 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. West Nile Virus Activity --- United States, September 26--October 2, 2002, and Investigations of West Nile Virus Infections in Recipients of Blood Transfusion and Organ TransplantationThis report summarizes West Nile virus (WNV) surveillance data reported to CDC through ArboNET and by states and other jurisdictions as of 7 a.m. Mountain Daylight Time, October 2, 2002, and updates preliminary demographic and clinical information on cases of WNV infections in recipients of blood transfusion and organ transplantation reported to CDC during August 28--October 2, 2002. WNV SurveillanceDuring the reporting period of September 26--October 2, a total of 409 laboratory-positive human cases of WNV-associated illness were reported from Illinois (n=81), Michigan (n=73), Ohio (n=56), Indiana (n=53), Nebraska (n=32), Louisiana (n=26), Missouri (n=17), Kentucky (n=13), Pennsylvania (n=eight), Iowa (n=seven), Minnesota (n=seven), Mississippi (n=six), Alabama (n=five), New York (n=five), Tennessee (n=five), Wisconsin (n=five), Maryland (n=four), Colorado (n=two), New Jersey (n=two), South Dakota (n=one), and Texas (n=one). During the same period, WNV infections were reported in 684 dead crows and 441 other dead birds. A total of 1,027 veterinary cases were reported (1,026 equine and one other species). During the same period, 521 WNV-positive mosquito pools were reported. During 2002, a total of 2,530 human cases with laboratory evidence of recent WNV infection have been reported from Illinois (n=599), Michigan (n=343), Ohio (n=288), Louisiana (n=287), Mississippi (n=163), Indiana (n=157), Missouri (n=131), Texas (n=92), Nebraska (n=80), New York (n=51), Kentucky (n=40), Tennessee (n=31), Alabama (n=30), Minnesota (n=26), Pennsylvania (n=26), Iowa (n=25), South Dakota (n=24), Georgia (n=19), Wisconsin (n=19), Virginia (n=16), North Dakota (n=15), Arkansas (n=11), Maryland (n=10), Massachusetts (n=10), Florida (n=eight), Connecticut (n=seven), the District of Columbia (n=six), New Jersey (n=six), Oklahoma (n=four), Colorado (n=three), California (n=one), North Carolina (n=one), and South Carolina (n=one) (Figure). Among the 2,132 patients for whom data were available, the median age was 56 years (range: 1 month--99 years); 1,150 (54%) were male, and the dates of illness onset ranged from June 10 to September 23. A total of 116 human deaths have been reported. The median age of decedents was 79 years (range: 27--99 years); 70 (60%) deaths were among men. In addition, 5,633 dead crows and 4,216 other dead birds with WNV infection were reported from 42 states, New York City, and the District of Columbia; 4,377 WNV infections in mammals (4,369 equines, three canines, and five other species) have been reported from 33 states (Alabama, Arkansas, Colorado, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, and Wyoming). During 2002, WNV seroconversions have been reported in 310 sentinel chicken flocks from Florida, Iowa, Nebraska, Pennsylvania, and New York City; 3,874 WNV-positive mosquito pools have been reported from 26 states (Alabama, Arkansas, Connecticut, Delaware, Georgia, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Vermont, and Virginia), New York City, and the District of Columbia. WNV Infections in Recipients of Blood Transfusion and Organ TransplantationCDC, the Food and Drug Administration, and the Health Resources and Services Administration, in collaboration with blood collection agencies and state and local health departments, continue to investigate WNV infections in recipients of blood transfusion and organ transplantation. During August 28--October 2, CDC received reports from 10 states of 15 patients with confirmed West Nile meningoencephalitis (WNME) or meningitis diagnosed after receiving blood components within 1 month of illness onset. CDC has been notified of additional cases among transfusion recipients, but demographic and clinical information is pending. All 15 of these patients resided in areas with high levels of WNV activity. Investigations are ongoing to determine whether transfusion was the source of WNV transmission. Of the 15 cases, eight (53%) were reported since September 25. One patient, an organ donor from Georgia, was positive for WNV at the time of organ recovery following receipt of multiple blood transfusions (1). The onset of symptoms for the remaining 14 patients began in July (two patients), August (five patients), and September (seven patients). The reasons for hospitalization included a surgical procedure or obstetric delivery (four patients) and solid organ transplantation (three patients who received an organ from different donors who did not have evidence of WNV infection at the time the organs were recovered). Five patients had hematologic conditions, three patients had myelodysplasia, and two patients had acute myelogenous leukemia. These 15 patients received blood components from a median of 18 donors (range: 2--185 donors). WNME was the probable cause of death for at least three of the four patients who died. Some of these investigations provide evidence that WNV can be transmitted through blood transfusion. Two patients tested positive for WNV infection after receiving different blood products derived from a single blood donation subsequently found to have evidence of WNV (2). In another case, WNV was isolated from a unit of frozen plasma that had been withdrawn as a result of the investigation, indicating that the virus can survive in some blood components (1). In addition to these patients, investigations in Georgia and Florida have demonstrated transmission of WNV in four recipients of solid organs from a single donor (1,3,4). Patients with WNV infection who have received blood transfusions or organs within the 4 weeks preceding the onset of symptom should be reported to CDC through local public health authorities. Serum or tissue samples should be retained for later studies. In addition, the Public Health Service is expanding an earlier recommendation (1) to request that cases of WNV infection in patients who had onset of symptoms within 2 weeks of blood or organ donation be reported. Prompt reporting of these cases will facilitate withdrawal of potentially infected blood components. Additional information about WNV activity is available from CDC at http://www.cdc.gov/ncidod/dvbid/westnile/index.htm and http://www.cindi.usgs.gov/hazard/event/west_nile/west_nile.html. References
Figure 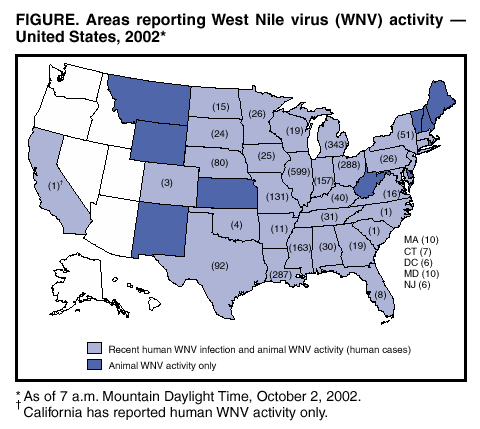 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/3/2002 |
|||||||||
This page last reviewed 10/3/2002
|