 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Preliminary Assessment of the Effectiveness of the 2003--04 Inactivated Influenza Vaccine --- Colorado, December 2003Influenza activity started earlier than usual in the United States this season, with widespread influenza activity* reported in 10 states by November 22, 2003 (1). The predominant influenza viruses (A/Fujian/411/2002 [H3N2]-like viruses) circulating this season differ antigenically from the 2003--04 influenza A (H3N2) vaccine strain (2). A retrospective cohort study was conducted among workers at a Colorado hospital to provide preliminary data on the effectiveness of trivalent inactivated influenza vaccine (TIV) against influenza-like illness (ILI). This report summarizes the results of that study, which indicated that TIV had no or low effectiveness against ILI. However, additional studies are needed to evaluate the effectiveness of the 2003--04 vaccine against laboratory-confirmed influenza and influenza-related complications, including hospitalization and death. Influenza vaccine continues to be recommended, particularly for persons at increased risk for influenza-related complications, their household contacts, and health-care personnel. The Children's Hospital (TCH) is a 233-bed pediatric hospital located in Denver. During October--December 2003, TCH promoted employee influenza vaccination with TIV. In early November, the weekly number of positive influenza test results from patient specimens tested through the TCH laboratory began to increase (Figure). To evaluate the effectiveness of TIV against ILI, CDC, the Colorado Department of Public Health and Environment, and TCH conducted a retrospective cohort study among hospital staff during December 11--17. An anonymous survey was distributed to hospital workers by paper questionnaire at worksites and via e-mail messages. Respondents were asked whether they had received the influenza vaccine, had certain medical conditions associated with increased risk for influenza-related complications (3), had any illness with fever, cough, or sore throat on or after November 1, or had contact with any patients. Data also were collected on age group, sex, and occupation. Persons who had received vaccine were asked when they were vaccinated, and those who reported illness were asked for date of illness onset. The survey was distributed to approximately 3,100 TCH workers. Responses were received from 1,886 (61%) of persons surveyed. Respondents who did not report influenza vaccination or illness status (3% and 1%, respectively) and those who were vaccinated but did not report a vaccination date (<1%) were excluded from the study. Of the 1,818 persons included in the study, 1,424 (78%) were vaccinated, including 1,009 (71%) vaccinated before November 1 and 415 (29%) vaccinated on or after November 1. ILI was defined as an illness with subjective fever (i.e., temperature not taken) plus cough or sore throat from November 1 through the questionnaire completion period (December 11--17). Overall, 289 (16%) respondents reported having ILI; 28 persons with ILI reported being tested for influenza, 13 (46%) of whom tested positive. Statistically significant factors associated with receiving vaccination included age group, occupation, and contact with patients (Table 1). Two methods were used to estimate vaccine effectiveness to account for changes in vaccination status during the influenza outbreak period: a categorical analysis and a person-time analysis. For each method, two estimates of vaccine effectiveness were calculated to account for the unknown protection from vaccine among persons vaccinated <2 weeks before onset of illness, because peak antibody response after influenza vaccination takes approximately 2 weeks and the level of protection during the 1--13 days after vaccination is not known (4,5). In the first estimate, persons vaccinated <2 weeks before illness onset were counted as unvaccinated; in the second estimate, those vaccinated <2 weeks before illness onset were excluded from the analyses. Categorical AnalysisPersons vaccinated on or after November 1 (the outbreak period) were excluded from the categorical analysis. ILI attack rates were compared between vaccinated and unvaccinated groups by using the Cochran-Mantel-Haenszel chi-square test to adjust for age group, patient contact, and high-risk conditions. Vaccine effectiveness against ILI was calculated as 1 -- vaccinated attack rate / unvaccinated attack rate. Point estimates for vaccine effectiveness were 14% and 3%, respectively, with 95% confidence intervals for both estimates that included zero (Table 2). Person-Time AnalysisTo determine vaccination status in the person-time analysis, the exposure cohort time was calculated from the time of the outbreak, November 1, 2003, until onset of a self-reported illness or until the date of survey completion (December 11--17). In this analysis, persons reporting vaccination during the outbreak period contributed both vaccinated and unvaccinated time. Incidence rates (i.e., the number of cases divided by person-months) for ILI were estimated separately for vaccinated and unvaccinated person-times. Vaccine effectiveness against ILI was calculated as 1 -- vaccinated incidence rate / unvaccinated incidence rate. A Cox proportional hazards model was used to estimate an incidence rate ratio adjusted for age group, patient contact, and high-risk conditions (6). Adjusted vaccine effectiveness for both estimates were not statistically significantly different from zero (Table 2). Reported by: S Dolan, MS, AC Nyquist, MD, D Ondrejka, PhD, J Todd, MD, The Children's Hospital, Denver; K Gershman, MD, Colorado Dept of Public Health and Environment. J Alexander, MD, C Bridges, MD, J Copeland, MS, F David, MS, G Euler, DrPH, P Gargiullo, PhD, K Kenyan, MPH , Z Moore, MD, J Seward, MBBS, Epidemiology Surveillance Div, National Immunization Program; N Jain, MD, EIS Officer, CDC. Editorial Note:The preliminary findings presented in this report demonstrated no or very low effectiveness of TIV against ILI. However, these findings do not provide a basis for assessing the effectiveness of TIV against more severe illness outcomes or against influenza B or influenza A (H1N1), nor do they assess the effectiveness of live attenuated influenza vaccine (LAIV). Despite a suboptimal antigenic match, TIV can still provide protection against influenza complications. In a study conducted among patients aged >65 years, TIV was effective in preventing 61% of influenza-related deaths when the vaccine and circulating strains were well matched and 35% when they were not well matched (7). Estimates of vaccine effectiveness generally are lower against ILI than against laboratory-confirmed influenza. During the 1998--99 season, when the vaccine and circulating strains were well matched, TIV effectiveness among healthy adults was 86% against laboratory-confirmed influenza and 34% against ILI; during the 1997--98 season, when the vaccine and circulating strains were not well matched, TIV effectiveness was 50% against laboratory-confirmed influenza and zero against ILI (8). Further studies are under way or planned to estimate the effectiveness of the 2003--04 influenza vaccine against laboratory-confirmed influenza and influenza-related complications. The person-time analysis included all persons in the cohort regardless of when they were vaccinated. These estimates probably are more precise than those obtained in the categorical analysis. Some negative effectiveness estimates (i.e., estimates lower than zero) were obtained in this analysis; because vaccine effectiveness cannot be lower than zero, such estimates should be considered zero. These results suggest that the study had unknown or uncorrected disparities between the vaccinated and unvaccinated groups in terms of risk for disease or other factors. The findings in this report are subject to at least five limitations. First, study participants were not selected at random to receive influenza vaccination, and persons with greater patient exposure and possibly greater exposure to influenza viruses were more likely to be vaccinated. Second, a greater percentage of persons aged >50 years and persons with one or more conditions associated with increased risk for influenza-related complications were vaccinated. Third, other biases, including participation in the study and reporting illness based on vaccination, might have occurred. Such biases and other differences between the vaccinated and unvaccinated groups are very likely to have occurred, given the disparities noted. For example, persons who were vaccinated and became ill might have been more likely to complete the questionnaire, biasing the study to indicate lower effectiveness. Fourth, influenza vaccination and illnesses were self-reported. Finally, the sample size might not have been large enough to detect vaccine effectiveness against ILI, particularly because a large proportion of the respondents were vaccinated. Many of these limitations can be avoided by using a prospective cohort study design with laboratory-confirmed disease as an outcome. Conducting annual prospective studies would provide consistent and comparable vaccine effectiveness data to assist with public health decision making. This study does not provide data that permits an assessment of the effectiveness of TIV against laboratory-confirmed influenza and its complications. Additional studies to provide such data are under way. Because TIV was effective against laboratory-confirmed influenza and influenza-related complications in previous years in which it was not effective against ILI (8,9), and because influenza B and influenza A (H1N1) viruses might cause serious illness later this season, influenza vaccine continues to be recommended for persons at increased risk for influenza-related complications, their household contacts, and health-care personnel (Box. References
* Outbreaks of influenza or increases in influenza-like illness (ILI) cases and recent laboratory-confirmed influenza in at least half the regions of a state.
Table 1 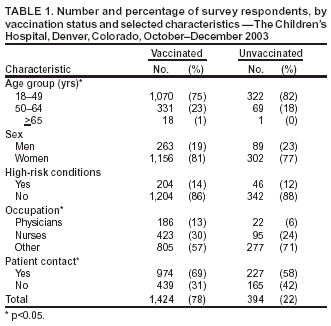 Return to top. Table 2 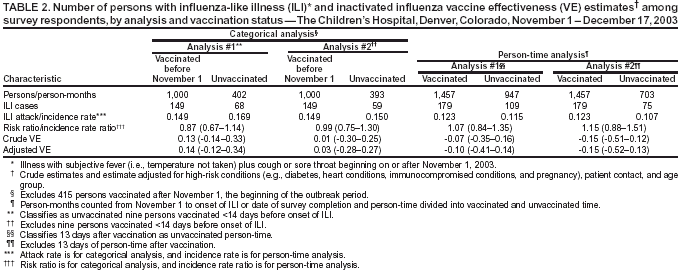 Return to top. Figure 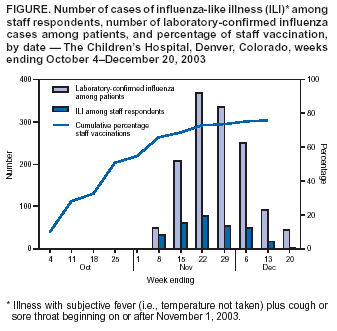 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/15/2004 |
|||||||||
This page last reviewed 1/15/2004
|