 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Seroprevalence of Poliovirus Antibodies Among Children in a Dominican Community --- Puerto Rico, 2002Please note: An erratum has been published for this article. To view the erratum, please click here.Although the Region of the Americas was certified as polio-free in 1994, an outbreak of paralytic poliomyelitis associated with circulating vaccine-derived poliovirus (cVDPV) occurred during July 2000--July 2001 on the Caribbean island of Hispaniola. A total of 21 cases of paralytic polio associated with type 1 oral poliovirus vaccine (OPV) strain were reported in Haiti and the Dominican Republic (DR) (1). Outbreaks from cVDPV occur among children in communities with low immunity levels to polioviruses and the absence of circulation of wild poliovirus (WPV) (2,3). The U.S. territory of Puerto Rico (PR), located approximately 72 miles east of DR, has not had a case of paralytic polio since 1974. However, because of its proximity to DR and concerns that visitors and immigrants from DR (who tend to live in a separate community in PR) might not be fully vaccinated against polioviruses, the PR Department of Health (PRDH) and CDC assessed the seroprevalence of poliovirus antibodies among children aged 7--60 months in a predominantly DR community of PR. This report describes the results of that assessment, which indicated high levels of seropositivity for all three poliovirus serotypes. If vaccination rates remain high, the risk for a polio outbreak in this community is low. However, until all threats of poliovirus are eliminated globally, high rates of vaccination among preschool children must be ensured to prevent outbreaks of paralytic polio from any source (e.g., imported WPV, laboratory strains, or cVDPV) in the United States and its territories. By using data from the U.S. 2000 Census and input from the Dominican Consulate in PR, a community of 3,958 households was selected in the San Juan metropolitan area, where a high concentration of Dominican families lived. During July--August 2002, community liaisons hired by PRDH approached households in this community in a nonsystematic way. Households with children aged 7--60 months were eligible for the study regardless of nationality. Sociodemographic surveys and serum samples from the children were obtained from consenting parents. Parents were offered a monetary incentive for their time and an additional incentive for serum samples. Parents could agree to be interviewed but decline permitting serum samples of their children. If more than one child in a household was eligible, the Kish table (4) was used to randomly select a child. Parents/guardians in 320 households agreed to be interviewed, and 180 (56%) consented to their children giving serum samples. Sera were tested for neutralizing antibodies to poliovirus (PV) types 1, 2, and 3 by using a modified micro-neutralization assay. Each serum specimen was run in triplicate, with the final titer estimated by Spearman-Karber method (5). Antibody levels were considered protective if titers were >1:8. Families with children who did not have antibodies to all three PV serotypes were offered counseling about immunization and a referral for free vaccination. The 320 children surveyed had a median age of 25 months (range: 7--58 months); 163 (51%) were female. Only two children (0.6%) were born in DR, but mothers of 48 (15%) children and fathers of 65 (20%) children were born in DR; both parents of 43 (13%) children were born in DR. The group that consented to a serum sample differed from the group that only consented to an interview: families with annual incomes of <$10,000 or who did not own a car were more likely to consent to a blood sample (68% versus 49% and 51% versus 33%, respectively). The number and prevalence of children with neutralizing antibodies against PV serotypes 1, 2, and 3 were 170 (94.4%), 176 (97.8%), and 168 (93.3%), respectively; 162 (90%) had antibodies to all three PV serotypes (Table). Of the 18 children who did not have neutralizing antibodies to all three PV types, 13 tested positive for two PV types (seven, one, and five to serotypes 1 and 2, 1 and 3, and 2 and 3, respectively); two were seropositive to one PV (both to serotype 2); and three were negative to all three PV serotypes. The latter three children were aged 7, 18, and 43 months; the first child reportedly had received 2 doses of inactivated poliovirus vaccine (IPV), and the other two children received 3 doses of IPV. To identify factors associated with poliovirus immunity, children who had poliovirus antibodies >1:8 for all three serotypes were compared with those who did not. No statistically significant difference was noted between these two groups with respect to median age (26 versus 30 months), place of birth of child or parents (DR or PR), polio vaccination schedule followed (sequential, all IPV, or all OPV), medical insurance status, or participation in the Women, Infants, and Children (WIC) Program. Children who had a history of >3 poliovirus vaccine doses were more likely to have protective levels for all three polio serotypes than children who had a history of <3 poliovirus vaccine doses, but this difference was not statistically significant (prevalence ratio = 1.88; 95% confidence interval = 0.60--5.74). Reported by: E Segarra, MPH, Y Garcia-Guadalupe, MPH, J Rullan, MD, Puerto Rico Dept of Health. L Alexander, MPH, T Murphy, MD, J Alexander, MD, J Seward, MBBS, Epidemiology and Surveillance Div; C Thames, MSc, Data Management Div, National Immunization Program; M Pallansch, PhD, National Center for Infectious Diseases; F Alvarado-Ramy, MD, Div of State and National Partners, National Center for Health Marketing, CDC. Editorial Note:The majority of children surveyed in this metropolitan San Juan community had neutralizing antibodies to all three PV serotypes and were considered protected against polio. These findings suggest that this community is at low risk for a polio outbreak from either cVDPV or WPV. This conclusion is supported by data from the Puerto Rico 2002 Immunization Survey, which reported 99% coverage levels with 3 doses of poliovirus vaccine among children aged 24 months (6). The findings in this report are subject to at least two limitations. First, because this assessment relied on a convenience sample, whether the seroprevalence of children surveyed was representative of the community is uncertain. Second, selection bias might have been introduced when interviewed parents were given the option of permitting a serum sample to be obtained from their child. Because parents were offered an additional monetary reimbursement if blood was drawn, the sero-study included families who were poorer than those who refused blood sampling. If vaccine coverage is inversely associated with poverty, then seroprevalence rates would be lower among poorer children and, therefore, would suggest that this survey underestimated the true seroprevalence in this community. Puerto Rico follows the immunization recommendations of the Advisory Committee on Immunization Practices (e.g., administering IPV at ages 2, 4, 6--18 months, and 4--6 years (7). The study described in this report included children who were vaccinated during the period of transition from OPV to IPV (1997--1999) and children who were vaccinated after the all-IPV schedule was implemented. The results of the study suggest that the schedule change was accepted in Puerto Rico and that PV vaccine coverage was not compromised. References
Table 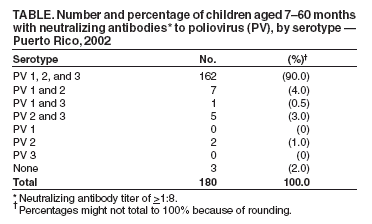 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/16/2005 |
|||||||||
|