 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Transmission of Hepatitis B Virus in Correctional Facilities --- Georgia, January 1999--June 2002Incarcerated persons have a disproportionate burden of infectious diseases (1), including hepatitis B virus (HBV) infection. Among U.S. adult prison inmates, the overall prevalence of current or previous HBV infection ranges from 13% to 47%. The prevalence of chronic HBV infection among inmates is approximately 1.0%--3.7%, two to six times the prevalence among adults in the general U.S. population (1). Incarcerated persons can acquire HBV infection in the community or in correctional settings (1). This report summarizes the results of 1) an analysis of hepatitis B cases among Georgia inmates reported to the Georgia Department of Human Resources, Division of Public Health (DPH) during January 1999--June 2002, including a retrospective investigation of cases reported during January 2001--June 2002; and 2) a prevalence survey conducted in prison intake centers during February--March 2003. These efforts identified cases of acute hepatitis B in multiple Georgia prisons and documented evidence of ongoing transmission of HBV in the state correctional system. The findings underscore the need for hepatitis B vaccination programs in correctional facilities. The Georgia correctional system houses approximately 45,000 inmates in 68 correctional facilities; approximately 16,000 new inmates are admitted each year and processed through one of five intake centers. The correctional system does not routinely screen inmates for HBV infection, and diagnostic testing is left to the judgment of individual physicians. In August 2000, in response to two hepatitis B outbreaks at one Georgia correctional facility (2,3), DPH began to monitor reports of acute hepatitis B cases among inmates at all Georgia correctional facilities, as determined by the inmates' addresses on laboratory reports. A case of acute HBV infection was defined as a positive serologic test for IgM antibodies to hepatitis B core antigen (IgM anti-HBc) on at least one occasion and at least one additional supporting finding (e.g., compatible symptoms, liver enzyme elevation, or another positive hepatitis B serologic test), received by DPH during January 1999--June 2002. Cases reported during January 2001--June 2002 were confirmed by retrospective review of the inmate's medical and laboratory records. The date of diagnosis of acute HBV infection was defined as the date that alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were elevated at least two times greater than the upper limit of normal in conjunction with a positive test for IgM anti-HBc. When ALT or AST levels were not available, the date of the blood draw with a positive IgM anti-HBc result was used as the approximate date of diagnosis. Incarceration histories of inmates with acute HBV infections reported during January 2001--June 2002 were reviewed to identify inmate locations and number of transfers between correctional facilities before illness onset. Persons with asymptomatic and symptomatic cases were considered to have been infected while incarcerated if they were in prison or jail during the 12 months or 6 months, respectively, before illness onset. A prevalence survey to assess the HBV infection status of prisoners on entry was conducted at three Georgia prison intake centers for males and one intake center for females during February--March 2003. Consenting inmates underwent HBV serologic testing; all inmates at intake when the survey was conducted were offered hepatitis B vaccine. During January 1999--June 2002, a total of 92 cases of acute HBV infection were identified, of which 57 (62%) were reported during January 2001--June 2002 and included in the retrospective investigation (Figure). Among the 57 inmates with HBV infection, the median age was 34 years (range: 18--59 years); 52 (91%) were male, and 35 (61%) were non-Hispanic blacks. Ten (18%) had symptoms that included jaundice, abdominal pain, fever, and vomiting. Seven (12%) subsequently were determined to have chronic infections. The chronic infection status of four inmates was not assessed. Among the 57 inmates included in the retrospective investigation, the most frequently reported reason for HBV testing was the presence of symptoms or elevated liver enzymes (21 cases [37%]). Other reasons included reported characteristics and behaviors that might be associated with HBV transmission (e.g., tattoos or unprotected sex contacts) (14 [24%]), serologic testing performed as part of initial medical evaluation (13 [23%]), and being positive for human immunodeficiency virus (five [9%]). Prison staff reported counseling and providing medical follow-up for 52 (91%) of the 57 inmates. The 57 cases were reported from 27 prisons and four probation detention centers in Georgia, with a mean of 1.8 cases per facility and a range of one to three cases for the 30 facilities that were not involved in the previously recognized outbreaks (2,3). The 57 inmates had been incarcerated for a median of 2.2 years (range: 0--23.7 years) before illness onset and had been transferred 1.4 times on average (median: one time; range: one to seven times) during the 12 months before diagnosis. The majority of HBV infections (41 [72%]) were acquired in prison. Of the remaining 16 cases, 13 (81%) occurred in persons who had been in prison or jail for 1--6 months before receiving a diagnosis. The remaining three (19%) inmates were asymptomatic and had been in prison or jail for 10--11 months before receiving a diagnosis. As of August 2002, the seven inmates who had chronic infections had been transferred among prison facilities 13 times during the cumulative 89 months of incarceration that followed their diagnosis, resulting in a mean of 1.8 transfers per person-year of incarceration (median: two transfers; range: zero to five transfers). Three inmates with chronic infection were released from prison. Of 546 inmates surveyed at intake during February--March 2003, a total of 489 (90%) consented to serologic testing, and 428 (78%) consented to hepatitis B vaccination. Of the 489 inmates tested, three (0.6%) had acute HBV infections, four (0.8%) had chronic infections, 64 (13%) had evidence of resolved infections, and 374 (76%) were susceptible to HBV infection. Two of three inmates with acute infection had spent 5.5--11.0 months in jail before intake. Reported by: K Arnold, MD, Georgia Dept of Human Resources, Div of Public Health; M LaMarre, MN, J Taussig, MPH, Georgia Dept of Corrections. BP Bell, MD, L Farrington, MS, Div of Viral Hepatitis, National Center for Infectious Diseases; S Vong, MD, PR Patel, MD, EIS officers, CDC. Editorial Note:HBV is a bloodborne pathogen, transmitted by percutaneous or permucosal exposure to infectious blood or body fluids. The prevalence of chronic infection is higher among prison inmates (1.0%--3.7%) than among the general U.S. population (0.5%) (1), reflecting an overrepresentation of persons entering prison who are at high risk for HBV infection (e.g., injection-drug users and those with reported histories of multiple sex partners). The prevalence of chronic infection among the intake population in this report (0.8%) suggests that high-risk behaviors practiced within the community before incarceration might not account entirely for the burden of HBV infection in correctional facilities. Although studies are limited, transmission of HBV infection within correctional settings has been documented, with incidence ranging from 0.8% to 3.8% per year (2,4--6). The retrospective investigation described in this report identified an increase in HBV infections in Georgia correctional facilities, beginning in January 2001. This increase likely was related to multiple factors, including enhanced surveillance and increased diagnostic testing by correctional medical staff. Changes in diagnostic practices might have occurred because of increased awareness of hepatitis B among medical staff after outbreaks at a Georgia correctional facility in June 2000 and again in June 2001. Nonetheless, the number of reported cases probably underestimates the extent of HBV transmission in the correctional system because the majority of persons with acute HBV infection are asymptomatic and investigations of single cases are not conducted routinely. In the first previous outbreak, one symptomatic patient reported to DPH was associated with a cluster of 11 acute cases, and four chronic HBV infections were identified (2). The majority of inmates with identified acute HBV infections were housed in multiple Georgia correctional facilities and were infected during their incarceration, suggesting widespread ongoing transmission in multiple facilities. Inmates infected with HBV were transferred frequently among facilities. Thus, potential sources of HBV transmission were distributed throughout the prison system. In the Georgia correctional system, approximately one third of inmates are released each year (7). Inmates who become chronically infected and subsequently are released represent potential sources of infection for others in the community. In addition, susceptible inmates who are released continue to be at increased risk for HBV infection (1). The majority of inmates in the intake survey were susceptible to HBV infection and consented to vaccination, suggesting that vaccination efforts in correctional facilities might effectively capture susceptible, high-risk populations. Although data are lacking regarding the overall burden of HBV infection in correctional systems, the ongoing transmission demonstrated in Georgia prisons might be occurring in other states, where similar conditions are likely to exist. All inmates who receive a medical evaluation should be vaccinated to prevent HBV infection (1). However, the majority of state correctional systems in the United States, including the Georgia system, do not have hepatitis B vaccination programs (1). Implementation of such programs in correctional settings nationwide could result in a considerable reduction in the hepatitis B--associated disease burden, not only by eliminating transmission among the incarcerated population, but also by reducing transmission in the community (8). References
Figure 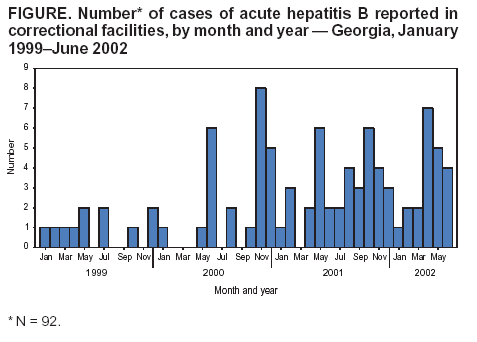 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 8/5/2004 |
|||||||||
This page last reviewed 8/5/2004
|