 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Notice to Readers: Guidelines for Maintaining and Managing the Vaccine Cold ChainIn February 2002, the Advisory Committee on Immunization Practices (ACIP) and American Academy of Family Physicians (AAFP) released their revised General Recommendations on Immunization (1), which included recommendations on the storage and handling of immunobiologics. Because of increased concern over the potential for errors with the vaccine cold chain (i.e., maintaining proper vaccine temperatures during storage and handling to preserve potency), this notice advises vaccine providers of the importance of proper cold chain management practices. This report describes proper storage units and storage temperatures, outlines appropriate temperature-monitoring practices, and recommends steps for evaluating a temperature-monitoring program. The success of efforts against vaccine-preventable diseases is attributable in part to proper storage and handling of vaccines. Exposure of vaccines to temperatures outside the recommended ranges can affect potency adversely, thereby reducing protection from vaccine-preventable diseases (1). Good practices to maintain proper vaccine storage and handling can ensure that the full benefit of immunization is realized. Recommended Storage TemperaturesThe majority of commonly recommended vaccines require storage temperatures of 35°F--46°F (2°C--8°C) and must not be exposed to freezing temperatures. Introduction of varicella vaccine in 1995 and of live attenuated influenza vaccine (LAIV) more recently increased the complexity of vaccine storage. Both varicella vaccine and LAIV must be stored in a continuously frozen state <5°F (-15°C) with no freeze-thaw cycles (Table 1). In recent years, instances of improper vaccine storage have been reported. An estimated 17%--37% of providers expose vaccines to improper storage temperatures, and refrigerator temperatures are more commonly kept too cold than too warm (2,3). Freezing temperatures can irreversibly reduce the potency of vaccines required to be stored at 35°F--46°F (2°C--8°C). Certain freeze-sensitive vaccines contain an aluminum adjuvant that precipitates when exposed to freezing temperatures. This results in loss of the adjuvant effect and vaccine potency (4). Physical changes are not always apparent after exposure to freezing temperatures and visible signs of freezing are not necessary to result in a decrease in vaccine potency. Although the potency of the majority of vaccines can be affected adversely by storage temperatures that are too warm, these effects are usually more gradual, predictable, and smaller in magnitude than losses from temperatures that are too cold. In contrast, varicella vaccine and LAIV are required to be stored in continuously frozen states and lose potency when stored above the recommended temperature range. Vaccine Storage RequirementsVaccine storage units must be selected carefully and used properly. A combination refrigerator/freezer unit sold for home use is acceptable for vaccine storage if the refrigerator and freezer compartments each have a separate door. However, vaccines should not be stored near the cold air outlet from the freezer to the refrigerator. Many combination units cool the refrigerator compartment by using air from the freezer compartment. In these units, the freezer thermostat controls freezer temperature while the refrigerator thermostat controls the volume of freezer temperature air entering the refrigerator. This can result in different temperature zones within the refrigerator. Refrigerators without freezers and stand-alone freezers usually perform better at maintaining the precise temperatures required for vaccine storage, and such single-purpose units sold for home use are less expensive alternatives to medical specialty equipment. Any refrigerator or freezer used for vaccine storage must maintain the required temperature range year-round, be large enough to hold the year's largest inventory, and be dedicated to storage of biologics (i.e., food or beverages should not be stored in vaccine storage units). In addition, vaccines should be stored centrally in the refrigerator or freezer, not in the door or on the bottom of the storage unit, and sufficiently away from walls to allow air to circulate. Temperature MonitoringProper temperature monitoring is key to proper cold chain management. Thermometers should be placed in a central location in the storage unit, adjacent to the vaccine. Temperatures should be read and documented twice each day, once when the office or clinic opens and once at the end of the day. Temperature logs should be kept on file for >3 years, unless state statutes or rules require a longer period. Immediate action must be taken to correct storage temperatures that are outside the recommended ranges. Mishandled vaccines should not be administered. One person should be assigned primary responsibility for maintaining temperature logs, along with one backup person. Temperature logs should be reviewed by the backup person at least weekly. All staff members working with vaccines should be familiar with proper temperature monitoring. Different types of thermometers can be used, including standard fluid-filled, min-max, and continuous chart recorder thermometers (Table 2). Standard fluid-filled thermometers are the simplest and least expensive products, but some models might perform poorly. Product temperature thermometers (i.e., those encased in biosafe liquids) might reflect vaccine temperature more accurately. Min-max thermometers monitor the temperature range. Continuous chart recorder thermometers monitor temperature range and duration and can be recalibrated at specified intervals. All thermometers used for monitoring vaccine storage temperatures should be calibrated and certified by an appropriate agency (e.g., National Institute of Standards and Technology). In addition, temperature indicators (e.g., Freeze Watch™ [3M, St. Paul, Minnesota] or ColdMark™ [Cold Ice, Inc., Oakland, California]) can be considered as a backup monitoring system (5); however, such indicators should not be used as a substitute for twice daily temperature readings and documentation. All medical care providers who administer vaccines should evaluate their cold chain maintenance and management to ensure that 1) designated personnel and backup personnel have written duties and are trained in vaccine storage and handling; 2) accurate thermometers are placed properly in all vaccine storage units and any limitations of the storage system are fully known; 3) vaccines are placed properly within the refrigerator or freezer in which proper temperatures are maintained; 4) temperature logs are reviewed for completeness and any deviations from recommended temperature ranges; 5) any out-of-range temperatures prompt immediate action to fix the problem, with results of these actions documented; 6) any vaccines exposed to out-of-range temperatures are marked "do not use" and isolated physically; 7) when a problem is discovered, the exposed vaccine is maintained at proper temperatures while state or local health departments, or the vaccine manufacturers, are contacted for guidance; and 8) written emergency retrieval and storage procedures are in place in case of equipment failures or power outages. Around-the-clock monitoring systems might be considered to alert staff to after-hours emergencies, particularly if large vaccine inventories are maintained. Additional information on vaccine storage and handling is available from the Immunization Action Coalition at http://www.immunize.org/izpractices/index.htm. Links to state and local health departments are available at http://www.cdc.gov/other.htm. Especially detailed guidelines from the Commonwealth of Australia on vaccine storage and handling, vaccine storage units, temperature monitoring, and stability of vaccines at different temperatures (6) are available at http://immunise.health.gov.au/cool.pdf. References
Table 1 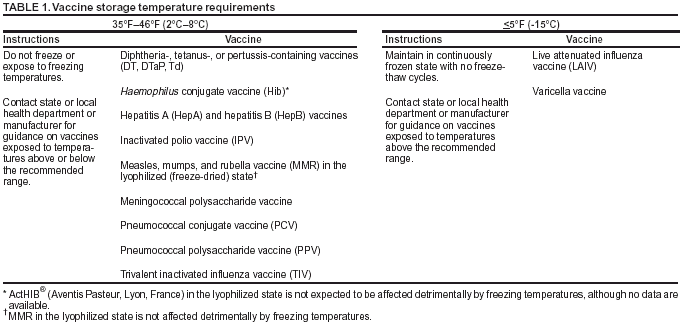 Return to top. Table 2 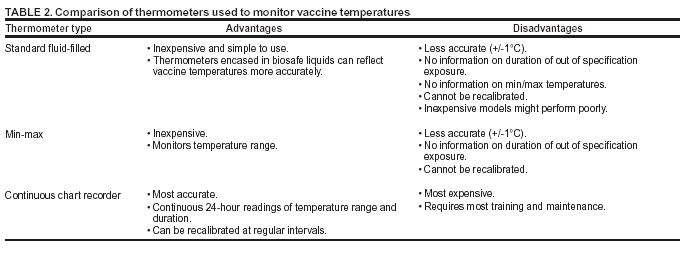 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/23/2003 |
|||||||||
This page last reviewed 10/23/2003
|