 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Notice to Readers: Recommended Childhood Immunization Schedule --- United States, 2002Each year, CDC's Advisory Committee on Immunization Practices (ACIP) reviews the recommended childhood immunization schedule to ensure that it is current with changes in manufacturers' vaccine formulations, has revised recommendations for the use of licensed vaccines, and has recommendations for newly licensed vaccines. This report presents the recommended childhood immunization schedule for 2002, which has remained the same in content since January 2001 (1) but has a redesigned format (Figure 1). The format of the 2002 schedule is based on a design developed by the Minnesota Department of Health immunization program; the recommendations and format have been approved by ACIP, the American Academy of Family Physicians, and the American Academy of Pediatrics. The new design highlights the importance of catch-up vaccination, the preadolescent visit, the preference for administering the first dose of the hepatitis B vaccine series at birth, and three vaccines for selected at-risk groups. The importance of assessing whether children aged 24 months--18 years require any catch-up vaccination is emphasized by the use of hatched bars. The schedule also underscores the visit at age 11--12 years when immunization status should be reviewed and all necessary vaccines administered. Hepatitis B VaccineThe schedule indicates a preference for administering the first dose of hepatitis B vaccine to all newborns soon after birth and before hospital discharge. Administering the first dose of hepatitis B vaccine soon after birth should minimize the risk for infection because of errors in maternal hepatitis B surface antigen (HBsAg) testing or reporting, or from exposure to persons with chronic hepatitis B virus (HBV) infection in the household, and can increase the likelihood of completing the vaccine series. Only monovalent hepatitis B vaccine can be used for the birth dose. Either monovalent or combination vaccine can be used to complete the series. Four doses of hepatitis B vaccine, including the birth dose, may be administered if a combination vaccine is used to complete the series. In addition to receiving hepatitis B immune globulin (HBIG) and the hepatitis B vaccine series, infants born to HBsAg-positive mothers should be tested for HBsAg and antibody to HBsAg (anti-HBs) at age 9--15 months to identify those with chronic HBV infection or those who may require revaccination (2). Vaccines for Selected PopulationsThe area below the dashed line (Figure 1) displays certain vaccines recommended for use in selected populations. High-risk children aged 24--59 months should receive catch-up pneumococcal conjugate vaccine (PCV) doses, if indicated (3). Pneumococcal polysaccharide vaccine (PPV) is recommended in addition to PCV for certain high-risk groups (3). The recommendation to administer annual influenza vaccine to high-risk children also appears on the schedule (4). Vaccine SupplyAs a result of the vaccine supply shortage, deferral of some doses of tetanus and diphtheria toxoids (Td), diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP), and pneumococcal conjugate vaccine (PCV) has been recommended (5--7); health-care providers should record patients for whom vaccination has been deferred and should contact them once the supply has been restored. Vaccine Information StatementsThe National Childhood Vaccine Injury Act requires that all health-care providers give parents or patients copies of Vaccine Information Statements before administering each dose of the vaccines listed in the schedule. Additional information about Vaccine Information Statements is available from state health departments and at http://www.cdc.gov/nip/publications/VIS. Detailed recommendations for using vaccines are available from the manufacturers' package inserts, ACIP statements on specific vaccines, and the 2000 Red Book (2--4,8). ACIP statements for each recommended childhood vaccine can be viewed, downloaded, and printed from the CDC National Immunization Program at http://www.cdc.gov/nip/publications/ACIP-list.htm; instructions on the use of the Vaccine Information Statements are available at http://www.cdc.gov/nip/publications/VIS/vis-Instructions.pdf. References
Figure 1 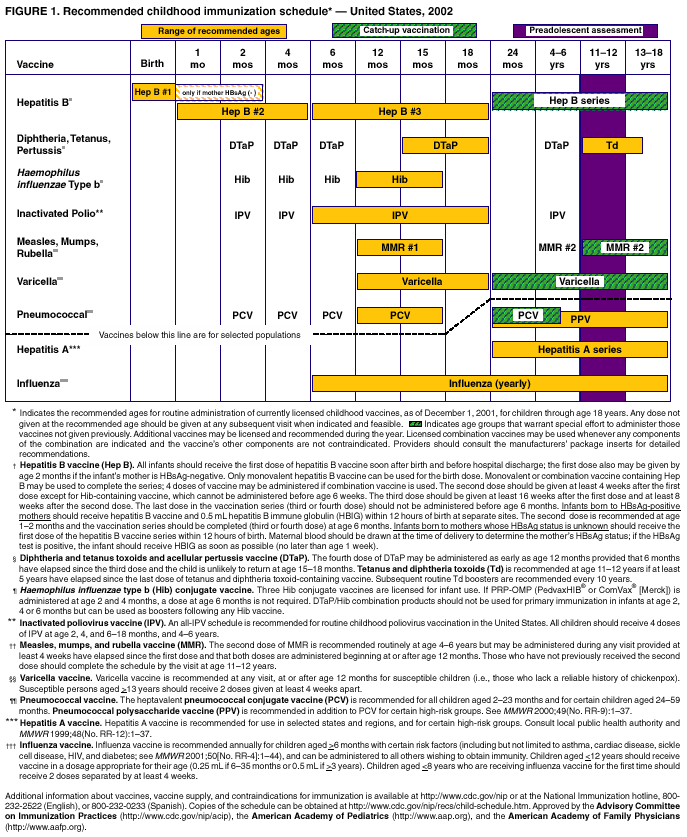 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/17/2002 |
|||||||||
This page last reviewed 1/17/2002
|