitalization with no apparent remaining impairments. Case 2. A man aged 23 years visited the ED at the same New Jersey hospital on January 29, 2005, a day after the patient in case 1. The man had headache, nausea, palpitations, chest pain, and anxiety after intranasal exposure to heroin the night before. He had no known connection to the patient in case 1. While in the ED, he was tachypneic and hypotensive; he had a widened pulse pressure (120/48 mmHg) and was persistently tachycardic (120--122 bpm). He was noted to have agitation and mydriasis on physical examination. Laboratory serum values included potassium, 2.9 mmol/L, and blood glucose, 157 mg/dL. The patient was admitted to the intensive care unit and discharged from the hospital on the fifth day with no known impairments. New YorkNine cases of atypical reactions to intranasally insufflated heroin were reported to the New York City Poison Control Center during February 5--March 14, 2005. Tachycardia (89%), palpitations (78%), chest pain (67%), and hypotension (56%) were the most common abnormal findings. Of the seven patients for whom potassium and glucose measurements were available, seven (100%) were hypokalemic, and five (71%) were hyperglycemic. Clenbuterol was detected in the urine by liquid chromatography mass spectrometry in four of the patients and ranged from 1.7 to 969 ng/mL. Testing for clenbuterol was not conducted on the other five patients. Cases 3 and 4. A man aged 43 years and a man aged 29 years visited an ED on February 8, 2005, after intranasally insufflating heroin together. Both patients noted the heroin "smelled like vanilla." Within 15 minutes of exposure, both complained of palpitations, chest pain, and shortness of breath. After arrival in the ED, both patients were noted to be tachycardic, hypotensive, and tremulous. One patient complained of tinnitus, and the other complained of "ear throbbing." A mild leukocytosis, hypokalemia, and hyperglycemia were noted on the initial laboratory results for both men. Both patients were admitted to the hospital but left against medical advice. North Carolina, South Carolina, and ConnecticutDuring February 19--22, 2005, two cases of suspected adulterated heroin exposure were reported to Carolinas Poison Center in North Carolina and two cases were reported to Palmetto Poison Center in South Carolina. In April, the Connecticut Poison Control Center received information on four more patients who had developed atypical reactions after using heroin. Five patients had intranasally insufflated the heroin, and three had injected it. All eight patients complained of palpitations with maximum heart rates of 120--141 bpm in the ED and were hypokalemic with serum potassium values ranging from 1.9--2.8 mmol/L. Six of the eight patients were noted as hypotensive in the ED. Seven patients had serum glucose testing performed, and all had results higher than 150 mg/dL. Urine from all four Connecticut patients tested positive for clenbuterol. A drug sample involved in one of the Connecticut cases was tested and was found to contain heroin, procaine, and clenbuterol. Provisional Case Definition for Future CasesTo facilitate uniform reporting of future cases of heroin adulterated with clenbuterol, a provisional case definition (Box) was created by CDC, in coordination with PCCs and public health agencies involved with this investigation. Because the assay for clenbuterol is not available in the majority of laboratories, only eight of the 26 cases described in this report were confirmed; 16 cases were classified as probable and two as suspected. Reported by: RS Hoffman, MD, LS Nelson, MD, GM Chan, MD, SE Halcomb, MD, NC Bouchard, MD, BY Ginsburg, MD, New York City Poison Control Center; J Cone, MD, Y Jean-Francois, MD, S Voit, MSPH, New York City Dept of Health and Mental Hygiene. S Marcus, MD, New Jersey Poison Information and Education System, Newark. M Ford, MD, Carolinas Poison Center, Charlotte; C Sanford, MSPH, North Carolina Dept of Health and Human Svcs. JE Michels, PharmD, WH Richardson, MD, Palmetto Poison Center, Columbia; LM Bretous, MD, South Carolina Dept of Health and Environmental Control. K Johnson-Arbor, MD, Connecticut Poison Control Center, Farmington. J Thomas, MD, Div of Laboratory Sciences, C Barthold, MD, M Belson, MD, M Patel, MD, J Schier, MD, A Wolkin, MSPH, C Rubin, DVM, Div of Environmental Hazards and Health Effects, National Center for Environmental Health; Z Duprey, DVM, EIS Officer, CDC. Editorial Note:Clenbuterol is a b2 adrenergic receptor agonist with a rapid onset and long duration of action approved for limited veterinary use in the United States (2,3). Clenbuterol is also used illicitly as an alternative to anabolic steroids in humans and livestock because it can increase muscle mass (4,5). Most adverse health effects are related to its stimulation of b2 adrenergic receptors and clinical manifestations, including hypokalemia, hyperglycemia, hyperlactemia, agitation, tachycardia, and hypotension (6). Adverse human health effects have been reported previously in a case of clenbuterol ingestion (7) and from ingestion of meat from livestock fed clenbuterol (3). However, the 26 cases described in this report are the first published accounts of poisoning from clenbuterol associated with reported heroin use. Whether these cases represent adulteration of a single source of heroin before widespread distribution or adulteration of multiple sources is unknown. Also unclear is whether the substance used by each patient was heroin contaminated with clenbuterol or pure clenbuterol sold as heroin. The presence of adulterants in heroin is common. In some years, substances such as caffeine were detected in more than half of samples tested (8). Widespread poisoning secondary to adulterated heroin has occurred before as in the case of scopolamine-adulterated heroin reported in four states during the mid-1990s (9). For various reasons, the 26 cases described in this report likely represent a fraction of actual cases of clenbuterol poisoning. Patients might not have medical evaluation for fear of legal repercussions. Passive reporting to public health agencies or PCCs might not have occurred because ED physicians, hospital intensivists, and the patients themselves might have presumed that the effects were related to a known coingestant. The identification of potential cases during the PCC record review process might have been limited by each center's database classification. The etiologic agent in suspicious cases might have been coded by using words other than "heroin" or "clenbuterol," such as "unknown drug" or "presumed coingestant." Communication and cooperation among PCCs, EDs, CDC, and local public health agencies allowed for coordination of an appropriate response to the clenbuterol incidents. Local public health agencies and PCCs (available 24 hours a day at telephone 800-222-1222) should be notified of any case of suspected or known human exposure to an adulterated product. Early and rapid collaboration among local, state, and federal public health and law enforcement agencies might be necessary to identify, respond to, and minimize the effects of unintentional or intentional adulteration of substances used by the public. Acknowledgments This report is based, in part, on contributions from the New Jersey State Police, West Trenton; B Ruck, PharmD, Z Vassilev, MD, New Jersey Poison and Education System, Newark; E Bresnitz, MD, New Jersey Dept of Health and Senior Svcs. N Kim, PhD, P Smith, MD, New York State Dept of Health. D Drociuk, MSPH, South Carolina Dept of Health and Environmental Control. E Tan, MBBS, EIS Officer, CDC. References
Figure 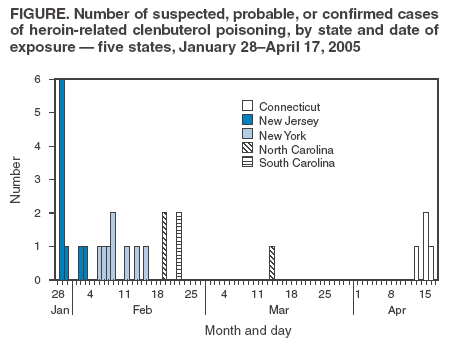 Return to top. Box 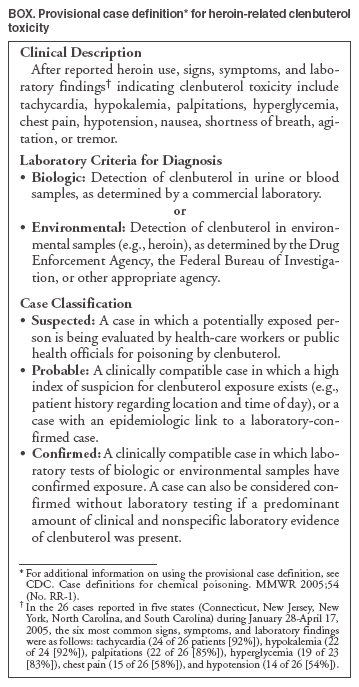 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/17/2005 |
|||||||||
|