 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Probable Transfusion-Transmitted Malaria --- Houston, Texas, 2003Malaria transmitted by blood transfusion is rare in the United States, with an estimated incidence of <0.3 cases per million transfused blood units. The last reported case of transfusion-transmitted malaria occurred in January 1998 (1); during 1990--1998, a total of 12 cases were identified (2). This report summarizes a case of malaria in Houston, Texas, that was likely transmitted from a blood donor. Because no laboratory test exists in the United States to screen donated blood for malaria, this case highlights the importance of effective donor screening to help prevent transfusion-transmitted malaria. In March 2003, a patient aged 69 years with a history of diabetic nephropathy and hypertension was admitted to a Houston-area hospital with malignant hypertension and acute renal failure. After 3 days, the patient was transfused with two units of packed red blood cells (PRBCs) for severe anemia. The patient reported having no other blood transfusions during the 12 months preceding hospitalization. The patient was started on hemodialysis and discharged; 17 days after the transfusion, the patient had fever and mental confusion, and 3 days later was admitted to the intensive care unit at a second Houston hospital. Blood cultures and cerebrospinal fluid testing did not reveal the presence of a bacterial pathogen. However, a blood smear demonstrated Plasmodium falciparum parasites. The patient was hospitalized for 21 days, treated successfully with intravenous quinidine and doxycycline, and discharged. Epidemiologists from the Houston Department of Health and Human Services interviewed members of the patient's household to obtain risk factor information and to ascertain the source of exposure. The patient, who was retired and spent most hours indoors, had last traveled outside Houston in 1995 to visit Laredo, Texas, on the Mexican border. The Texas Department of Health, in collaboration with CDC and the local blood collection center, conducted a donor traceback investigation of the two units of PRBCs used for the patient's transfusions. One donor was a woman, a U.S.-born Texas resident aged 47 years who had never traveled outside the United States. The other donor was a man, a native of Ghana aged 18 years whose March 2003 blood donation record stated he had arrived from Ghana 2 years earlier and had never had malaria. The investigation determined that the Ghanaian donor had immigrated to Houston in May 2002. He denied having any febrile illness during the 12 months preceding the blood donation. However, his mother recalled that her son had been treated for malaria in Ghana 2.5 years earlier. No segments from the two donors' original blood collection bags were available for testing; however, both donors submitted blood specimens for malaria smears, DNA polymerase chain reaction (PCR) testing, and indirect immunofluorescence (IFA) testing for malarial antibodies 4--5 weeks after their blood donations. The blood smear examination, PCR test, and IFA test performed on the specimen from the U.S.-born donor all were negative. The blood smear examination and PCR performed on the specimen from the Ghanaian donor also were negative for the presence of malaria parasites or parasite DNA. However, the IFA test found elevated titers of antibodies to malaria (1:256 for P. falciparum, 1:64 for P. malariae, 1:64 for P. ovale, and 1:64 for P. vivax), indicating previous malaria infection at an indeterminate time. Reported by: R Arafat, MD, S Long, MD, M Perry, MPH, S Marsh, Houston Dept of Health and Human Svcs, Houston, Texas. M Wilson, MS, Div of Parasitic Diseases, National Center for Infectious Diseases; S Avashia, MD, S Filler, MD, EIS officers, CDC. Editorial Note:In the case described in this report, the incubation period from transfusion to illness was consistent with previously reported cases of transfusion-transmitted P. falciparum malaria (2). The presence of malarial antibodies in samples taken from the Ghanaian donor 1 month after donation indicate previous infection; this infection might have been active at the time of blood donation. Donors who have been implicated as infection sources in transfusion-transmitted malaria cases usually have had undetectable levels of parasitemia; therefore, antibody detection has been the method of choice to identify infected donors in CDC investigations of transfusion-transmitted malaria cases. Malaria antibody testing is 95% sensitive and 99% specific. Because the donor emigrated from an area with endemic malaria, the predictive value positive for this test is high (3). The majority of malaria cases in the United States are associated with previous travel to areas where malaria is endemic. Although mosquito-borne transmission of malaria has occurred occasionally in the United States, including in Texas, those cases occurred during summer months (4). In this case, factors including positive donor serology, absence of travel by the patient, low likelihood of local transmission in early spring, and an appropriate incubation period support transfusion as the likely mechanism for malaria transmission. No available laboratory test is suitable for screening donated blood for malaria. Such a test would require 1) large-scale use design, 2) high sensitivity and specificity, and 3) ability to detect all four species of Plasmodium that affect humans. In the United States, prevention of transfusion-transmitted malaria largely depends on careful questioning of prospective donors to defer those at increased risk for malaria. The Food and Drug Administration (FDA) recommends deferring residents of malaria-endemic areas for 3 years after they emigrate from those areas and deferring persons who have had malaria for 3 years after they become asymptomatic; the American Association of Blood Banks has published standards consistent with FDA recommendations (Box. In this case, the donor from Ghana should have been deferred for both reasons. However, the donor did not tell the screener about having malaria during the previous 3 years, and the screener did not defer the donor for immigrating within 3 years from an area with endemic malaria. During 1963--1999, approximately two thirds of the 93 transfusion-transmitted malaria cases in the United States could have been prevented if the implicated donors had been deferred according to established guidelines (2). To facilitate the donor screening process, CDC is developing an Internet-based map to help screeners identify areas with endemic malaria. Instances of transfusion-related transmission of malaria should be carefully reviewed to determine whether improvements to the donor screening process are needed. The case described in this report underscores the importance of close cooperation between managers of blood collection centers and state and federal public health officials whenever transfusion-related illness occurs. Such cooperation can facilitate traceback investigations and ensure prompt care of both donors and recipients, helping to strengthen the screening process, making blood transfusion as safe as possible, and ensuring an adequate supply of a lifesaving resource. References
Box 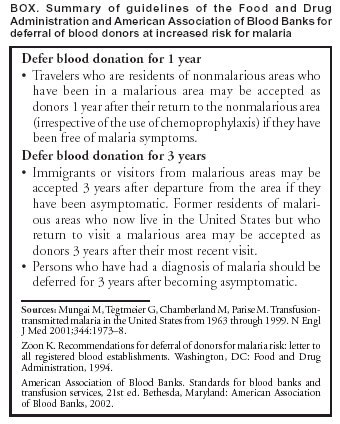 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/6/2003 |
|||||||||
This page last reviewed 11/6/2003
|