 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Laboratory-Acquired Meningococcal Disease --- United States, 2000Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis among older children and young adults in the United States. N. meningitidis usually is transmitted through close contact with aerosols or secretions from the human nasopharynx. Although N. meningitidis is regularly isolated in clinical laboratories, it has infrequently been reported as a cause of laboratory-acquired infection. This report describes two probable cases of fatal laboratory-acquired meningococcal disease and the results of an inquiry to identify previously unreported cases. The findings indicate that N. meningitidis isolates pose a risk for microbiologists and should be handled in a manner that minimizes risk for exposure to aerosols or droplets. Case ReportsCase 1. On July 15, 2000, an Alabama microbiologist aged 35 years presented to the emergency department of hospital A with acute onset of generalized malaise, fever, and diffuse myalgias. The patient was given a prescription for oral antibiotics and released. On July 16, the patient returned to hospital A, became tachycardic and hypotensive, and died 3 hours later. Blood cultures were positive for N. meningitidis serogroup C. Three days before the onset of symptoms, the patient had prepared a Gram's stain from the blood culture of a patient who was subsequently shown to have meningococcal disease; the microbiologist also had handled and subcultured agar plates containing cerebrospinal fluid (CSF) cultures of N. meningitidis serogroup C from the same patient. Co-workers reported that in the laboratory, aspiration of materials from blood culture bottles was performed at the open laboratory bench; biosafety cabinets, eye protection, or masks were not used routinely for this procedure. Results of pulsed-field gel electrophoresis (PFGE) and multilocus enzyme electrophoresis (MEE) testing at CDC indicated that the two isolates were indistinguishable. The laboratory at hospital A infrequently processed isolates of N. meningitidis and had not processed another meningococcal isolate during the previous 4 years. Case 2. On December 24, 2000, a Michigan micro-biologist aged 52 years had acute onset of sore throat, vomiting, headache, and fever; by December 25, the patient had developed a petechial rash on both legs, which quickly evolved to widespread purpura. The patient presented to the emergency department of hospital B and died later that day of overwhelming sepsis. Blood cultures were positive for N. meningitidis serogroup C. The patient was a micro-biologist in the state public health laboratory and had worked on several N. meningitidis serogroup C isolates during the 2 weeks before becoming ill. That laboratory had handled a median of four meningococcal isolates per month (range: 0--11) during the previous 4 years. Co-workers reported that the patient had performed slide agglutination testing and recorded colonial morphology using typical biosafety level 2 (BSL 2) precautions; this did not entail the use of a biosafety cabinet. PFGE was performed at the state public health laboratory and at CDC on all four specimens handled by the microbiologist; results of this testing indicated that the isolates from the patient and from one of the recently handled laboratory samples were indistinguishable. To detect additional cases, on November 11, 2000, a request for information was posted on selected electronic mail discussion groups (i.e., listservs) to members of several infectious disease, microbiology, and infection control professional organizations. A probable case of laboratory-acquired meningococcal disease was defined as confirmed or probable meningococcal disease (1) in a laboratory scientist who had had occupational exposure to a N. meningitidis isolate during the 14 days before onset of illness and who had illness with a serogroup that matched the source isolate. In addition to the two cases described in this report, CDC received an additional 14 reports of probable laboratory-acquired meningococcal disease worldwide during the preceding 15 years; six cases occurred in the United States during 1996--2001. The source isolates from five of these six U.S. cases were from either blood or CSF; the source of the sixth isolate could not be definitively determined but was most likely CSF or middle ear fluid. Of these 16 previously unreported cases, nine (56%) were caused by N. meningitidis serogroup B, and seven (44%) were caused by serogroup C; eight cases (50%) were fatal (three from serogroup B and five from serogroup C). Case-fatality rates did not differ significantly by serogroups (serogroup C: 71%; serogroup B: 33%; p=0.16). In the 10 cases for which data were available, a median of 4 days (range: 2--10 days) passed between handling the source isolate and symptom onset. Procedures performed on the 16 source isolates included reading plates (50%), making subcultures on agar plates (50%), and performing serogroup identification at the bench (38%). In 15 of the 16 cases, the laboratory reportedly did not perform procedures within a biosafety cabinet. All 16 cases occurred among workers in the microbiology section of the laboratory; no cases were reported among workers in hematology, chemistry, or pathology. Reported by: J Lofgren, MD, B Whitley, MPH, Alabama Dept of Health. D Johnson, MD, F Downes, DrPH, State Public Health Laboratory; P Somsel, DrPH, B Robinson-Dunn, PhD, J Massey, DrPH, G Stoltman, PhD, MG Stobierski, DVM, S Bidol, MPH, Michigan Dept of Community Health. C Hahn, MD, L Tengelson, DVM, Idaho Dept of Public Health. P Murray, PhD, American Society for Microbiology, Washington, DC. The Infectious Diseases Committee of the American Public Health Laboratories Association, Washington, DC. The College of American Pathologists, Waukegan, Illinois. D Sewell, PhD, National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania. W Schaffner, MD, Vanderbilt Univ School of Medicine, Nashville, Tennessee. D Stephens, Div of Infectious Diseases, Emory Univ School of Medicine, Atlanta, Georgia. M Miller, Div of Healthcare Quality Promotion; J Sejvar, MD, T Popovic, MD, B Perkins, MD, N Rosenstein, MD, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; National Institute for Occupational Safety and Health; Div of Laboratory Systems; Office of Health and Safety, CDC. Editorial Note:Although the risk for disease remains low (2), laboratory-acquired meningococcal disease represents an occupational hazard to microbiologists. The findings in this report were self-reported and required respondents to have access to electronic media. However, the identification of 14 previously unreported cases and the additional two cases reported to CDC in 2001 suggest that either cases of laboratory-acquired meningococcal disease are underreported or the incidence of laboratory-acquired meningococcal disease has increased. The case-fatality rate of 50% in this report is substantially higher than that observed among community-acquired cases; this might reflect underreporting of mild cases or might be a result of the highly virulent strains and high concentration of organisms encountered in the laboratory setting. Each year in the United States, approximately 3,000 isolates of invasive N. meningitidis are cultured (3); on the basis of standard practices used for isolation and identification of N. meningitidis, each of the clinical samples and isolates is handled by an average of three microbiologists during the course of a laboratory investigation, resulting in an estimated 9,000 microbiologists exposed per year. During 1996--2000 in the United States, six cases of probable laboratory-acquired meningococcal disease were detected, for an attack rate of 13 per 100,000 population (95% confidence interval [CI]= 5--29) at risk per year, compared with approximately 0.2 per 100,000 population among adults aged 30--59 in the United States (CDC, unpublished data, 2001), the age group of most laboratory scientists. If the three cases from 2000 are excluded from this estimate, the attack rate is seven (95% CI=1--19). N. meningitidis is classified as a biosafety level 2 organism (4). Guidelines recommend the use of a biosafety cabinet for mechanical manipulations of samples that have a substantial risk for droplet formation or aerosolization such as centrifuging, grinding, and blending procedures (4,5). Less is known about the risk associated with routine isolate manipulation. The exclusive occurrence of probable laboratory-acquired cases in microbiologists suggests that exposure to isolates of N. meningitidis, and not patient samples, increases the risk for infection. Nearly all the microbiologists in this report were manipulating isolates and performing subplating with an inoculation loop on an open laboratory bench. A recent study indicated that manipulating suspensions of N. meningitidis outside a biosafety cabinet is associated with a high risk for contracting disease (3). Isolates obtained from a respiratory source are in general less pathogenic and represent a lower risk for microbiologists. Although the exact mechanism of transmission in the laboratory setting is unclear, use of a biosafety cabinet during manipulation of sterile site isolates of N. meningitidis would ensure protection. Alternative methods of protection (e.g., splash guards and masks) from droplets and aerosols require additional assessment. If a biosafety cabinet or other means of protection is unavailable, manipulation of these isolates should be minimized, and workers should consider sending specimens to laboratories possessing this equipment. Education of microbiologists and strict adherence to these safety precautions when manipulating meningococcal isolates should further minimize the risk for infection. To address these safety issues, the governing bodies of organizations responsible for setting policy for laboratory safety will be reassessing current guidelines about the handling of N. meningitidis. Although primary prevention should focus on laboratory safety, laboratory workers also should make informed decisions about vaccination. The quadrivalent meningococcal polysaccharide vaccine, which includes serogroups A, C, Y, and W-135, will decrease but not eliminate the risk for infection (6). Research and industrial laboratory scientists who are exposed routinely to N. meningitidis in solutions that might be aerosolized also should consider vaccination (6--8). In addition, vaccination might be used as an adjunctive measure by microbiologists in clinical laboratories. Laboratory scientists with percutaneous exposure to an invasive N. meningitidis isolate from a sterile site should receive treatment with penicillin; those with known mucosal exposure should receive antimicrobial chemoprophylaxis (6) (Table 1). Microbiologists who manipulate invasive N. meningitidis isolates in a manner that could induce aerosolization or droplet formation (including plating, subculturing, and serogrouping) on an open bench top and in the absence of effective protection from droplets or aerosols also should consider antimicrobial chemoprophylaxis. CDC has instituted prospective surveillance for laboratory-acquired meningococcal disease. Hospitals, laboratories, and public health departments that are aware of suspected cases should report these cases through their state public health department to CDC, telephone 404-639-3158. References
Table 1 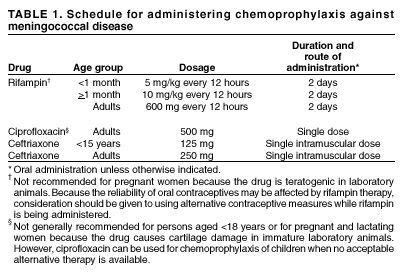 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/21/2002 |
|||||||||
This page last reviewed 2/21/2002
|